Abstract
The application of equipment and tools that produce a magnetic field is increasing in aquatic ecosystems. In the present study, the effects of acute (1 week) and subacute (3 weeks) exposures to different static magnetic fields (SMFs) of 2.5, 5, 7.5 mT on stress indices (cortisol and glucose), sex steroid hormones (17β-estradiol and 17-α hydroxy progesterone) and fecundity of the zebrafish (Danio rerio) were investigated. The obtained results showed a significant change in cortisol, glucose, 17β-estradiol (E2) and 17-α hydroxy progesterone (17-OHP) levels by enhancing the intensity and time of exposure to SMFs. In conclusion, the SMFs, especially at higher levels of intensities, showed physiologically harmful effects on the reproductive biology of the zebrafish during acute and subacute exposures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Static magnetic fields (SMFs) are time-independent fields of constant strength (Hashish et al. 2008). Recently, increasing environmental exposure to magnetic fields is coincidental with rapid improvements in science and technology (World Health Organization 2006). The induced magnetic fields in the aquatic environments have many different origins but mainly generate from infrastructures such as underwater power and telecommunication cables, electrical heating cables as well as generating units and submerged substations (Gill and Kimber 2005). Electric current in the cables creates the magnetic fields, which can be hazardous to the behavior or viability of fish and benthic invertebrates (Gill et al. 2009; Gill and Kimber 2005). Many studies have investigated the effect of SMFs on cell biology, genetics, reproduction and development, physiological and behavioral response (World Health Organization 2006; Health Protection Agency 2008). Several studies reviewed the responses of marine organisms to electromagnetic fields from undersea electricity cables; some suggested changes in biomass of macrofauna (Gill et al. 2009) while others did not (Andrulewicz et al. 2003). Loghmannia et al. (2015) exposed the Caspian kutum (Rutilus frisii kutum) fry to SMFs at different intensities during acute and subacute exposures; they detected some physiological changes in the samples compared to the control. Biological indices including enzymes, hormones, and biochemical parameters can be used to evaluate the health of aquatic organisms and ecosystems (Loghmannia et al. 2015). A delay in the hatching of embryos of Danio rerio was detected during exposure to the electromagnetic field with an intensity of 1 mT (Skauli et al. 2000). A fixed magnetic field (2.0 mT) increased the water permeability of the egg envelope in the bull trout, rainbow trout, and Atlantic salmon from family Salmonidae and subsequently, developmental stages of the egg were changed (Sadowski et al. 2007). The sperm motility of the trout prolonged for 12 days during exposure to SMF with intensities of 1, 5 and 10 mT whereas the control sperm had motile only for 3 days (Formicki et al. 2015).
Cortisol as a stress indicator is a steroid glucocorticoid hormone that increases the glucose level in fish (Moon et al. 1988). 17β-estradiol (E2) is an estrogen and the primary sex hormone produced by granulosa cells of the ovary (Saldanha et al. 2011). E2 is the main hormone of fish for oocyte vitellogenesis (Heidari et al. 2010). 17α-Hydroxyprogesterone (17-OHP) is another steroid hormone, which is increased by elevating the level of plasma gonadotropins and it is involved in the oocyte maturation (Heidari et al. 2010).
Zebrafish (Danio rerio) is a tropical freshwater fish from the family Cyprinidae that originates from waters in the southeastern Himalayan region (Mayden et al. 2007). The zebrafish is one of the most widely used animals in biological laboratory studies. The effects of magnetic fields on the reproductive physiology of the zebrafish as a laboratory model have not been studied in detail. Therefore, in the present study, the effects of acute and subacute exposure of SMFs on zebrafish were investigated by assaying glucose, cortisol, E2, 17-OHP, and fecundity.
Materials and Methods
Adult zebrafish (n = 240, body weight 0.8 ± 0.08 g, total length 4.75 ± 0.63 cm) were obtained from a commercial supplier (Persian aquarium, Rasht, Iran) and maintained for 2 weeks for acclimation. The experimental conditions were kept constant at pH 7.0 and temperature to 29 ± 0.7°C. The samples were fed twice a day (Fernanda et al. 2018) with the feeding powder purchased from Isfahan Mokammel Co. (Isfahan, Iran).
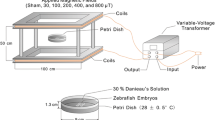
To generate magnetic field based on the Helmholtz coil theory, a device designed and made in the research laboratory of the University of Guilan as described by Loghmannia et al. (2015). They explained the details of how the device made and how the samples exposed to the magnetic field. Briefly, the SMF was generated from two coils that contain a constant electric current with a frequency of 50 Hz (Fig. 1). A Gaussmeter (model HT201, Hengtong, China) was used for daily evaluation of SMFs during the experiments. All fish except the controls were exposed to a uniform magnetic field with different intensities (2.5, 5.0 and 7.5 mT).
Device used to produce the constant static magnetic field (Loghmannia et al. 2015)
The experiment was conducted in two separate exposures, acute and subacute. In the acute experiment, the zebrafish was exposed to 2.5, 5.0 or 7.5 mT intensity of the SMF (exposures were done at the same time each day) for 1 week as well as a control (without SMF). In the subacute experiment, duration of the exposure lasted for 3 weeks with the same intensities. Therefore, ten samples (5 females and 5 males) with three replications for each treatment were kept in small glass aquariums containing 3 L of water. For exposure, the zebrafish was placed between the coils set to a specific intensity (2.5, 5.0 and 7.5 mT) for 1 h per day. At the end of the exposure period, the fish were immediately frozen at − 70°C to be used for analysis.
Whole bodies of frozen samples (n = 5 for each treatment) were homogenized (Misonix Sonicator 3000, USA) with 0.1 M phosphate buffer saline (PBS, pH 7.2) (1W:10V) for 2 min. After centrifuging at 4°C at 7000 rpm for 10 min, the supernatant was aliquoted into sterile microtubes and stored at − 70°C.
Monobind ELISA kits (Monobind Inc., Lake Forest, USA) via the ELISA method (Nash et al. 2000), were used to measure the whole-body sex steroids (17β-estradiol and 17α-Hydroxyprogesterone) and whole-body cortisol. Briefly, 25 µL of the sample was pipetted into the wells and 50 µL of the Estradiol Biotin Reagent was added to all wells. After shaking for 30 s, the wells were incubated for 30 min at room temperature and then 50 µL of the estradiol enzyme reagent was added to all wells. After shaking and incubating for 30 s and 90 min, respectively, 350 µL of wash buffer was added into the wells. Then, 100 µL of substrate solution was added to all wells and incubated at room temperature for 20 min. Finally, 50 µL of stop solution (0.5M H2SO4) was added to each well and gently mixed; the absorbance was read at the wavelength of 450 nm by ELISA reader (BioTek, ELx800, Germany) and compared with the control. Whole-body 17-OHP and cortisol were measured according to the Monobind ELISA kit protocol as above.
Whole-body glucose concentration was measured using the by o-toluidine method (Liquid O-toluidine, Sigma–Aldrich, America) (Genba et al. 1963). The optical density (OD) was read at 630 nm by a spectrophotometer (UNICO, s 2100 UV, USA). The data values were expressed as mg/g.
To calculate fecundity, the broodstocks (5 females and 5 males) were kept in smaller aquariums containing 1 L of the water (pH 7.0) at a temperature of 29 ± 0.7°C. After spawning, the broodstocks were removed from the aquarium and then all eggs were counted. In order to prevent egg feeding by the breeders, a fine net separated the bottom of the aquarium.
The obtained data were analyzed using SPSS version 20 in Windows 8. The raw values of whole-body glucose, cortisol, E2 and 17-OHP were initially analyzed assuming the normality and homogeneity of variance by Kolmogorov–Smirnov test. Then, the differences between various treatments were evaluated using one-way ANOVA followed by Duncan’s test at a confidence level of 95%. To find the correlation between parameters in acute and subacute exposures, the results were compared with Pearson’s correlation coefficient. The comparison of parameters between the acute and subacute exposure was carried out by independent T-test. In addition, the combined effects of the exposure time and intensities of the SMFs were analyzed by two-way ANOVA. All data are presented as the mean ± standard deviation.
Results and Discussion
In acute exposure, the whole-body cortisol concentration showed a significant increase at 7.5 mT intensity of the SMF (p < 0.05), whereas the other intensities were not significantly different with each other (p > 0.05) (Fig. 2). In subacute exposure, the increasing trend of cortisol level was remarkably from the control to 7.5 mT intensity of SMF (p < 0.05). In acute and subacute exposures, the highest and lowest values of the cortisol were recorded at 7.5 mT and the control treatments, respectively. The independent T-test showed a significant difference between acute and subacute exposure only in the 5 mT treatment (sig = 0.01, t = − 3.832). In addition, there was no significant Pearson’s correlation coefficient between variations of the cortisol level in the acute and subacute exposures (r = 0.37, p > 0.05).
The cortisol level in the female zebrafish (n = 5) during acute and subacute exposure to the different SMF intensities. The letters above columns are showing significantly different based on one-way ANOVA and the symbol, *, is showing a significant difference between acute and subacute in each intensity based on independent T-test (p < 0.05)
The trend of whole-body glucose variations was similar to the cortisol ones (r = 0.7, p < 0.05). There was a significant change in the concentration of glucose between the 7.5 mT and other treatments (p < 0.05) (Fig. 3). The highest value of glucose was observed at 7.5 mT (2.22 ± 0.14 mg/g). In subacute exposure, glucose concentration showed a significant increase from 2.5 to 7.5 mT (p < 0.05) (Fig. 3). The statistical comparison of the glucose concentration between the acute and subacute exposures in each treatment showed a significant difference at 5 mT (sig = 0.00, t = − 10.970) and 7.5 mT (sig = 0.01, t = − 4.254) (Fig. 3). In addition, there was no significant Pearson’s correlation coefficient of changes in the glucose level between the acute and subacute exposures (r = 0.56, p > 0.05).
The glucose level in the female zebrafish (n = 5) during acute and subacute exposure to different SMF intensities. The letters above columns are showing significantly different based on one-way ANOVA and the symbols, *, are showing a significant difference between acute and subacute in each intensity based on independent T-test (p < 0.05)
In the acute exposure, a remarkable decrease of the whole-body E2 concentration was observed between the controls and the different intensities of the SMF (p < 0.05) (Fig. 4). The decreasing trend of the E2 level was significantly recorded from the control to 7.5 mT in subacute exposure (p < 0.05) (Fig. 4). In both exposures, the minimum value of E2 was recorded at 7.5 mT intensity. The variation trends of the E2 level were similar during acute and subacute exposures and significant Pearson’s correlation coefficient was obtained (r = 0.78; p < 0.05). The independent T-test showed the significant difference between E2 in acute and subacute exposures only in the 5 mT treatment (sig = 0.00, t = 16.8) (Fig. 4).
The E2 level in the female zebrafish (n = 5) during acute and subacute exposure to different SMF intensities. The letters above columns are showing significantly different based on one-way ANOVA and the symbol, *, is showing a significant difference between acute and subacute in each intensity based on independent T-test (p < 0.05)
There was a considerable decrease in the whole-body 17-OHP concentration at 7.5 mT in the acute exposure (p < 0.05) (Fig. 5). The significant decreasing trend of the 17-OHP level was observed from the control to the 7.5 mT intensity of the SMF (p < 0.05) (Fig. 5). The variations of 17- OHP level during acute exposure were similar to subacute with a significant Pearson’s correlation coefficient (r = 0.83; p < 0.05). The statistical comparison of the 17-OHP concentration between the acute and subacute exposures in each treatment showed the significant difference at 5 mT (sig = 0.50, t = 0.727) and 7.5 mT (sig = 0.00, t = 53.600) (Fig. 5).
The 17-OHP level in the female zebrafish (n = 5) during acute and subacute exposure to different SMF intensities. The letters above columns are showing significantly different based on one-way ANOVA and the symbols, *, are showing a significant difference between acute and subacute in each intensity based on independent T-test (p < 0.05)
In the acute and subacute exposures, the zebrafish did not show any spawning at the intensity of 7.5 mT. In the acute exposure, the fecundity value showed a significant decrease at 5 and 7.5 mT in comparison to control broodstock, whereas in the subacute exposure, this decrease was observed from the control to 7.5 mT (p < 0.05) (Fig. 6). The variation trends of the fecundity level were completely similar during acute and subacute exposures with a high significant Pearson’s correlation coefficient (r = 0.96, p < 0.05). At 2.5 and 5 mT intensities of the SMF, there was significant difference in the fecundity value between acute and subacute exposure (2.5 mT; sig = 0.00, t = 10.352 and 5 mT; sig = 0.04, t = 6.094) (Fig. 6).
The absolute fecundity value in the zebrafish during acute and subacute exposure to different SMF intensities. The letters above columns are showing significantly different based on one-way ANOVA and the symbols, *, are showing a significant difference between acute and subacute in each intensity based on independent T-test (p < 0.05)
Based on two-way ANOVA, there was no interaction between exposure and intensity of the SMF and variations of cortisol and glucose levels (p > 0.05) (Table 1). For E2, 17-OHP and absolute fecundity, the interaction occurred sharply between exposure and intensity of the SMF (p < 0.05) (Table 1). The analysis of the combined effects of exposure (time) and intensity showed that the 7.5 mT intensity plus acute and subacute exposure had the most influence on the fluctuations of the E2, 17-OHP, and absolute fecundity (Table 1).
Studies have been carried out to evaluate the effects of the magnetic fields on steroid hormones of the animals (Al-Akhras et al. 2006; Zare et al. 2005; Bonhomme-Faivre et al. 1998; Gorczynska and Wegrzynowicz 1991), but scarce research has been carried out on SMF effects on the sex hormones of fish. Al-Akhras et al. (2006) revealed a significant increase of the LH (luteinizing hormone) in the adult male rat after 4.5 months of exposure while testosterone levels were remarkably decreased only after 1.5 and 3 months of the exposure period (Al-Akhras et al. 2006). Guinea pigs showed a significant decrease in cortisol level after exposure to a field of 50 Hz and 0.2 T, whereas no significant changes were observed in the cortisol in those treated with 0.01 T for 2 h and 4 h per day over a period of 5 days (Zare et al. 2005). A reduction of the cortisol value was observed in the Swiss mice continuously exposed to a low frequency (50 Hz) field on day 190 of the experiment (Bonhomme-Faivre et al. 1998). There was an increase of the cortisol level in rats during exposure to constant magnetic fields of 3–10 T and 2–10 T, 1 h per day for a period of 10 days (Gorczynska and Wegrzynowicz 1991).
In the present study, increasing levels of cortisol were detected in the adult female zebrafish during exposure to different intensities of SMF. This increase is directly related to the intensity and duration of SMF, so that the maximum level of cortisol was recorded at 7.5 mT on day 21 of the experiment. It seems that the magnetic field plays the role of stress, which can elevate the amount of cortisol level. It was suggested that exposure to weak electromagnetic fields (EMFs) could disturb the production of the hormone melatonin by the pineal gland (Noonan et al. 2002a, b) that eventually leads to a disorder in the synthesis of cortisol. In this study, the trend of glucose variations in the zebrafish after exposition to different magnetic fields was similar to the changes in the cortisol trend. Increased cortisol levels lead to elevation of glucose levels in the fish because glucose is the most important energy substrate for coping with stress (Barton and Schreck 1987).
In the female zebrafish, the sex hormones and, consequently, the fecundity values were influenced by the magnetic fields. The increased intensity of the SMF caused a significant reduction both in E2 and in 17-OHP levels compared to the control. The lowest values of E2 and 17-OHP were measured at the highest intensity value of the SMF (7.5 mT). Larger drops in levels of E2 and 17-OHP were observed in the subacute experiment in comparison to the acute treatment, which indicated a more severe effect of the SMF over longer exposure time. It seems that SMF, as a stress inducer, increases the concentration of cortisol and glucose and eventually decreases the concentration of the sex hormones in the broodstock of the zebrafish. Kubokawa et al. (1999) showed that the increase in cortisol level is followed by a decrease in sex hormone levels. The disruption in production of E2 and 17-OHP led to a decline in the fecundity of zebrafish.
The results of our experiments showed that the SMFs could disrupt the physiology of zebrafish, especially the reproductive system. In conclusion, it seems that the SMFs, especially at higher intensities and as an important inducer of stress, could disrupt the endocrine reproductive system in the zebrafish. The damaging effects on the reproductive system increase with prolonged exposure of the fish to the magnetic field.
References
Al-Akhras MA, Darmani H, Elbetieha A (2006) Influence of 50 Hz magnetic field on sex hormones and other fertility parameters of adult male rats. Bioelectromagnetics 27:127–131
Andrulewicz E, Napierska D, Otremba Z (2003) The environmental effects of the installation and functioning of the submarine SwePol Link HVDC transmission line: a case study of the Polish Marine Area of the Baltic Sea. J Sea Res 49(4):337–345
Barton BA, Schreck CB (1987) Metabolic cost of acute physical stress in juvenile steelhead. Trans Am Fish Soc 116:257–263
Bonhomme-Faivre L, Mac´e A, Bezie Y (1998) Alterations of biological parameters in mice chronically exposed to low-frequency (50 Hz) electromagnetic fields. Life Sci 62(14):1271–1280
Fernanda SD, Débora F, Renan I, João GSR, Michele F, Alessandra M, Rodrigo EB, Angelo P, Leonardo JG (2018) Barcellos feeding regimen modulates zebrafish behavior. PeerJ 6:e5343
Formicki K, Szulc J, Korzelecka-Orkisz A, Tanski A, Kurzydłowski JK, Grzonka J, Kwiatkowski P (2015) The effect of a magnetic field on trout (Salmo trutta Linnaeus, 1758) sperm motility parameters and fertilisation rate. J Appl Ichthyol 31(1):136–146
Genba A, Taninaka M, Oda K, Kondo H (1963) Blood sugar determination by the o-toluidine method, with special reference to the use of the direct quantitative method. Rinsho Byori 11:116–119
Gill AB, Kimber JA (2005) The potential for cooperative management of elasmobranchs and offshore renewable energy development in UK waters. J Mar Biol Assoc UK 85(5):1075–1081
Gill AB, Huang Y, Gloyne-Philips I, Metcalfe J, Quayle V, Spencer J, Wear-mouth V (2009) EMF-sensitive fish response to EM emissions from sub-sea electricity cables of the type used by the offshore renewable energy industry. Commissioned by Cowrie Ltd p. 68 (project reference COWRIE-EMF-1-06)
Gorczynska E, Wegrzynowicz R (1991) Glucose homeostasis in rats exposed to magnetic fields. Invest Radiol 26(12):1095–1100
Hashish AH, El-Missiry MA, Abdelkader HI, Abou-Saleh RH (2008) Assessment of biological changes of continuous whole body exposure to static magnetic field and extremely low frequency electromagnetic fields in mice. Ecotoxicol Environ Saf 71(3): 895–902
Health Protection Agency (2008) Static Magnetic Fields. Report of the independent Advisory Group on Non-ionising Radiation. RCE-6. Documents of the Health Protection Agency. Radiation, Chemical and Environmental Hazards. Chilton UK
Heidari B, Roozati SA, Yavari L (2010) Changes in plasma levels of steroid hormones during oocyte development of Caspian Kutum (Rutilus frisii kutum, Kamensky, 1901). Anim Reprod 7(4):373–381
Kubokawa K, Watanabe T, Yoshizaki M, Iwama M (1999) Effect of acute stress on plasma cortisol, sex steroid hormone and glucose level in male and female sockeye salmon during the breeding season. Aquaculture 172:335–349
Loghmannia J, Heidari B, Rozati SA, Kazemi S (2015) The physiological responses of the Caspian kutum (Rutilus frisii kutum) fry to the static magnetic fields with different intensities during acute and subacute exposures. Ecotoxicol Environ Saf 111:215–219
Mayden RL, Tang KL, Conway KW, Freyhof J, Chamberlain S, Haskins M, Schneider L, Sudkamp M (2007) Phylogenetic relationships of Danio within the order Cypriniformes: a framework for comparative and evolutionary studies of a model species. J Exper Zoo Part B Mol Dev Evol 308(5):642–654
Moon TW, Walsh PJ, Perry SF, Mommsen TP (1988) Effects of in vivo beta-adrenoceptor blockade on hepatic carbohydrate metabolism in rainbow trout. J Exp Zool 248:88–93
Nash JP, Davail- CuissetS B, Bhattacharyya S, Suter HC, Le Menn F, Kime DE (2000) An enzyme linked immunosorbant assay (ELISA) for testosterone, estradiol, and 17,20β-dihydroxy-4-pregenen-3-one using acetylcholinesterase as tracer: application to measurement of diel patterns in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 22(4):355–363
Noonan CW, Reif JS, Burch JB, Ichinose TY, Yost MG, Magnusson K (2002a) Relationship between amyloid beta protein and melatonin metabolite in a study of electric utility workers. J Occup Environ Med 44(8):769–775
Noonan CW, Reif JS, Yost M, Touchstone J (2002b) Occupational exposure to magnetic fields in case-referent studies of neurodegenerative diseases. Scand J Work Environ Health 28(1):42–48
Sadowski M, Winnicki A, Formicki K, Sobotinski A, Tanski A (2007) The effect of magnetic field on permeability of egg shells of salmonid fishes. Acta Ichthyol Piscat 37(2):129–135
Saldanha CJ, Luke RH, Schlinger BA (2011) Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev 32(4):532–549
Skauli KS, Reitan JB, Walther BT (2000) Hatching in zebrafish (Danio rerio) embryos exposed to a 50 Hz magnetic field. Bioelectromagnetics 21(5):407–410
World Health Organization (2006) Static Fields (Environmental Health Criteria: 232). Geneva, Switzerland
Zare S, Hayatgeibi H, Alivandi S, Ebadi AG (2005) Effects of whole-body magnetic field on changes of glucose and cortisol hormone in quinea pigs. Am J Biochem Biotechnol 4:209–211
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sedigh, E., Heidari, B., Roozati, A. et al. The Effect of Different Intensities of Static Magnetic Field on Stress and Selected Reproductive Indices of the Zebrafish (Danio rerio) During Acute and Subacute Exposure. Bull Environ Contam Toxicol 102, 204–209 (2019). https://doi.org/10.1007/s00128-018-02538-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-02538-1