Abstract
The aim of this study was to investigate the responses in filtration and grazing rates of five rotifer strains of the species Brachionus calyciflorus under different temperatures and MC-LR concentrations. The results showed that strain identity, MC-LR concentration, temperature, and the interactions of these factors significantly affected both response variables, with the exception of the interaction of strain and MC-LR on the grazing rates. At low MC-LR concentrations and for the control group, the filtration and grazing rates increased with increasing temperature. The filtering and grazing rates of B. calyciflorus exposed to higher MC-LR concentrations, however, showed no evident enhancement with increasing of temperature. At high temperatures, the filtration and grazing rates of all rotifer strains decreased significantly with increasing concentration of MC-LR, however B. calyciflorus exhibited a refractory stability in the presence of increased MC-LR levels at lower temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Bloom proliferation and the spread of cyanobacteria are well-known phenomena in both fresh and brackish water systems around the world (Codd et al. 2005; Frazão et al. 2010). Owing to the production of cyanotoxins, most cyanobacteria strains of the genus Microcystis are considered to be of serious health risks (Carmichael et al. 1990). Out of 80 variants, Microcystis aeruginosa is usually the dominant species and the largest producer of microcystins which are the most common and abundant cyanotoxins, particularly the variant microcystin-LR (MC-LR) (Barrios et al. 2015). Following the collapse of blooms, microcystin concentrations in water sources vary from trace levels up to 1.8 (mg/L) or higher (Chorus and Bartram 1999). These toxins may affect local organisms (Whitton and Potts 2000) and pose a threat to humans, the tertiary consumers of aquatic organisms, due to the potential for bioaccumulation (Falconer 2005; Berry and Lind 2010).
Aquatic animals, particularly zooplankton, are often used as test organisms to assess for potential adverse effects of chemicals. This is supported by their rapid growth, high reproductive rate, short generation time, and general sensitivity to toxic substances, making them excellent bioindicators of water quality (Snell and Janssen 1995; Landsberg 2002; Sarma and Nandini 2006; Kostopoulou et al. 2012). Cyanobacterial toxins, mainly microcystins, have been suggested to cause rapid mortality of herbivorous zooplankton, as well as long-term chronic effects on zooplankton growth and reproduction (Paerl et al. 2001; Lürling 2003; Zhang and Geng 2012). In addition, these toxic compounds may act as feeding deterrents and as such inhibit feeding of grazers especially cladocerans, copepods, and rotifers (Ostrofsky et al. 1983; Burns et al. 1989; Shaw et al. 1997). The effects of cyanotoxins are often determined by extracting the active compounds from cyanobacteria cultured under laboratory conditions or directly exposing the test organisms to cyanobacterial cells (Barrios et al. 2015). These bacteria have, however, been well documented to simultaneously produce more than one cyanotoxin as well as secondary metabolites that may also be toxic (Jungmann 1992). It is therefore not clear whether all of the observed toxicological effects found in zooplankton exposed to cyanobacterial cells or their crude extracts are attributable to any particular intracellular toxin or other toxins or even substances attached to the cyanobacterial cell wall (Ghadouani et al. 2004). The use of purified toxins could provide direct evidence of the effects of individual cyanotoxins in organisms.
Besides cyanotoxins, temperature has, moreover, a major impact on zooplankton physiology, ecology, and behavior by changing metabolic rates and activity level (Claska and Gilbert 1998). High temperatures can also trigger outbreaks of toxic algal blooms, cyanotoxin production (Jang et al. 2003; El-Shehawy et al. 2012), and therefore play a vital role in modifying the responses of zooplankton to toxic cyanobacteria (Gilbert 1996; Montagnes et al. 2001). Nevertheless, very little information on combined effects of purified MC-LR and temperature on rotifer feeding behavior is available.
In addition to exogenous variables, the identity of the rotifers’ strain is one of the most significant endogenous factor determining their feeding strategy under changing environmental conditions (Awaiss and Kestemont 1992; Hu and Xi 2008; Snell 2014). Different strains tend to be characterized by different traits, allowing them to adapt to their ecological niches. An increasing number of studies has documented the effects of environmental factors on demographic traits and population growth among different rotifer strains (Halbach 1973; Awaïss and Kestemont 1992; Xi et al. 2005; Gama-Flores et al. 2014). Nevertheless, the effects of such hidden differentiations among strains are usually overlooked in studies focusing on the feeding behavior and responses to cyanotoxin exposure. Accordingly, the current study aimed to quantify the filtration and grazing rates of the five Brachionus calyciflorus rotifer strains when exposed to purified MC-LR at different temperatures.
Materials and Methods
Zooplankton samples were collected using a 30-μm vertical plankton net towed in lentic water bodies from four cities; XN2 was from Xi’ning (36°39′N, 101°42′E), LZC1 and LZB1 were from Lanzhou (36°05′N, 103°45′E), KMC23 was from Kunming (25°03′N, 102°42′E), and BNB3 was from Xishuangbanna (22°00′N, 100°47′E). These cities were located in four different climate zones, namely mountain plateau zones, temperate, subtropical and tropical zones in China (Fig. 1). Observations under microscope revealed B. calyciflorus samples to be free of the other zooplankters, and that the clonal populations for each B. calyciflorus strain were established from a single parthenogenetic female, in order to reduce variation within strains.
Rotifers were cultured in a fluorescent illumination incubator at a temperature of 24 ± 1°C on a 14:10 h light:dark photoperiod under 130 lx for over 6 months. During the routine culture and pre-culture before experiment, all animals were fed daily with Scenedesmus obliquus at 1.0 × 106 cells/mL, and maintained using EPA medium (prepared by dissolving 96 mg NaHCO3, 60 mg CaSO4, 60 mg MgSO4, and 4 mg KCl in 1 L of distilled water) (Peltier and Weber 1985). Scenedesmus obliquus was grown semi-continuously in HB-4 medium (Li et al. 1959), which was replenished daily at 20% with a 16:8 light:dark photoperiod under a 3000 lx fluorescent light. In the exponential phase, fresh algae were harvested and stored at 4°C for use within two days. The density of the S. obliquus suspension was ascertained using a haemocytometer.
The MC-LR (95% purity) used in this study was purchased from Express Technology Co., Ltd. (Beijing, China), split charging from the certified MC-LR standard (Microcystin-LR, MCYL1GAM004, Taiwan Algal Science Inc.). According to the range of dissolved MCs in natural waters worldwide and the climate features (especially temperature) in the four sampled climate zones, the MC-LR concentrations were set at 0.0, 2.0, 4.0, and 6.0 mg/L and tested at 16, 20, 24, and 28°C. Before the experiment, microcystin-LR was dissolved in distilled water to achieve a stock solution of 50 μg/mL, and serially diluted to reach nominal concentrations using EPA medium for the experiments. The exposure concentrations were verified by high performance liquid chromatography (HPLC) in each of three replicates collected immediately prior to the addition of the rotifers.
In the experiment, 30 neonates (<4 h old), non-fed but active, were introduced into 8-ml glass beakers containing 3 ml test solution with 1.0 × 106 cells/ml S. obliquus, after which the beakers were covered with plastic boards to prevent the evaporation of water from the test medium, and then placed on shakers set at 80 rpm in complete darkness at four different temperatures to prevent the algae from settling and growth, respectively. All the experiments were conducted for 20 h with three replicates per treatment, so that there were 240 total units (4 concentrations × 4 temperatures × 5 strains × 3 replicates). After feeding, the density of unconsumed algae cells in each beaker was quantified using a haemocytometer. The EPA medium, containing algae S. obliquus at 1.0 × 106 cells/mL (but no B. calyciflorus), served as reference.
Grazing rates were calculated as the quantity of particles gathered from a food suspension in a specified period of time, while filtration rates showed the volume of medium passing through the filter to gather food. The filtering rate (F), i.e., the average volume of liquid filtered by each B. calyciflorus per unit time (ml ind−1 h−1), the grazing rate (G), i.e., the average number of algae cells ingested by each B. calyciflorus per unit time (cell ind−1 h−1), were calculated according to the following equations given by Frost (1972).
where V is the solution volume (ml); t is the experimental time (h); n is the number of rotifers in each replicate; C 0 and C tf are the initial and final densities of a given microalgae; and C t is the final microalgal density of the control group in which no rotifer is present and the algae S. obliquus is cultured synchronously with the test groups.
Data on the grazing (G) and filtration (F) rates were statistically tested using SPSS 19.0 to quantify the differences among the treatments. All data were tested for normality using the one-sample Kolmogorov–Smirnov procedure. The homogeneity of variances was checked using Levene’s test. In order to evaluate the effect of MC-LR concentrations on G and F, one-way ANOVAs were performed for each temperature and strain separately. The combined effects of MC-LR concentrations, temperature and their interactions on G and F were conducted using the two-way ANOVA for each strain separately. Finally, the three-way ANOVA was used to assess the effects of strain, MC-LR concentration, temperature and their interactions. Multiple comparisons were performed using SNK-q method to determine which groups were significantly different among groups. p < 0.05 was considered to be statistically significant.
Results and Discussion
The analysis of the chemical concentrations at the beginning of the exposure period revealed that the nominal and actual exposure levels were similar for all treatments. Control levels were found to be below the detection limit (0.05 µg/L LOD) in all cases. The nominal MC-LR concentrations in EPA medium were 2.0, 4.0, and 6.0 mg/L, and the exposure concentrations were 1.95 ± 0.07, 3.93 ± 0.09, and 5.83 ± 0.12 mg/L, respectively. As difference between measured and nominal concentrations are marginal the present study was based on the latter.
The results of the three-way ANOVA exploring the feeding behavior depending on strains, MC-LR concentrations, and temperatures are presented in Table 1. The interaction of strain × temperature and MC-LR × temperature were significant for both the filtration and grazing rates (p < 0.001). Similarly, the interaction of strain × MC-LR × temperature was significant for filtration and grazing rates (p < 0.05). It is noteworthy that the strain × MC-LR interaction was not significant for the grazing rate (p > 0.05), but did affect the filtration rate (p < 0.05) (Table 1). The significant interactions of exogenous and endogenous factors are complex: Rotifer sensitivity can be increased by increasing temperature or by using a more sensitive strain (Snell et al. 1991).
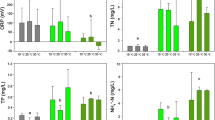
Temperature had a significant effect on the filtration and grazing rates of all five B. calyciflorus strains (p < 0.001, Table 1). Increasing temperature caused an increase in all filtration and grazing rates in the control and the low MC-LR concentration group (2.0 mg/L). Except for the BNB3 and LZC1 strains, the filtration and grazing rates of B. calyciflorus at 28°C were the highest among all four temperatures in the controls and the three treatment groups (Fig. 2). Increasing temperature may directly influence the grazing rate by increasing the metabolism, for instance, by altering enzyme kinetics. Indirect implications could be seen by increasing toxicant solubility or reducing membrane and water viscosity, thereby increasing diffusion levels (Cossins and Bowler 1987).
The average filtration and grazing rates in the five B. calyciflorus strains at three MC-LR concentrations and four temperatures. Asterisk indicates that there are significant differences in the filtration and grazing rates between the MC-LR treatments and the control at the same temperature (*p < 0.05; **p < 0.01; ***p < 0.001). The error bars represent standard error calculated based on three replicates
The two-way ANOVA also indicated that both the filtration and grazing rates of all B. calyciflorus strains were significantly affected by temperature and MC-LR (p < 0.05, Table 2). The interaction of MC-LR × temperature was significant for the filtration rates of all rotifer strains (p < 0.05, Table 2). Except for the LZB1 strain (p > 0.05), the grazing rates of all strains showed a significant MC-LR × temperature interaction (p < 0.05, Table 2). In general, the effects of MC-LR increased significantly with increasing temperature. Bloom frequency is known to generally increase with rising global temperatures, which accelerates the growth of toxic Microcystis compared to its non-toxic strains (Paerl et al. 2011; El-Shehawy et al. 2012; Liu et al. 2014). Huang et al. (2012) found that the population growth and reproduction of B. calyciflorus was markedly affected by MC-LR with the concentrations of 0.001–200 µg/L at 30°C, but not at 20 or 25°C. In this study, we observed a similar response for this rotifer species, and the significant effects of MC-LR on the feeding behavior of the five B. calyciflorus strains were detected at the highest temperature (28°C).
The different rotifer strains from distinct climatic zones have adapted to local environmental fluctuation. As a result, feeding activity is finely tuned to these conditions. In this study, the filtration and grazing rates in rotifers responded diversely to changes in MC-LR concentration and temperature among different B. calyciflorus strains (p < 0.001, Table 1). At a high temperature (28°C), the feeding behavior of the KMC23 strain from subtropics was the most active among all strains, regardless of MC-LR concentration. At a low temperature, however, LZC1 from temperate zone was the most active strain compared to the other four in relation to filtration and grazing rates (Fig. 2). The key reason could be attributed to their different life history strategies and the long-term adaptation of their feeding activity to specialized temperature regimes.
The results of one-way ANOVA with SNK-q method indicated that MC-LR significantly depressed the filtration and grazing rates of all five B. calyciflorus strains at 28°C, as well as those of four of the strains (KMC23, XN2, LZB1, and LZC1) at 24°C (p < 0.05), but had no influence on BNB3 at 24°C (p > 0.05) (Fig. 2). At 24 and 28°C, the presence of MC-LR at higher concentrations (4.0 and 6.0 mg/L) inhibited the filtration and grazing rates of the five B. calyciflorus strains. However we detected no significant differences between the treatments and the controls for any rotifer strain at 16 and 20°C (Fig. 2). Previous studies have shown that MCs inhibited feeding, reduced growth, and increased mortality in cladocera and copepods with increasing concentration (Lürling and van der Grinten 2003; Ghadouani et al. 2004; Wilson et al. 2006). The 24-h LC50 in B. plicatilis for MC-LR was 124.87 mg/L, and the survival and reproductive rates and population growth parameter of B. plicatilis were lower at each test group than those at the controls (Chen et al. 2002). In the present study, the results showed that MC-LR significantly depressed the filtration and grazing rates of B. calyciflorus at higher temperatures, especially in treatments with higher concentrations of MC-LR (4.0 and 6.0 mg/L) at 24°C and 28°C, except for the tropical strain BNB3.
In summary, strain, MC-LR concentration, temperature, and the interactions among these factors significantly affected the filtration and grazing rates of the five B. calyciflorus strains, except that no significant effect was found for the strain × MC-LR interaction on rotifer grazing rates. Increasing temperatures stimulated the feeding behavior of B. calyciflorus in the control and low MC-LR concentration groups, and the filtration and grazing rates of all rotifer strains were significantly inhibited at different concentrations of MC-LR at 28°C. The feeding activities of the Kunming (KM) and Lanzhou (LZ) strains were the most active at high and low temperatures, respectively, regardless of MC-LR concentration, which could be attributed to the long-term adaptation of their feeding behavior to specialized local temperature regimes.
References
Awaiss A, Kestemont P (1992) An investigation into the mass production of the freshwater rotifer Brachionus calyciflorus Pallas 2. Influence of temperature on the population dynamics. Aquaculture 105:337–334
Barrios CAZ, Nandini S, Sarma SSS (2015) Effect of crude extracts of Dolichospermum planctonicum, on the demography of Plationus patulus (Rotifera) and Ceriodaphnia cornuta (Cladocera). Ecotoxicology 24(1):85–93
Berry JP, Lind O (2010) First evidence of “paralytic shellfish toxins” and cylindrospermopsin in a Mexican freshwater system, Lago Catemaco, and apparent bioaccumulation of the toxins in “tegogolo” snails (Pomacea patula catemacensis). Toxicon 55(5):930–938
Burns CW, Forsyth DJ, Haney JF, Lampert W, Pridmore RD (1989) Coexistence and exclusion of zooplankton by Anabaena minutissima var. attentuata in Lake Rotongaio, New Zealand. Arch Hydrobiol Beih Ergebn Limnol 32:63–82
Carmichael WW, Mahmood NA, Hyde EG (1990) Natural toxins from cyanobacteria (blue–green) algae. In: Hall S, Strichartz G (eds) Marine toxins: origins, structure and molecular pharmacology. American Chemical Society, Washington, DC, pp87–106
Chen Y, Wang JQ, Wang Y, Yu SZ (2002) Toxicity and population growth effects of microcystin on the rotifer Brachionus plicatilis. China Environ Sci 22(3):198–201
Chorus I, Bartram J (1999) Toxic cyanobacteria in water. A guide to their public health consequences, monitoring, and management. E & FN Spon, London
Claska ME, Gilbert JJ (1998) The effect of temperature on the response of Daphnia to toxic caynobacteria. Freshw Biol 39:221–232
Codd GA, Morrison LF, Metcalf JS (2005) Cyanobacterial toxins: risk management for health protection. Toxicol Appl Pharm 203(3):264–272
Cossins AR, Bowler K (1987) Temperature Biology of Animas. Chapman and Hall, London
El-Shehawy R, Gorokhova E, Fernández-Piñas F, Del Campoc FF (2012) Global warming and hepatotoxin production by cyanobacteria: what can we learn from experiments? Water Res 46(5):1420–1429
Falconer IR (2005) Cyanobacterial toxins of drinking water supplies: cylindrospermopsins and microcystins. CRC Press, Boca Raton
Frazão B, Martins R, Vasconcelos V (2010) Are known cyanotoxins involved in the toxicity of picoplanktonic and filamentous north Atlantic marine cyanobacteria? Mar Drugs 8:1908–1919
Frost BW (1972) Effects of size and concentration of food particles on the feeding behavior of the maring planktonic copepod Calanus pacificus. Limnol Oceanogr 17(6):805–815
Gama-Flores JL, Huidobro-Salas ME, Sarma SSS, Nandini S (2014) Combined effects of temperature (level and oscillation) and cadmium concentration on the demography of Brachionus calyciflorus (Rotifera). Int Rev Hydrobiol 99:173–177
Ghadouani A, Pinel-Alloul B, Plath K, Codd GA, Lampert W (2004) Effects of Microcystis aeruginosa and purified microcystin-LR on the feeding behavior of Daphnia pulicaria. Limnol Oceanogr 49(3):666–679
Gilbert JJ (1996) Effect of temperature on the response of planktonic rotifers to a toxic cyanobacterium. Ecology 77(4):1174–1180
Halbach U (1973) Life table data and population dynamics of the rotifer Brachionus calyciflorus Pallas as influenced by periodically oscillating temperature. In: Wieser W (ed) Effects of temperature on ectothermic organisms. Springer, Berlin, pp 217–228
Hu HY, Xi YL (2008) Demographic parameters and mixis of three Brachionus angularis Gosse (Rotatoria) strains fed on different algae. Limnologica 38:56–62
Huang L, Xi Y, Xu X, Wen X (2012) Responses in population growth and reproduction of the freshwater rotifer Brachionus calyciflorus to microcystin-LR at different temperatures. Ann Limnol- Int J Lim 48(4):383–390
Jang MH, Kyong H, Gea-Jae J, Takamura N (2003) Toxin production of cyanobacteria is increased by exposure to zooplankton. Freshw Biol 48:1540–1550
Jungmann D (1992) Toxic compounds are isolated from Microcystis PCC786 that are more active to Daphnia than two microcystins. Limnol Oceanogr 37(8):1777–1783
Kostopoulou VE, Carmona MJ, Divanach P (2012) The rotifer Brachionus plicatilis: An emerging bio-tool for numerous applications. J Biol Res 17:97–112
Landsberg JH (2002) The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci 10(2):113–390
Li SH, Zhu H, Xia YZ, Yu MJ, Lin KE, Liu KS, Yue ZY, Chen YX (1959) The mass culture of unicellular green algae. Acta Hydrobiol Sin 4:462–472
Liu FF, Feng MH, Shang LX (2014) Effects of temperature on the growth and generation of extracellular organic matter of Microcystis aeruginosa and Anabeana sp. J Lake Sci 26(5):780–788
Lürling M (2003) Daphnia growth on microcystin-producing and microcystin-free Microcystis aeruginosa in different mixtures with the green alga Scenedesmus obliquus. Limnol Oceanogr 48(6):2214–2220
Lürling M, van der Grinten E (2003) Life-history characteristics of Daphnia, exposed to dissolved microcystin-LR and to the cyanobacterium Microcystis aeruginosa, with and without microcystins. Environ Toxicol Chem 22(6):1281–1287
Montagnes D, Kimmance S, Tsounis G, Gumbs J (2001) Combined effect of temperature and food concentration on the grazing rate of the rotifer Brachionus plicatilis. Mar Biol 139(5):975–979
Ostrofsky ML, Jacobs FG, Rowan J (1983) Evidence for the production of extracellular herbivore deterrents by Anabaena flos-aquae. Freshwater Biol 13:501–506
Paerl HW, Fulton RS, Moisander PH, Dyble J (2001) Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci World J 1:76–113
Paerl HW, Hall NS, Calandrino ES (2011) Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci Total Environ 409(10):1739–1745
Peltier WH, Weber CI (1985) Methods for measuring the acute toxicity of effluents to freshwater and marine organisms. In: EPA/600/4–85/013. Environmental monitoring and support laboratory, 3rd edn. US Environmental Protection Agency, Cincinnati
Sarma SS, Nandini S (2006) Review of recent ecotoxicological studies on cladocerans. J Environ Sci Health B 41(8):1417–1430
Shaw BA, Andersen RJ, Harrison PJ (1997) Feeding deterrent and toxicity effects of apo-fucoxanthinoids and phycotoxins on a marine copepod (Tigriopus californicus). Mar Biol 128:273–280
Snell TW (2014) Rotifers as models for the biology of aging. Int Rev Hydrobiol 99:84–95
Snell TW, Janssen CR (1995) Rotifers in ecotoxicology: a review. Hydrobiologia 313(1):231–247
Snell TW, Moffat BD, Janssen C, Persoone G (1991) Acute toxicity tests using rotifers. III. Effects of temperature, strain, and exposure time on the sensitivity of Brachionus plicatilis. Environ Toxicol 6:63–75
Whitton BA, Potts M (2000) The ecology of cyanobacteria: their diversity in time and space. Kluwer Academic Publishers, Dordrecht¸ pp 669
Wilson AE, Sarnelle O, Tillmanns AR (2006) Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: meta-analyses of laboratory experiments. Limnol Oceanogr 51(4):1915–1924
Xi YL, Ge YL, Chen F, Wen XL, Dong LL (2005) Life history characteristics of three strains of Brachionus calyciflorus (Rotifera) at different water temperatures. J Freshw Ecol 20:707–713
Zhang X, Geng H (2012) Effect of Microcystis aeruginosa on the rotifer Brachionus calyciflorus at different temperatures. B Environ Contam Tox 88(1):20–24
Acknowledgements
We are grateful to the anonymous referees for their valuable comments and constructive suggestions, which have greatly improved the manuscript. We thank the Shenzhen Nobel Science and Technology Service Co., Ltd. for the language editing service. This research was funded by the Natural Science Foundation of China (31200324, 31470015), Natural Science Foundation of Anhui Province (1708085MC79), and Foundation of Provincial Key Laboratory of Conservation and Utilization for Important Biological Resources in Anhui.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, XL., Chen, YY., Xu, QL. et al. Combined Effects of Temperature and the Microcystin MC-LR on the Feeding Behavior of the Rotifer Brachionus calyciflorus . Bull Environ Contam Toxicol 99, 493–499 (2017). https://doi.org/10.1007/s00128-017-2172-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2172-9