Abstract
Methodology using solid phase extraction and high performance liquid chromatography (SPE-C18/HPLC–DAD) was applied to pesticide determinations in ten water reservoirs in the semidarid region of northeastern Brazil. The validated method was suitable for determination of herbicides and insecticide in surface water. The recovery efficiency of atrazine, methyl-parathion and simazine was approximately 70 %. The method also showed good linearity and selectivity with correlation coefficients (R) greater than 0.99. The limits of detection were below the maximum residue limits (MRLs) established by government agencies. Studied reservoirs showed presence of atrazine at mean levels from 7.0 to 15.0 µg/L. Simazine and methyl parathion were not detected during the period. The atrazine levels measured from this semiarid region are of the same magnitude as those found in regions with moderate to high agricultural activity. According to detected atrazine concentrations, the annual health risk to humans was insignificant. However, the control of herbicides is important to maintain the quality of water in the reservoirs of Ceará, Brazil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The use of pesticides in Brazil has grown significantly in recent decades. Since 2008, the country of Brazil has been considered the largest consumer of pesticides in the world (Milhome et al. 2015). In Ceará State, Brazil, more than 150 active ingredients of different classes are used in agriculture (Gama et al. 2013). Based on the classification from the Ministry of Agriculture, 48 % of the pesticides used in Ceará State are in the extremely and highly toxic classification, while 60 % are considered to be extremely and highly hazardous to the environment (MAPA 2015). Most active ingredients used in Ceará State present great contamination risks to surface and ground waters (Milhome et al. 2009; Gama et al. 2013).
Currently, there is growing concern in the scientific community about the contamination of water, soil and food by pesticides, especially in relation to substances that exhibit chemical stability in the environment and toxicity (Barlas 2002; Soares and Porto 2007; Cavalcante et al. 2012; Sousa et al. 2013; Yang et al. 2015). Singh and Gupta (2002) conducted pesticide residues monitoring on different sources of drinking water in Jaipur, India. The indiscriminate use of pesticides has also been shown in several countries to be a cause of increased incidences of cancer (Rigotto 2009).
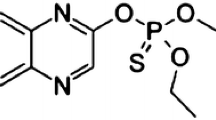
Alachlor, atrazine, simazine and methyl-parathion are herbicides and insecticides widely used in various major crops, such as maize, cotton, beans and sugar cane. These products present high/moderate toxicity and persistence in the environment, causing risks to aquatic organisms and to humans. Several cases of contamination in surface and groundwater have been reported worldwide (Palma et al. 2004; Claver et al. 2006; Bortoluzzi et al. 2007; Ribeiro et al. 2013; Milhome et al. 2015). The herbicides (atrazine, simazine, and clomazone) and insecticides (imidacloprid, chlorpyrifos) were found by Bortoluzzi et al. (2007) in waters (surface and wells) related to the production of tobacco in Rio Grande do Sul, Brazil. In addition to the World Health Organization (WHO), several environmental agencies have encouraged the establishment of monitoring programs to assess the quality of water resources in order to ensure the protection of human health. For this purpose, analytical methods have been developed and validated, often aimed at detecting the level of trace contaminants (Sabin et al. 2009; Albaseer et al. 2010). Maximum residue limits (MRLs) are established by government agencies and therefore the methods must have detection limits below these levels.
A large amount of pesticides are used in State of Ceará, but few studies have reported the contamination risk of the water resources. Thus, the main objective was to determine validation parameters and apply a method using solid phase extraction and high performance liquid chromatography (SPE-C18/HPLC–DAD) for the determination of pesticides in environmental aqueous matrices. The validated method was applied to the determination of herbicides and insecticides in the water reservoirs situated in State of Ceará to assess the impact of agricultural activity in the semiarid region, with scarce water resources. The quality of these water resources is fundamental to the development of the region. Thus, the results reported in this paper should help the environmental agencies develop monitoring programs and control the levels of contamination.
Materials and Methods
Standards of the alachlor, atrazine, simazine and methyl-parathion pesticides (purity >99 %) were purchased from Sigma–Aldrich (São Paulo, Brazil). Stock and working solutions were prepared with ultrapure water (Milli-Q system, Millipore). The standard solutions were used for calibration and determination of the validation parameters. Acetonitrile and methanol (used in SPE) were of chromatographic grade.
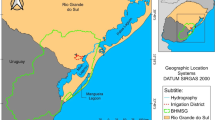
Surface water samples were collected from the ten main reservoirs (Pacatuba, Baturité, Itapiúna, Palhano, Oróz, Crateús, Parambu, Iguatu, Assaré, Lavras da Mangabeira) of Ceará State, as illustrated in Fig. 1. The samples were collected in 1 L volumes, stored in amber vials and kept under refrigeration (4°C) until analysis. The cleaning of bottles and glassware and quality control measures were conducted as described by standard methods (APHA et al. 2005).The extraction of pesticides and pre-concentration of samples were performed using SPE method. Sorbent cartridges (C18, 500 mg) were conditioned with 10 mL of methanol, followed by 10 mL of Milli-Q water. A volume of 1 L of water sample was passed through the cartridge at flow rate of 10 mL/min. The cartridge was then eluted with 2 mL of acetonitrile for extraction of analytes.
A Shimadzu liquid chromatograph (LC-10 AD model, Kyoto, Japan) equipped with a diode array detector, DAD (SPD-10AVP), and column oven (CTO-10AS) system for the low-pressure pump (SL-10AVP) was used for pesticide analysis. The pesticide separation was performed on a Supelco C18 column (25 cm × 4.6 mm i.d.; particles of 5 µm diameter) with the following chromatographic conditions: gradient system with acetonitrile:water mobile phase (initially 65:35 v/v, increasing to 85:15 v/v in 7 min, then decreased to 70:30 v/v in 10 min); flow rate of 0.8 mL/min; detection at 220 and 270 nm; and injection volume of 20 µL.
Method validation was optimized by studying the following parameters: selectivity, linearity, limit of detection (LOD), limit of quantification (LOQ), precision and recovery, according to ABNT (2005) and CITAC/EURACHEM (2002).The linearity of a method was observed by the correlation coefficient (R) of the linear regression curve (calibration curve). LODs and LOQs of method were estimated as the lowest concentration of fortified water that yielded S/N (signal to noise) ratios of 3 and 10, respectively (ABNT 2005). Precision was estimated in terms of repeatability of the measure (relative standard deviation, RSD) from 10 determinations with a sample of known concentration. Recovery was investigated with fortified samples. A volume of 100 mL of solution containing atrazine, simazine, methyl-parathion and alachlor (10 μg/L) was percolated in C18 cartridges (pre-conditioned). Then, the analyte was eluted with 2 mL of acetonitrile, and 20 µL were injected into the HPLC.
A health risk assessment model (non-carcinogenic chemicals) was used based on chronic daily exposures to evaluate the exposure doses for humans (EPA 1992, 2014). This model connected the level of environmental contamination of the studied area to human health, showing the potential risk of ingestion. Pesticide levels were converted into doses using an exposure equation, considering the exposure time, ingestion rate and body weight of an average individual, according to methods of the U.S. Environmental Protection Agency (EPA 1992, 2014).
The value of the oral reference dose (RfD) was used as the threshold parameter for evaluating the exposure level of ingested water (RfD Atrazine = 0.035 mg/kg day) (EPA 2014). The \(R_{i}^{n}\) is assigned as the non-carcinogenic contamination to the average individual (i) health hazard annual risk by intake pathway. Then, the individual average health risk of chemical non-carcinogens \(R_{i}^{n}\) is expressed by \(R_{i}^{n} = \left( {D_{i} /RfD_{i} } \right) \times 10^{ - 6} /70\), where RfD i is the oral reference dose; the lifetime of residents was assumed to be the standard 70 years; and D i is the unit weight exposure dosage of chemical non-carcinogens through water ingested. D i can be obtained by: \(D_{i} = 2 \times C_{i} /70\), considering 2 L as the average daily intake of drinking water for adults; C is the concentration of contamination and 70 is the average weight (kg) of people in the studied area.
Results and Discussion
Validation parameters (linearity, LOD, LOQ, precision and recovery) are summarized in Table 1. The linearity of the calibration curves were evaluated by the correlation coefficient (R) ranging from 0.9922 to 0.9995. According to ABNT (2005) and CITAC/EURACHEM (2002), R must be greater than 0.99. The 2–20 µg/L linearity range was satisfactory (1–20 µg/L for alachlor). The LOD was determined based on the results obtained for the lowest fortified level detected in the method and LOQ was the lowest fortification level that resulted in good accuracy and precision. LODs and LOQs of the method ranged from 0.004 to 0.24 µg/L and 0.012 to 0.72 µg/L, respectively (Table 1). The LODs and LOQs have similar values compared to those reported in other studies using liquid chromatography (Ribeiro et al. 2013). RSD values (precision) were obtained ranging from 3.9 % to 15.2 % (Table 1), which are satisfactory considering that values up to 20 % are acceptable for chromatographic methods (ABNT 2005).
Recovery efficiency (RE) for alachlor, atrazine, parathion-methyl and simazine pesticides was determined by spiking the samples (concentration of 10 µg/L). Alachlor had the lowest recovery (approximately 30 %), while atrazine, methyl-parathion and simazine exhibited satisfactory recoveries (>60 %). According to ABNT NBR 14029 (2005), RE of 60 %–115 % is acceptable for a concentration level of 10 µg/L. Milhome et al. (2011) found recoveries varying from 70 % to 110 % using C18 cartridges applied to determination of residues in water spiked with molinate, parathion methyl, malathion, chlorpyrifos, fenitrothion, pendimethalin and triazophos. Ribeiro et al. (2013) conducted experiements using solid phase extraction (styrene divinylbenzene-SDVB cartridges) for method validation of 16 pesticides in environmental aqueous matrices (Rio São Lourenço-MT). The method showed average recovery efficiency between 61 % and 120 % (CV ≤ 15 %) for 14 pesticides, with only two active principles being considered to exhibit unsatisfactory recoveries.
Various parameters, such as log Kow, log Koc and solubility, govern the recovery efficiency using adsorption, e.g.: SPE, for the determination of hydrophobic organic contaminants (Cavalcante et al. 2007; Jeanneau et al. 2007). Water solubility seems to be the parameter governing the extraction of the studied pesticides using SPE because atrazine, simazine and methyl-parathion have similar physico-chemical properties and alachlor has a solubility (240 mg/L), approximately 7 to 48 times higher than the other studied pesticides.
The alachlor recovery efficiency was relatively low (36 %) and it was not quantified in the real samples. Simazine and parathion-methyl were not present at concentrations above their detection limits. Atrazine was detected in 60 % of the samples, at levels as high as 15.0 µg/L. Its concentrations in the study are compared to concentrations reported in other studies in Table 2. The Ministry of Health of Brazil (Portaria MS No 2914 DE 12/12/2011) established the MRL for atrazine of 2 µg/L. The European Community (Directive 98/83/EC) established a limit of 0.1 µg/L for individual pesticides and 0.5 µg/L for a total concentration of pesticides and metabolites in drinking water (European Community 1998). In six of the reservoirs (Fig. 2), atrazine levels exceeded the limits, making the water inappropriate for human consumption. Atrazine levels ranging from 2.0 to 9.95 µg/L had been previously reported in the municipality of Tianguá in the western part of the Ceará state (Arraes et al. 2008). Similar atrazine levels were reported in areas in Brazil with moderate to high agricultural activity (Armas et al. 2007; Arraes et al. 2008; Nogueira et al. 2012). The high levels of atrazine in Tejo Basin (Portugal) were due to the application of atrazine over decades of extensive agriculture in these regions. Pesticides levels detected in the water resources of the municipalities studied are justified because atrazine is one of the most heavily used herbicide (Milhome et al. 2009; Gama et al. 2013).
The risk to human health was evaluated based on the atrazine level detected in the water samples. Figure 2 shows the relation between the pesticide level and health risk for water reservoirs in Ceará State. Although the atrazine levels were more than three times above the Brazilian and International MRLs, annual health risks were insignificant (Fig. 2). The water reservoirs located in Crateús, followed by Lavras da Mangabeira, presented the highest risks.
The human health hazard, estimated from annual health risk, caused by atrazine levels has a magnitude ranging from 10−10 to 10−11. This means that the human health hazard or the risk of death is one in ten billion people. This indicates that the human health hazard caused by atrazine levels in water resources from the studied area is minimal and will not be harmful to human health. Ni et al. (2009) found an even lower human health hazard promoted by non-carcinogenic substance levels in drinking water.
Atrazine as a photosynthesis inhibitor is more toxic to plants than animals. Toxic effects of triazine herbicides on aquatic plants were studied by Vryzas et al. (2009). Toxicity levels of atrazine to phytoplankton is 3 µg/L. Ventura et al. (2008) found mutagenic effects in aquatic organisms exposed to atrazine (at level of approximately 25 µg/L). The detected levels of atrazine in this paper (up to 15 µg/L) would be considered as unlikely to cause observable adverse effects in reservoirs (Solomon et al. 1996; Diana et al. 2000). Ecological risk for freshwater benthos and fish have been associated with higher concentrations (greater than 220 µg/L) for atrazine (Solomon et al. 1996) m g/L for a saltwater macrophyte, and greater than 220 mg/L for freshwater benthos and fish.
The validated method using SPE/HPLC–DAD for atrazine, methyl-parathion and simazine determination in environmental aqueous matrices was satisfactory and applicable to environmental studies. Six out of ten reservoirs showed the presence of atrazine. Levels were similar to those reported in other areas of Brazil with intense agricultural activities. Although the atrazine levels were about 3–7 times above the Brazilian and international MRLs, annual health risks were insignificant. However, due to the presence of herbicides in the samples, it is important to monitor and control pesticide residue levels in the waters of the reservoirs of Ceará.
References
ABNT-Associação Brasileira de Normas Técnicas (2005) Agrotóxicos e Afins -Validação de métodos analíticos, NBR14029
Albaseer SS, Rao RN, Swamy YV, Mukkanti K (2010) An overview of sample preparation and extraction of synthetic pyrethroids from water, sediment and soil. J Chromatogr A 1217(35):5537–5554
APHA, Awwa, WEF (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Administration, American Water Works Association, Water Environment Federation, Washington, DC
Armas ED, Monteiro RTR, Antunes PM, Santos M, Camargo PB, Abakerli RB (2007) Spatial-temporal diagnostic of herbicide occurrence in surface waters and sediments of Corumbataí River and main affluents. Quim Nova 30(5):1119–1127
Arraes AA, Barreto FMS, Araújo JC (2008) XIII World Water Congress, Montpellier. Proceedings. International Water Resources Association, Johanesburg
Barlas NE (2002) Determination of organochlorine pesticide residues in water and sediment samples in Inner Anatolia in Turkey. Bull Environ Contam Toxicol 69(2):236–242
Bortoluzzi EC, Rheinheimer DS, Gonçalves CS, Pellegrini JBR, Maroneze AM, Kurz MHS, Bacar NM, Zanella R (2007) Investigation of the occurrence of pesticide residues in rural wells and surface water following application to tobacco. Quim Nova 30(8):1872–1876
Cavalcante RM, Viana RB, Oliveira I, Nascimento RF, Silveira ER, Freire GSS (2007) Utilization of solid-phase extraction (SPE) for the determination of polycyclic aromatic hydrocarbons in environmental aqueous matrices. Quim Nova 30(3):560–564
Cavalcante RM, Lima DM, Fernandes GM, Duaví WC (2012) Relation factor: a new strategy for quality control in the determination of pesticides in environmental aqueous matrices. Talanta 93:212–218. doi:10.1016/j.talanta.2012.02.015
Cerejeira M, Viana P, Batista S, Pereira T, Silva E, Valério M, Silva A, Ferreira M, Silva-Fernandes A (2003) Pesticides in portuguese surface and ground waters. Water Res 37(5):1055–1063
CITAC/EURACHEM (2002) Guide to quality in analytical chemistry. URL: http://www.citac.cc/CITAC_EURACHEM_GUIDE.pdf
Claver A, Ormad P, Rodríguez L, Ovelleiro JL (2006) Study of the presence of pesticides in surface waters in the Ebro River basin (Spain). Chemosphere 64(9):1437–1443
Diana SG, Resetarites WJ Jr, Schaeffer DJ, Beckmen KB, Beasley BR (2000) Effects of atrazine on amphibian growth and survival in artificial aquatic communities. Environ Toxicol Chem 19(12):2961–2967
dos Santos Neto AJ, Siqueira MEPB (2005) Analysis of organophosphorus pesticides in water using SPE C18 disks and gas chromatography: evaluation of Furnas dam contamination. Quím. Nova 28(5):747–750
Environmental Protection Agency (EPA) (1992) Guidelines for exposure assessment FRL-4129-5. URL: http://www.epa.gov/ncea/pdfs/partmatt/April1996/guidline.pdf. Accessed on 20 Mar 2015
Environmental Protection Agency (EPA) (2014) Integrated risk information system (IRIS). URL: http://www.epa.gov/iris/iris-nrc.htm
European Community (EC) (1998) Council Directive 98/83/EC. URL http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1998:330:0032:0054:EN:PDF
Gama AF, Oliveira AHB, Cavalcante RM (2013) Inventário de agrotóxicos e risco de contaminação química dos recursos hídricos no semiárido cearense. Quim Nova 36(3):462–467
Garmouma M, Teil MJ, Blanchard M, Chevreuil M (1998) Spatial and temporal variations of herbicide (triazines and phenylureas) concentrations in the catchment basin of the Marne River (France). Sci Total Environ 224(1–3):93–107
Jeanneau L, Faure P, Jardé E (2007) Influence of natural organic matter on the solid-phase extraction of organic micropollutants: application to the water-extract from highly contaminated river sediment. J Chromatogr A 1173(1–2):1–9
MAPA- Ministério da Agricultura, Pecuária e Abastecimento (2015) Agrofit. URL: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 05 Aug 2014
Milhome MAL, Sousa DOB, Lima FAF, Nascimento RF (2009) Assessment of surface and groundwater potential contamination by agricultural pesticides applied in the region of Baixo Jaguaribe, CE, Brazil. Eng Sanit Ambient 14(3):363–372
Milhome MAL, Sousa PLR, Keukeleire D, Nascimento RF (2011) Multiresidue methods for determination of pesticides using SPME and SPE followed by GC-NPD System: a comparative study. J Braz Chem Soc 22(11):2048–2055
Milhome MAL, Sousa PLR, Lima FAF, Nascimento RF (2015) Assessment of pesticides contamination in water resources of the irrigated areas of Jaguaribe, Ceará, Brazil. Int J Environ Res 9(1):255–262
Ministry of Health of Brazil (2011) Portaria MS No 2914 DE 12/12/2011
Ni F, Liu G, Ren H, Yang S, Ye J, Lu X, Yang M (2009) Health risk assessment on rural drinking water safety—a case study in rain city district of Ya’an city of Sichuan Province. J Water Resource and Protection 1(2):128–135
Nogueira EM, Dores EFGC, Pinto AA, Amorim RSS, Ribeiro ML, Lourencetti C (2012) Currently used pesticides in water matrices in Central-Western Brazil. J Braz Chem Soc 23(8):1476–1487
Ribeiro ACA, Dores EFGC, Amorim RSS, Lourencetti C (2013) Resíduos de pesticidas em águas superficiais de área de nascente do Rio São Lourenço-MT: validação de método por extração em fase sólida e cromatografia líquida. Quim Nova 36(2):284–290
Rigotto RM (2009) Exploring fragility: industrial delocalization, occupational and environmental risks, and non-governmental organizations. Int J Environ Res Public Health 6(3):980–998
Sabin GP, Prestes OD, Adaime MB, Zanella R (2009) Multiresidue determination of pesticides in drinking water by gas chromatography-mass spectrometry after solid-phase extraction. J Braz Chem Soc 20(5):918–925
Singh B, Gupta A (2002) Monitoring of pesticide residues in different sources of drinking water of Jaipur, India. Bull Environ Contam Toxicol 69(1):49–53
Soares WL, Porto MF (2007) Atividade agrícola e externalidade ambiental: uma análise a partir do uso de agrotóxicos no cerrado brasileiro. Ciência Saúde Coletiva 12(1):131–143
Solomon K, Baker D, Richards RP, Dixon KR, Klaine SJ, La Point TW, Kendall RJ, Weisskopf CP, Giddings JM, Giesy JP, Hall LW, Williams WM (1996) Ecologial risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 15(1):31–76
Sousa JS, Castro RC, Andrade GA, Lima CG, Lima LK, Milhome MAL, Nascimento RF (2013) Evaluation of an analytical methodology using QuEChERS and GC-SQ/MS for the investigation of the level of pesticide residues in Brazilian melons. Food Chem 141(3):2675–2681
Ventura BC, Angelis DF, Marin-Morales MA (2008) Mutagenic and genotoxic effects of the atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pestic Biochem Phys 90(1):42–51
Vryzas Z, Vassiliou G, Alexoudis C, Papadopoulou-Mourkidou E (2009) Spatial and temporal distribution of pesticide residues in surface waters in northeastern Greece. Water Res 43(1):1–10
Yang H, Zhou S, Li W, Liu O, Tu Y (2015) Residues, sources and potential biological risk of organochlorine pesticides in surface sediments of Qiandao Lake, China. Bull Environ Contam Toxicol. doi:10.1007/s00128-015-1553-1
Acknowledgments
The authors thank FUNCAP- Fundação Cearense de Apoio ao Desenvolvimento cinetífico e tecnológico and CNPQ- Conselho Nacional de Desenvolvimento Científico e Tecnológico for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sousa, A.S., Duaví, W.C., Cavalcante, R.M. et al. Estimated Levels of Environmental Contamination and Health Risk Assessment for Herbicides and Insecticides in Surface Water of Ceará, Brazil. Bull Environ Contam Toxicol 96, 90–95 (2016). https://doi.org/10.1007/s00128-015-1686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1686-2