Abstract
Gold mining is known to generate important economic products but also to produce several types of contamination/pollution. We report here the first data about Hg concentrations in the soils of the Yacuambi River in the Ecuadorian Amazon. We analyzed soil samples to assess the extent of contamination caused by gold placer mining in this area. Hg concentrations in soils exceeded the local background concentrations. High concentrations of Mn, As, Pb, Cr, Cu, Fe and Zn in some soil samples were probably derived from the geology of the site, which is rich in polysulfides and metamorphic rocks. Placer mining may accelerate the natural release of these elements to the environment by the exposure of the bedrock to the atmosphere. Accumulation of Hg in the river soils may be a potential source of toxicity for aquatic life and a risk to human health in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Wastes generated by mining activities are much greater than the economic products they yield (Oyarzun et al. 2011). As a well documented example, in 1997, waste products from the mining industry in South Africa were about 468 million tons per year. Of that total, wastes from gold mining were estimated at around 221 million tons, or 47 % of all mining wastes in the country (DWARF 2001).
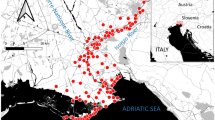
Mining is one of the main sources of income in Ecuador. Illegal and legitimate mining alike have been used for years to extract mineral resources in the south of the country. The regions of Loja, Azuay, El Oro and Zamora Chinchipe have a high economic potential for polymetallic deposits (Ramirez-Requelme et al. 2003; Fig. 1). Most of the gold extraction is originated from artisanal and small-scale mining, and it comes from the Portovelo-Zaruma and Zamora batholith areas (Litherland et al. 1994). However, methods used to extract gold are different in each area depending on whether it is underground or placer mining. Underground mining is used in the Portovelo-Zaruma region and placer mining in the Yacuambi region (Zamora batholitic), whereas in the Nambija region (Zamora batholith) both methods are applied (Fig. 1).
Location map of the Yacuambi River. a Geographic location of the main mining districts in Ecuador (Ponce Enriquez, Portovelo-Zaruma, Nambija, and Yacuambi, the last ones in the Zamora batholic), b Sample sites and geological map. Sources National Research Institute in Geology, Mining and Metallurgy (2015). Preliminary Geological Map Paquisha. Scale 1:100.000. Ecuadorian Institute of Mining (1985). Geological Map of Gualaquiza. Scale 1:100.000
Placer mining is the simplest way to extract gold. It starts by opening trails and cutting vegetation where terraces will be exploited. The next steps are the desencape and the destape, which consist of removing the upper layer of organic matter and the sterile gravels, respectively, using a digger. This process finishes with the arranque, which involves gold gravel removal until the bedrock. At the same time that gold gravel is extracted, washing is done with a gravimetric sluice. Hg is added onto the heaviest material to retain most of the Au in the amalgam. Then, the amalgam is burnt with a blowtorch. This process is done on river shores, and the tailings generated are deposited and/or discharged into the river. Previous studies in Ecuador have mainly focused on underground mining in the Portovelo-Zaruma area (e.g., Ramirez-Requelme et al. 2003; Carling et al. 2013). However, no studies about the effects of placer mining in Yacuambi have yet been conducted. In this study we report the first results about trace-element concentrations in this Ecuadorian Amazon region.
The main aim of this article is to assess the accumulation degree of trace elements in soils around the Yacuambi River. This study has a high relevance for two reasons: (1) no study has been conducted in this area so far, and (2) the study site has high biological and cultural diversity since it is situated in the Amazonian region. These factors enhance its scientific value, and the study sets a precedent for future investigations about placer-mining impacts.
Methods and Materials
Yacuambi river is situated in southeast Ecuador (3º 50′ 53″ S, 78º 49′ 42″ W; Fig. 1) within the Sub-Andinian area, which is the morphostructural division between Amazonian catchment and the high Andes zone. It is a relatively narrow belt formed by rocks, with flats and deformations originating from Real Mountains and Amazonian sediments. This belt is defined by granite batholithic type “I” (Prodeminca 2000) in the western part of the river and by metamorphic rocks in the northeastern part of the study site (Fig. 1).
Most of the Yacuambi region has a humid tropical climate, which is characterized by season-long precipitation. Annual rainfall values are 2000 mm and concentrated from January to July. Mean annual temperature is 22°C with a minimum value of 10.2°C and a maximum of 33°C. Relative humidity is around 90 % (Mora and Torre 2013). Some parts of the region also show some patches of a warm, periodically dry climate.
In this area, seven composite soil samples were taken in September 2013 from stirred terrains around placer mines and one soil sample was recovered from a pristine site upstream the mining sites (Fig. 1). In this pristine site, no recent alteration of soils or vegetation cover was observed. Samples were recovered throughout the Yacuambi River following the methodology proposed by Sepúlveda et al. (2005). One composite sample was obtained by mixing and homogenizing three subsamples, which were taken at a 50-cm depth, until 1 kg of composite sample had been recovered. Samples were kept in hermetic bags at a low temperature (4°C) until their analysis. These composite samples have been analyzed without replicates. They were analyzed for main cations (Na, Ca and Mg) and trace elements (Mn, As, Cd, Pb, Cr, Cu, Fe and Zn) by using inductively coupled plasma atomic optical emission (ICP-OES Perkin Elmer Optima 8300) at the INIGEMM laboratory (detection limit <0.01 mg/kg). Hg was measured by inductively coupled plasma mass spectrometry (ICP-MS Agilent 7700x) (<0.5 ppb). Soil samples were similarly prepared for both instruments following the EPA 3051 A method. Humidity from samples was partially eliminated by oven-drying at 50°C, which prevents Hg volatilization.
This procedure is based on an acid digestion (nitric and hydrochloric acids) with a heated mixture of mineral acids. Analytical standards for each trace elements measured in the ICP-OES were prepared from single-elements standards (AccuStandard e Inorganic Ventures). From standards of 1000 mg/L, a set of standards were prepared and dilutions were made in function of the desire calibration curve. Calibration curve for major elements was between 1–10 mg/kg and between 0.05–1.6 mg/kg for minor elements. The INIGEMM laboratory holds Certificates of Laboratory Proficiency for metals in soils according to the ISO/IEC 1703:2010. Proficiency test PT for ICP-OES was obtained on August 2013 (ERA- A waters Company). For Hg analysis in the ICP-MS, Plasma Pure standards (100 mg/kg) were used and successive dilutions were done to obtain the required calibration curve. The calibration blank is used to establish the analytical curve. A quality control of the blank samples was performed and a detection limit of 0.077 mg/kg was found for Hg. Coefficient of variation inter samples was acceptable (7 %) and correlation of the analytical curve was 0.99997.
A preliminary Shapiro–Wilk test (α = 0.05) was performed on the data of trace-elements to determine the normality of the concentration distribution of each element. This test shows an absence of normal distribution, since the coefficient of skewness and kurtosis describe how the shapes of sample-frequency distribution curves differ from the ideal Gaussian curves. Hence, all data were square-root transformed, and a principal component analysis (PCA) was performed on these transformed data. A PCA was carried out to detect the cases of variation of multivariate data using Canoco 4.5. for Windows software. Biplot was performed by using Canoco Draw 4.5.
Results and Discussion
Trace elements, as such addressed in our study, are present in relatively low concentrations (<mg/kg) in the Earth’s crust, soils and plants. The occurrence of harmful concentrations (anomalies) in the soils is a particular degradation called contamination, which can be geogenic or anthropogenic (Galán and Romero 2008). Besides, pollution refers to the introduction by man, directly or indirectly, of substances into the environment. The Yacuambi River is a good example of both types of processes as a result of placer-mining activities. Pollution results from the use of Hg in amalgamation during ore recovery, whereas higher concentrations of Mn, Hg, As, Pb, Cr, Cu, Fe and Zn with respect to the background levels can indicate geogenic contamination resulting from mineral leaching and the bedrock in some points of the river basin.
Higher Hg concentrations than the local background were recorded in all the soil samples. However, those levels are still smaller than the recommended levels for environmental health (6.6 mg/kg; Canadian Council of Ministers of the Environment 2007). Mn and Fe concentrations were greater than the local background. However, the absence of recommended guidelines of Mn and Fe in soils prevented us from a comparison with international standards. For the rest of the elements (As, Pb, Cr, Cu, Fe and Zn), we have consistently found some of the samples exceeding the local background and/or the standards for soils. In the case of Pb, Cu, Cr, Fe and Zn, the highest levels were detected in the first two samples from upstream to downstream.
PCA results showed that the two principal components, F1 and F2, accounted for 99.8 % of the total variance of the data; the first factor explained 98.6 % of the total variance, and the second explained 1.2 % of the remaining data. Figure 2 shows the distribution of the trace elements on a biplot representation. Along the first principal component axis F1, four groups of elements show positive loadings (Cu–Fe, Hg, Pb–Zn and Mn–Cd–As), while Cr exhibits negative loading. Trace-element distribution along the second principal axis F2 also shows a similar distribution, Cr in the negative part of the axis and the rest of elements, except Cu–Fe, in the positive part. Hg occupied a more central position in the biplot. Trace elements from natural sources in the studied site, such as metamorphic rocks and sulfides, which are on the biplot periphery, explained the variability found in both axes. However, a more central position is occupied by allochthonous elements, such as Hg, indicated that they are common in all the samples, regardless of the natural composition of the bedrock (Fig. 2).
PCA graphic representation of studied soils in the biplot factorial plan of F1 and F2 principal components. Axis 1 represents metamorphic rocks on the negative side and porphyry copper on the positive side. Axis 2 seems also to be a representation of the different mineral compositions, metamorphic rocks and sulfides derived from the Zamora batholitic (see map in Fig. 1)
These results are in concordance with the characteristics of the geological setting and with the form of extraction in this area. Elementary Hg has been used extensively for the recovery of gold in the Yacuambi River. The loss of Hg during the gold recovery reported by several studies worldwide (e.g., Alpers et al. 2005; Getaneh and Alemayehu 2006) and the absence of natural sources of Hg in Zamora province point at placer mining as the main source of this element in the environment. Concentrations of Hg higher than the local background support this hypothesis. However, these concentrations are still below the recommended levels for protection of the environment (Canadian Council of Ministers of the Environment 2007).
On the other hand, when bedrock contains pyrite and other sulfides, as in the Yacuambi region, their oxidation can release great quantities of heavy metals, such as As, Cd, Mn, Pb and Zn. Additionally, the presence of granodiorite (Fig. 1) which is usually associated to porphyry copper deposits may explain the higher concentrations of Cu and Fe in sample 2 and their position in the biplot. Chromium is a less common element in the area but it is still present in alluvial deposits of metamorphic rocks (phyllites, schists, granodiorite/diorites) (Ball and Izbicki 2004), as noticed especially in sample 1 (Figs. 1, 3). Samples with negative values of PC1 are situated on metamorphic terrains. However, only three samples (1, 3 and 7) exceed the regulatory guidelines for this metal, which can be caused by a synergic effect of the rock’s origin and the extent of mining activities in each point. According to Motzer and Engineers (2004), average chromium concentrations in granites are about 20 mg/kg (samples 3 and 7) while average concentrations in basalts, which can lead to the formation of schist (sample 1), are about 220 mg/kg. Those data are very consistent with our results. All these metals can be released into the environment by natural processes like weathering. Flooding during the rainy season and sudden variations of temperature may exacerbate this process. The exposure of minerals to the atmosphere caused by mining operations can also accelerate their release. Thus, minerals, especially sulfides, can be oxidized or hydrated. Moreover, crushing, grinding and leaching can also contribute to unlocking metals from host minerals.
Trace-element concentrations (mg/kg) in soil samples from the Yacuambi River. Samples are ordered from upstream to downstream. Solid lines show the limits from the Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health (Canadian Council of Ministers of the Environment 2007), and dashed lines with dot symbols show background concentrations from the pristine site
Regarding the spatial distribution of the concentrations throughout the river, Hg concentrations are higher and more homogenous than the rest of elements due to the constant use of Hg in the amalgam and the continuous process of soil removal and mixing carried out by placer-mining operations. The rest of the elements show less homogenous concentrations since they primarily depend on the presence of each element in the bedrock and on other secondary factors such as the geochemistry of each element, the weather and the process of soil removal from each point.
Our results show a high degree of concordance with previous studies about the environmental release of Hg during placer-mining activities. Although Hg concentrations nowadays only indicate a pollution phenomenon, ongoing amalgamation processes in the Yacuambi catchment may lead on to contaminant concentrations. Studies worldwide have evidenced the effect of this dangerous contaminant in aquatic environments, due to its bioaccumulation and biomagnification along the food web (Boening 2000). Several studies have also demonstrated its risk to human health through the consumption of contaminated fish (Mason et al. 2000). Future studies should be initiated to determine levels of methylmercury (MeHg), which is the most toxic and persistent in the trophic chain, and to set measures to stop or diminish the release of Hg into the Yacuambi River.
References
Alpers CN, Hunerlach MP, May JT, Hothem RL (2005) Mercury contamination from historical gold mining in California. US Geological Survey Fact Sheet, April, 1–6. http://pubs.usgs.gov/fs/2000/fs06100/. Accessed 06 Oct 2014
Ball JW, Izbicki JA (2004) Occurrence of hexavalent chromium in ground water in the western Mojave Desert California. Appl Geochem 19:1123–1135
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40:1335–1351
Canadian Council of Ministers of the Environment (2007) Canadian soil quality guidelines for the protection of environmental and human health: Summary tables. Updated September, 2007. In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, Winnipeg
Carling GT, Díaz X, Ponce M, Pérez L, Nasimba L, Pazmino E, Rudd A, Merugu S, Fernández DP, Gale BK, Johnson WP (2013) Particulate and dissolved trace element concentrations in three southern Ecuador rivers impacted by artisanal gold mining. Water Air Soil Pollut 224:1415
DWARF (Department of Water Affairs and Forestry) (2001) Waste generation in South Africa. Water Quality Management Series, Pretoria
Ecuadorian Institute of Mining (1985) Geological Map of Gualaquiza. Scale 1:100.000
Galán Huertos E, Romero Baena A (2008) Contamination of soils by heavy metals. Macla 10:48–60
Getaneh W, Alemayehu T (2006) Metal contamination of the environment by placer and primary gold mining in the Adola region of southern Ethiopia. Environ Geol 50:339–352
Litherland M, Aspden J, Jemielita R (1994) The metamorphic belts of Ecuador: keyworth, U.K.: British geological survey, overseas. Memoir 11:147
Mason RP, Laporte JM, Andres S (2000) Factors controlling the bioaccumulation of mercury, methylmercury, selenium and cadmium by freshwater invertebrates and fish. Arch Environ Contam Toxicol 38:283–297
Mora D, Torre H (2013) Identification of the productive ecosystems and evaluation of the water demand for human use in the main towns in the Yacuambi region. National University of Loja, Loja
Motzer WE, Engineers T (2004) Chemistry, geochemistry and geology of chromium and chromium compounds. In: Guertin J, Avakian CP, Jacobs JA (eds) Chromium (VI) handbook. CRC Press, Florida, pp 23–88
National Research Institute in Geology, Mining and Metallurgy (2015) Preliminary Geological map of Paquisha. Scale 1:100.000
Oyarzun R, Higueras P, Lillo J (2011) Environmental mining. An introduction to impacts and remediation. Ediciones GEMM- la2puntonet, Madrid
Prodeminca (2000) Porfiric and epi-mesotermals deposits related to the intrusions of the Cordillera del Cóndor. Assessment of mining district in Ecuador. Coordination unit of Promeminca project. Proyect MEM BIRF 36–55, p. 223
Ramirez-Requelme ME, Ramos JFF, Angelica RS, Brabo ES (2003) Assessment of Hg-contamination in soils and streams sediments in the mineral district of Nambija, Ecuadorian Amazon (example of an impacted area affected by artisanal gold mining). Appl Geochem 18:371–381
Sepúlveda TJ, Velasco J, De la Rosa A (2005) Polluted soils for metals and metaloides: sampling and alternatives for remediation. National Institute of Ecology, México
Acknowledgments
CLB acknowledges her Grant to the Prometeo Project from the Secretaría de Educación Superior, Ciencia, Tecnología e Innovación de la República del Ecuador (SENESCYT). This work has been financed by the Project “Improvement of the conditions of small and artisanal mining in Ecuador” by the SENPLADES (Secretaría Nacional de Planificación y Desarrollo de Ecuador). We are also very grateful to an anonymous reviewer for his/her valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Blanco, C., Collahuazo, L., Torres, S. et al. Mercury Pollution in Soils from the Yacuambi River (Ecuadorian Amazon) as a Result of Gold Placer Mining. Bull Environ Contam Toxicol 95, 311–316 (2015). https://doi.org/10.1007/s00128-015-1604-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1604-7