Abstract
The present study investigated the effect of lead (0, 16, 40 and 80 mg L−1 Pb2+) exposure for 3, 12 and 24 h on root biochemistry in hydroponically grown Zea mays (maize). Pb2+ exposure (80 mg L−1) enhanced malondialdehyde content (239 %–427 %), reactive carbonyl groups (425 %–512 %) and H2O2 (129 %–294 %) accumulation during 3–24 h of treatment, thereby indicating cellular peroxidation and oxidative damage. The quantitative estimations were in accordance with in situ detection of ROS generation (using 2′,7′-dichlorodihydrofluorescein diacetate dye) and H2O2 accumulation. Pb2+ treatment significantly reduced ascorbate and glutathione content during 3–24 h of exposure. On the contrary, levels of non-protein thiols were enhanced by 3–11.8 time over control in response to 16–80 mg L−1 Pb2+ treatment, after 24 h. A dose-dependent induction in ascorbate peroxidase and lipoxygenase enzyme activity was observed in Z. mays roots. The activities of ascorbate-recycling enzymes (dehydroascorbate reductase and monodehydroascorbate reductase) were significantly increased in relation to concentration and duration of Pb2+ treatment. The study concludes that Pb2+-exposure induces ROS-mediated oxidative damage during early period of exposure despite the upregulation of enzymes of ascorbate–glutathione cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Lead (Pb), a contaminant of almost every ecosystem (soil, water, atmosphere and living organisms), enters the plant system through soil (Sharma and Dubey 2005) or via foliar uptake (Uzu et al. 2010). Pb is transmitted from soil to the living organisms, via – the food chain, and it has now become necessary to explore the phytotoxic effect of Pb, especially in the context of food quality. It induces a broad range of toxic effects in plants, such as, it impairs plant growth, affects root elongation, seed germination, seedling development, transpiration, photosynthesis and cell division (Sharma and Dubey 2005; Kaur et al. 2014). However, plants respond to different intensities of Pb stress in a different manner, since it depends on the amount and duration of the exposure, plant developmental stage and specific organ under observation. At low Pb levels, sometimes plants show adaptive response (Gupta et al. 2009); whereas, at high Pb levels plants fail to combat Pb-induced oxidative stress (Kaur et al. 2012). To deal with metal toxicity, plants selectively uptake metals, form complexes with specific ligands, follow excretion and do compartmentalization (Krzesłowska et al. 2010; Maestri et al. 2010). Different responses of plants towards Pb exposure serve as bioindicators and help us to understand the mechanisms involved in uptake, transfer and toxicity (Pourrut et al. 2011). Pb primarily targets biochemicals and enzymes involved in the physiological processes, thus, affecting plant growth and causing visible injury (Sengar et al. 2008). Pb toxicity has been investigated in various crop plants like wheat (Kaur et al. 2012); maize (Gupta et al. 2009), pea (Malecka et al. 2009) and onion (Kaur et al. 2014). Most of the studies have been conducted under longer periods of exposure, ranging from 24 h to few weeks. However, not much information is available for cellular peroxidation (lipid and protein), ROS (reactive oxygen species) generation, non-protein thiols (NPTs) and modulation of ascorbate–glutathione cycle in Zea mays (maize) during early period of exposure (3–24 h). We chose Z. mays as a test plant because it is one of the most important cereal crops of the world, has high biomass and an ability to tolerate heavy metals and remediate both organic and inorganic pollutants from the soil and water; and does not accumulate these in the fruits (Komárek et al. 2007).
Materials and Methods
Seeds of maize (var. Subeej) purchased locally from the market were surface sterilized with sodium hypochlorite (0.1 %, w/v), washed under running tap water, and then rinsed in distilled water. Pb2+ was supplied in the form of lead nitrate (MW = 331.21; purity = 99 %; Merck Ltd., India). Imbibed maize seeds (for 6 h) were germinated on a wet filter paper in enamel trays (32 cm × 23 cm × 7 cm) lined with a moist cotton wad. After 24 h, germinating seeds were transferred to nylon mesh floating on distilled water in glass beakers (500 mL capacity) in growth chamber set at 25/20 (±2)°C, 75 % ± 3 % RH, and a 12 h photoperiod of ~240 µmol photons m−2 s−1 PFD. On third day, seedlings were exposed to 16, 40 and 80 mg L−1 Pb2+ or distilled water (control) for 3, 12, and 24 h. Since the Pb2+-toxicity was more pronounced in roots; these were excised, washed with 10 mM CaCl2, and stored at −20°C for further studies. The amount of Pb in root tissues was determined using an Atomic Absorption Spectrophotometer (EC 4139; ECL, Hyderabad, India).
Pb2+-induced ROS accumulation was determined in terms of hydrogen peroxide (H2O2) generation according to Singh et al. (2008), and malondialdehyde (MDA) accumulation (an indicator of lipid peroxidation) as per Heath and Packer (1968). Effect of Pb2+on membrane integrity was determined in terms of electrolyte leakage from root tissue (Singh et al. 2008). Protein oxidation was measured in terms of formation of reactive carbonyl groups (Levine et al. 1994). Histochemical detection of ROS was done using H2DCFDA (2′,7′-dichlorodihydrofluorescein diacetate) (Ezaki et al. 2000). In vivo root plasma membrane integrity was detected using Evans Blue solution (Singh et al. 2008) and H2O2 was localized by 3,3′-Diaminobenzidine (DAB)–H2O2 complex (Thordal-Christensen et al. 1997). Non-protein thiols (NPTs) content was assayed with Ellman’s reagent (Ellman 1959). Changes in low molecular mass antioxidants such as ascorbate (AsA) and glutathione (GSH) were determined as per the method given by Law et al. (1983) and Griffith (1980), respectively. ROS scavenging enzymes such as ascorbate peroxidases (APX), dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDHAR) were estimated according to Singh et al. (2008), Hossain and Asada (1984), and Hossain et al. (1984), respectively. Lipoxygenases (LOX) activity was determined at 234 nm using an extinction coefficient (ε) of 0.25 mM−1 cm−1 for linoleic acid (Chowhan et al. 2013). For determining the activities of APX and LOX, frozen root tissue was homogenized in 100 mM PO4 3− buffer (pH 7.0) in a pre-chilled pestle mortar. For the assay of DHAR and MDHAR, frozen root tissue was ground with 5 mL of ice-cold 25 mM HEPES buffer (pH 7.8) containing 0.2 mM EDTA, 2 mM ascorbate and 2 % PVP. The homogenates were passed through three layers of cheesecloth followed by centrifugation at 15,000×g at 4°C rotor temperature for 30 min. The supernatant was stored at −20°C and used further for enzyme assays. Protein content in the homogenate was determined against a standard of bovine serum albumin (Lowry et al. 1951). The experiments were performed in a randomized block design with at least five replicates, each consisting of a beaker with 15 seedlings. The data are presented as mean ± SE, and analyzed by one-way ANOVA followed by the comparison of mean values using Tukey’s test at p ≤ 0.01 and p ≤ 0.05.
Results and Discussion
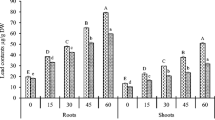
Exposure to 16, 40, and 80 mg Pb2+ L−1 for 24 h caused accumulation of 4.9 ± 0.37, 6.12 ± 0.49, and 6.89 ± 0.47 µg Pb2+ mg−1 root tissue (significant at p ≤ 0.01) compared to the control (0.001 µg Pb2+ mg−1). The difference in Pb2+ content between 16 and 40 mg Pb2+ L−1 was significant at p ≤ 0.01, whereas it was insignificant between 40 and 80 mg Pb2+ L−1 treatments. Maize roots absorb/uptake Pb (p ≤ 0.05) from the medium in a concentration-dependent manner; however, the uptake was limited at higher Pb dose. It suggests that beyond a limit (up to which a plant can withstand Pb exposure) plants activate resistance mechanisms that limit absorption/uptake of Pb, and consequently protect from injury ensued at higher doses. In other words, retarded absorption at high doses acts as defense strategy against metal-induced toxicity. Possibly, it results in a trade-off between metal resistance and plant fitness that enables plant to divert greater energy for survival in stressed environment and thus lesser energy is made available for growth and development (Maestri et al. 2010).
MDA content in Z. mays roots was enhanced by 149 %, 166 % and 337 % over their respective controls, after 3, 12 and 24 h of exposure to 16 mg L−1 Pb2+ (Fig. 1a). It increased further and was 194 %, 221 % and 363 % greater over the respective control in response to 40 mg L−1 Pb2+, after 3, 12 and 24 h, respectively. In response to 80 mg L−1 Pb2+, MDA content increased by 239 %–427 % over the controls during 3–24 h of exposure (Fig. 1a). MDA accumulation under Pb-treatment suggested lipid peroxidation. In support to present results, stimulation of lipid peroxidation was observed in onion roots exposed to Pb for 24 h (Kaur et al. 2014). Logani and Davies (1980) explained that lipid peroxidation initiates cyclic reactions that lead to the formation of short chain alkane and aldehydes, thereby causing dimerization and polymerization of proteins, thus damaging the membranes. Lipid peroxidation is the most common indicator of oxidative stress resulting in the disturbance in membrane integrity and consequently enhanced permeability (Heath and Packer 1968).
Effect of short-term Pb2+ exposure on a MDA content, b REL, c H2O2 accumulation and d carbonyl content in roots of 4-day-old Zea mays seedlings measured at 3, 12 and 24 h after Pb2+ treatment (16, 40 and 80 mg L−1). Data presented as mean ± SE. In figures a, b, different alphabets (lower case at a particular time period, upper case within a particular concentration at different time period) represent significant difference among them at p ≤ 0.05 applying Tukey’s test. In figures c, d, data were analyzed by linear regression models and r 2 represents the correlation coefficient
Membrane permeability was estimated on the basis of electrolyte leakage upon exposure to Pb2+. After 3 h, not much change in REL was observed in response to 16 mg L−1 Pb2+ (Fig. 1b). However, after 12 h of exposure REL increased by 31 %–65 % over the control upon treatment with 16–80 mg L−1 Pb2+. At 80 mg L−1 Pb2+, REL was greater over the control by ~77 % after 24 h of treatment (Fig. 1b). Increased REL in the present study, thus, suggests the toxic effect of Pb2+ in causing damage to the membrane in Z. mays roots. Seregin et al. (2004) explained that 10 mM Pb (lethal dose) penetrates all radicular tissues, and the cell membranes no longer play the role of physical barriers, thus causing disorganization of the membranes. Membrane disruption results in cellular disintegration leading to cell death (Baker and Mock 1994). The quantitative estimations in the present study were in agreement with the in situ histochemical localization where Pb2+ accumulation resulted in cell death as evidenced by Evans Blue staining (Fig. 2a).
Parallel to MDA accumulation, exposure to Pb2+ resulted in significant accumulation of H2O2 in root tissue. Accumulation of H2O2 reached up to 200 % after 24 h of exposure to 16 mg L−1 Pb2+ as compared to the control (Fig. 1c). At 40 mg L−1 Pb2+ exposure, 90 %–247 % greater accumulation of H2O2 over that in the control was observed during 3–24 h of treatment. H2O2 levels enhanced further with the increase in dose of the treatment. After 3, 12 and 24 h of treatment to 80 mg L−1 Pb2+, H2O2 content increased by ~129 %, 280 % and 294 % compared to their respective controls (Fig. 1c). H2O2 accumulation in Z. mays roots upon Pb2+ exposure was confirmed by in situ histochemical localization using DAB (Fig. 2b). Appearance of brown color in Pb2+-treated roots represented the formation of DAB–H2O2 complex (Fig. 2b). ROS production in roots was monitored by imaging the ROS-sensitive fluorescent dye, H2DCFDA (Fig. 2c).
In accordance with the quantitative measurement of ROS, fluorescence intensity was higher at 80 mg L−1 Pb2+ as the dye is oxidized by ROS to DCF, indicating increased ROS generation (Fig. 2c). H2O2, one of the ROS, acts as a signalling molecule and serves a dual role in plant defense mechanism (Stone and Yang 2006). Significant accumulation of H2O2 content in Z. mays roots in the present study suggests the induction of ROS-mediated oxidative stress. It is in agreement with previous findings reporting H2O2 accumulation in pea root cells (Malecka et al. 2012), wheat roots (Kaur et al. 2012) and onion roots (Kaur et al. 2014) exposed to Pb2+. Direct estimation of ROS indicates that Pb2+ exposure increases the production of H2O2, as reported by Pirslova et al. (2011) and Kaur et al. (2012).
Pb2+ exposure induced protein oxidation. Amount of carbonyls increased to 127 %, 181 % and 226 % after 3, 12 and 24 h of exposure to 16 mg Pb2+ L−1 (Fig. 1d). Further, upon increasing the dose of treatment to 80 mg L−1, ~512 % increase in carbonyl content was noticed after 24 h, compared to control (Fig. 1d). The results are in concordance with that reported by Biteur et al. (2011) who noticed carbonyl accumulation in radish exposed to 500–1000 mg L−1 of Pb2+. Carbonyl content is a sensitive marker of oxidative damage to proteins (Levine et al. 1994). Mitochondria are the main source of ROS in roots and regarded as the main target for oxidative damage to proteins (Møller and Kristensen 2004). Boscolo et al. (2003) and Pena et al. (2008) associated the levels of carbonylated proteins in plants with the oxidative stress induced by heavy metals. The observed formation of carbonyl reactive groups paralleled the ROS generation, oxidative stress and cellular injury. Excessive ROS generated in mitochondria has been related to oxidative carbonylation of proteins such as heat shock proteins and enzymes of Krebs cycle in mitochondria, and results in structural/functional modification that affect its catalytic properties (Møller and Kristensen 2004). Enhanced carbonyl content indicate an imbalance between the quantity of free radicals generated and the capacity of antioxidant defensive system under Pb2+ stress.
Non-enzymatic radical scavengers such as ascorbate and glutathione are known for their antioxidant potential for the removal of H2O2. Both ascorbate and glutathione content showed a progressive decline with increasing concentrations and duration of Pb2+ treatment (Fig. 3). After 3 h, ascorbate content was reduced by ~20 %, 34 % and 50 % over the control on exposure to 16, 40 and 80 mg L−1 Pb2+, respectively (Fig. 3a). A continuous decline was noticed with increasing duration of the treatment. Exposure to 16, 40 and 80 mg L−1 of Pb2+ caused ~55 %, 65 % and 80 % reduction in the ascorbate content after 24 h. Likewise, we observed a decline in the glutathione content on exposure to Pb2+ (Fig. 3b). After 3 h, the glutathione content was decreased by 9 %–34 % as compared to control in response to 16–80 mg L−1 Pb2+. At 80 mg L−1 Pb2+, glutathione content decreased by 43 % and 52 % over control after 12 and 24 h, respectively (Fig. 3b). Non-enzymatic radical scavengers, such as ascorbate and glutathione, play an important role in endogenous protective mechanism and help the plant cells to tolerate ROS (Nakano and Asada 1987). Ascorbates affect the redox balances of other metabolites, such as glutathione, since, both are involved in the maintenance of cellular redox balance and a failure to maintain this equilibrium leads to signal amplification (Apel and Hirt 2004). Glutathione is a precursor of low molecular mass peptides such as phytochelatins, produced by the plants to immobilize heavy metals; and correlates with the plant’s ability to adapt to metal stress (Seth et al. 2012). In the current investigation, the concentration of ascorbate and glutathione, both, decreased with increasing dose of lead treatment. This indicates the failure of the plant to alleviate oxidative stress induced by Pb2+.
Effect of Pb2+ exposure on the content of ascorbates and glutathione in maize roots measured at 3, 12, 24 h of the treatment. Data represented as mean ± SE. Different alphabets (lower case at a particular time period, upper case within a particular concentration at different time period) represent significant difference among them at p ≤ 0.05 applying Tukey’s test
In our study, the levels of NPTs were enhanced by ~2 to 9 times over the control in response to 16–80 mg L−1 of Pb2+ after 3 h (R2 = 0.983) (Fig. 4). Higher concentrations of Pb2+–induced greater accumulation of thiol compounds with respect to increasing duration of the treatment. Treatment with 80 mg L−1 Pb2+ for 12 h, accumulated ten times higher NPTs with respect to control. After 24 h, the NPT levels were 3, 7.7 and 11.8 times higher than the control at 16, 40 and 80 mg L−1 Pb2+, respectively (Fig. 4). NPTs levels increase under metal stress and these play a significant role in detoxification of metals through the possible synthesis of phytochelatins (Nadgórska-Socha et al. 2011).
Pb2+ significantly enhanced the LOX enzyme activity in a concentration- and time-dependent manner (Fig. 5a). LOX activities ranged from 117 % to 222 % in response to 16–80 mg L−1 Pb2+, with respect to control, after 3 h. The highest LOX activity (271 % increase over the control) was observed at 80 mg L−1 Pb2+ after 24 h of treatment. Heavy metal stress is known to induce membrane lipid peroxidation by the initiation of enzyme LOX, which produces hydroperoxide and oxy-free radicals by catalyzing the peroxidation of unsaturated fatty acids of biomembranes (Kumar et al. 2013). Increased LOX activity in the present study is well supported by increased H2O2 generation and greater lipid peroxidation. LOX-mediated lipid peroxides accumulation has also been reported in roots of Talinum triangulare (Kumar et al. 2013) and Sedum alfredii (Huang et al. 2012) upon Pb-exposure. The possible cause of Pb-induced oxidative stress could be the production of hydroperoxide derivatives by the enhanced LOX activity that attacks the cell membranes in Z. mays root cells.
Effect of short- term Pb+2 exposure on alterations in the activities of a APX, b LOX, c DHAR and d MDHAR in roots of 4-day-old Zea mays seedlings measured before treatment (0 h stage), and after 3, 12 and 24 h of Pb2+ treatment (16, 40 and 80 mg L−1). Data presented as mean ± SE. Different alphabets (lower case at a particular time period, upper case within a particular concentration at different time period) represent significant difference among them at p ≤ 0.05 applying Tukey’s test
We observed alterations in the activities of APX, DHAR and MDHAR under Pb2+ stress (Fig. 5b–d). APX activity was slightly enhanced after 3 h exposure to 16–80 mg L−1 of Pb2+ compared to control. Further, with increasing dose and duration of Pb2+ treatment, the maximum increase by 112 % and 129 % was noticed at 80 mg L−1 Pb2+ after 12 and 24 h, respectively (Fig. 5b). These observations are supported by similar findings made earlier in Oryza sativa (Verma and Dubey 2003), onion roots (Kaur et al. 2014) and Cassia angustifolia (Qureshi et al. 2007) under Pb treatment. In the ascorbate–glutathione cycle (Foyer–Halliwell–Asada cycle) H2O2 produced by SOD is further detoxified by APX, thereby producing monodehydroascorbate. An increase in APX activity should probably decrease H2O2 accumulation and can help to tolerate metal stress, but an increase in APX activity alone is not sufficient to confer abiotic stress induced by Pb. More likely, a coordinated action of all enzymes of ascorbate–glutathione cycle is required for plant stress tolerance.
The activities of ASC-recycling enzymes (DHAR and MDHAR) also showed significant (p ≤ 0.05) increase with respect to increasing concentration and duration of the treatment (Fig. 5c, d). A significant increase of 1.2–3.2 fold in DHAR activity was observed with respect to control in the roots of Z. mays subjected to 16–80 mg L−1 of Pb2+ for 3 h (Fig. 5c). With increase in period of exposure, DHAR activity was almost 1.9–6.3 fold and 2.41–6.50 fold higher than the control in response to 16–80 mg L−1 Pb2+ after 12 and 24 h, respectively (Fig. 5c). Parallel to DHAR, a significant rise in MDHAR activity was observed in Z. mays roots exposed to Pb2+ (16–80 mg L−1) for different time periods (3, 12 and 24 h) (Fig. 5d). In response to 16, 40 and 80 mg L−1 Pb2+, ~1.22, 2.38, 3.04 fold increase in MDHAR activity, compared to control, was noted after 3 h of exposure. After 12 and 24 h, MDHAR activity increased by ~5.9 and 6.5 fold over the control, when exposed to 80 mg L−1 Pb2+ (Fig. 5d).
The enhanced DHAR activity in response to Pb in the current study is in agreement with previous finding demonstrating stimulation of DHAR by Pb2+ in Phaseolus vulgaris (Geebelen et al. 2002), rice (Verma and Dubey 2003), and lupin roots (Rucińska-Sobkowiak and Pukacki 2006). DHA is reduced to ascorbate by DHAR using glutathione, as a reducing power, which in turn is regenerated by glutathione reductase (Blokhina et al. 2003). MDHAR is responsible for the reduced form of ascorbate, where NAD(P)H or ferrodoxin acts as a reductant in the ascorbate–glutathione pathway (Foyer and Halliwell 1976). Enhanced MDHAR activity under metal stress has been been linked to the increased resistance of plants to metal stress (Schützendūbel et al. 2001).
After analyzing all the observations together, it can be suggested that Pb induces a transitory loss in ‘antioxidative capacity’, perhaps accompanied by stimulation of oxidant producing enzymatic machinery, thus causing H2O2 accumulation and an increase in the oxidative stress markers and alteration in the related enzymes of ascorbate and glutathione cycle. The used Pb concentrations are ecologically relevant and comparable to those found under natural field conditions (Kaur et al. 2014). The obtained findings suggest that maize could be successfully grown in Pb-contaminated soil without any visible injury symptoms, and used for Pb-phytoextraction under natural field conditions.
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tissue Organ Cult 39:7–12
Biteur N, Aoues A, Kharoubi O, Slimani M (2011) Oxidative stress induction by lead in leaves of radish (Raphanus sativus) seedlings. Not Sci Biol 3(4):93–99
Blokhina OB, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Boscolo PRS, Menossi M, Jorge RA (2003) Aluminum-induced oxidative stress in maize. Phytochemistry 62:181–189
Chowhan N, Singh HP, Batish DR, Kaur S, Ahuja N, Kohli RK (2013) β-Pinene inhibited germination and early growth involves membrane peroxidation. Protoplasma 250:691–700
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ezaki B, Gardner RC, Ezaki Y, Matsumoto H (2000) Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol 122:657–665
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Geebelen W, Vangronsveld J, Adriano DC, Van Poucke LC, Clijsters H (2002) Effects of Pb-EDTA and EDTA on oxidative stress reactions and mineral uptake in Phaseolus vulgaris. Physiol Plant 115(3):377–384
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106(1):207–212
Gupta DK, Nicoloso FT, Schetinger MRC, Rossato LV, Pereira LB, Castro GY, Srivastava S, Tripathi RD (2009) Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J Hazard Mater 172:479–484
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hossain MA, Asada K (1984) Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol 25:85–92
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Huang H, Gupta DK, Tian S, Yang X, Li T (2012) Lead tolerance and physiological adaptation mechanism in roots of accumulating and non-accumulating ecotypes of Sedum alfredii. Environ Sci Pollut Res 19:1640–1651
Kaur G, Singh HP, Batish DR, Kohli RK (2012) A time-course assessment of changes in reactive oxygen species generation and antioxidant defense in hydroponically grown wheat in response to lead ions (Pb2+). Protoplasma 249:1091–1100
Kaur G, Singh HP, Batish DR, Kohli RK (2014) Pb-inhibited mitotic activity in onion roots involves DNA damage and disruption of oxidative metabolism. Ecotoxicology 23:1292–1304
Komárek M, Tlustos P, Száková J, Chrastný V, Ettler V (2007) The use of maize and poplar in chelant-enhanced phytoextraction of lead from contaminated agricultural soils. Chemosphere 67:640–651
Krzesłowska M, Lenartowska M, Samardakiewicz S, Bilski H, Wόzny A (2010) Lead deposited in the cell wall of Funaria hygrometrica protonemata is not stable—a remobilization can occur. Environ Pollut 158(1):325–338
Kumar A, Prasad MNV, Achary VMM, Panda BB (2013) Elucidation of lead-induced oxidative stress in Talinum triangulare roots by analysis of antioxidant responses and DNA damage at cellular level. Environ Sci Pollut Res 20:4551–4561
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J 210(3):899–903
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assay for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Logani MK, Davies RE (1980) Lipid oxidation: biologic effects and antioxidants—a review. Lipids 15:485–495
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein estimation with Folin–phenol reagent. J Biol Chem 193:265–278
Maestri E, Marmiroli M, Visioli G, Marmiroli N (2010) Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot 68(1):1–13
Małecka A, Piechalak A, Tomaszewska B (2009) Reactive oxygen species production and antioxidative defense system in pea root tissues treated with lead ions: the whole roots level. Acta Physiol Plant 31:1053–1063
Malecka A, Piechalak A, Mensinger A, Hanc A, Baralkiewicz D, Tomaszewska B (2012) Antioxidative defense system in Pisum sativum rooots exposed to heavy metals (Pb, Cu, Cd, Zn). Pol J Environ Stud 21:1721–1730
Møller IM, Kristensen BK (2004) Protein oxidation in plant mitochondria as a stress indicator. Photochem Photobiol Sci 3(8):730–735
Nadgórska-Socha A, Kandziora-Ciupa M, Ciepał R, Walasek K (2011) Effects of Zn, Cd, Pb on physiological response of Silene vulgaris plants from selected populations. Pol J Environ Stud 20:599–604
Nakano Y, Asada K (1987) Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol 28:131–140
Pena LB, Zawoznik MS, Tornado ML, Gallego SM (2008) Heavy metals effects on proteolytic system in sunflower leaves. Chemosphere 72:741–746
Pirslova B, Kuna R, Libantova J, Moravcikova J, Matusikova I (2011) Biochemical and physiological comparison of heavy metal-triggered defense responses in the monocot maize and dicot soybean roots. Mol Biol Rep 38:3437–3446
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol 213:113–136
Qureshi MI, Abdin MZ, Qadir S, Iqbal M (2007) Lead induced oxidative stress and metabolic alterations in Cassia angustifolia Vahl. Biol Plant 51:121–128
Rucińska-Sobkowiak R, Pukacki PM (2006) Antioxidative defense system in lupin roots exposed to increasing concentrations of lead. Acta Physiol Plant 28:357–364
Schützendūbel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 127:887–898
Sengar RS, Gautam M, Sengar RS, Garg SK, Sengar K, Chaudhary R (2008) Lead stress effects on physiobiochemical activities of higher plants. Rev Environ Contam Toxicol 196:73–93
Seregin IV, Shpigun LK, Ivanon VB (2004) Distribution and toxic effects of cadmium and lead on maize roots. Russ J Plant Physiol 51:525–533
Seth CS, Remans T, Keunen E, Jozefczak M, Gielen H, Opdenakker K, Weyens N, Vangronsveld J, Cuypers A (2012) Phytoextraction of toxic metals: a central role for glutathione. Plant Cell Environ 35(2):334–346
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17(1):35–52
Singh HP, Batish DR, Kaur G, Arora K, Kohli RK (2008) Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ Exp Bot 63:158–167
Stone JR, Yang S (2006) Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8:243–270
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during barley–powdery mildew interaction. Plant J 11:1187–1194
Uzu G, Sobanska S, Sarret G, Munoz M, Dumat C (2010) Foliar lead uptake by lettuce exposed to atmospheric fallouts. Environ Sci Technol 44:1036–1042
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Acknowledgment
GK is thankful to University Grants Commission (New Delhi, India) for research fellowship.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, G., Kaur, S., Singh, H.P. et al. Biochemical Adaptations in Zea mays Roots to Short-Term Pb2+ Exposure: ROS Generation and Metabolism. Bull Environ Contam Toxicol 95, 246–253 (2015). https://doi.org/10.1007/s00128-015-1564-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1564-y