Abstract
This study examined the concentration of heavy metals in 13 fish species. The results indicated that shellfish species (clams) have the highest metal concentrations, followed by demersal and pelagic fishes. The mean concentration of metals in clams are Zn 88.74 ± 11.98 µg/g, Cu 4.96 ± 1.06 µg/g, Pb 1.22 ± 0.19 µg/g, Cd 0.34 ± 0.04 µg/g dry wt. basis, whereas the same measure in fish tissues was 58.04 ± 18.51, 2.47 ± 1.21, 0.58 ± 0.27 and 0.17 ± 0.08 µg/g dry wt. basis. The concentrations of heavy metals in clams and fish tissues were still lower than the maximum allowable concentrations as suggested by the Malaysian Food Act (1983) and are considered safe for local human consumption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fish are the main supply of protein besides meat and poultry products and contribute to a large percentage of dietary protein globally. In most Asian countries, especially in south-east Asia like Thailand, Indonesia and Malaysia, fish is consumed as the main dish of the diet. Fish is particularly valuable for providing food with nutritional value in the form of protein, and minerals such as calcium, phosphorus, iron and copper. In addition, consumption of fatty fish is recommended by nutritionists as a means of preventing cardiovascular diseases (Domingo et al. 2007). Heavy metals are serious pollutants in our natural environment due to their toxicity, persistence and bioaccumulation problems (Balachandran and Lalu 2005). In freshwater systems, fish is one of the aquatic products humans consume, and also provide a good indicator of trace element pollution. Copper and zinc are essential elements for growth and development and their uptake from the environment is regulated according to nutritional demand through homeostatic control (Couture and Rajotte 2003). However, Cu and Zn become toxic at concentrations above the limits of homeostatic control (McKim and Eaton 1978). Cadmium and lead are non-essential heavy metals that do not have dedicated regulatory mechanisms (Viarengo 1989) and are therefore more toxic to organisms. Some previous studies (Lim and Kiu 1995; DOE 2005) at various periods indicate that the Juru River Basin is grossly polluted by domestic wastes and discharges from pig farms. The objectives of this study are to determine the distribution of selected heavy metal concentrations (lead (Pb), zinc (Zn), cadmium (Cd) and nickel (Ni)) in 13 species of fish from the Juru River, Malaysia in based on living habitats, to estimate the health risk from these heavy metals related to the consumption of the edible parts (muscles) of fish and also to assess the safe dietary intake of these metals.
Materials and Methods
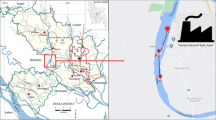
The Juru River originates from the Bukit Mertajan hills located at 05°22 N latitude and 100° 28 E longitude, Penang in the north-eastern part of Malaysia (Fig. 1) and drains approximately 7.95 km long. The Juru River receives water from several small tributaries that flow through urban settings that are grossly polluted with industrial, agriculture and domestic wastes which are discharged directly into these rivers. Besides high levels of organic loadings, these wastes are also contaminated with heavy metals (Lim and Kiu 1995).
Fish were randomly caught using the gill net and cast net. In the laboratory, the frozen samples were thawed at room temperature and dissected using stainless steel scalpels for analysis. Only tissues without bone were selected for extraction. Approximately 10 g of fish tissues of each sample were dried in an oven at 60°C for 2 days until dry weight was obtained (Nussey et al. 2000). The dry tissue samples were extracted with concentrated nitric acid (HNO3, 70 %) followed by perchlorid acid (HCLO4) and digestion was done on the hot plate (Agemian et al. 1980). After extraction sample mixtures (of yellowish-colour) were then filtered through 0.45 µm pore size and filtrates were then analyzed using inductively coupled plasma (ICP) model Perkin Elmer/Elan 9000. The Operational parameter setting used for ELAN 9000 Perkin Elmer is given in Table 1 and shows the Operational parameter settings used for the ELAN 9000 Perkin Elmer. One-way analysis of variance (ANOVA) was performed on all experimental data, and means were compared using Tukey’s test with SPSS for Windows (version 18) software.
The confidence level was p < 0.05. The detection limits for the studied elements were 0.00004d mg/kg for Pb, 0.0003d mg/kg for Zn, 0.00009d mg/kg for Cd and 0.0002c mg/kg for Cu; where d means run on ELAN DRC in DRC mode in a class-100 clean room using PT cones and quartz sample introduction system, c means run on ELAN DRC in standard mode in a class-100 clean room using PT cones and the quartz sample introduction system. For comparison purposes, a reference site at Lake Chini, Pahang was chosen because this site has low concentrations of metals in fish.
For the quality assurance purpose in fish, standard reference materials were analysed prior to the analysis. The lobster sample (TORT-2) (National Research Council Canada) was used to confirm the recovery of digestion and analysis. Percentage of recoveries (n = 5 for each metal) for certified and measured concentrations of those metals was satisfactory, with the recoveries being 80 for nickel, 90 for zinc, 96 for cadmium and 85 for copper. All experimental data were analysed using ANOVA, while the means of the two sampling times were compared using Tukey’s test.
Assessment of Human Health Risk
The daily intake of an element from food consumption is dependent on the element concentration in food and amount of food consumed. Statistics have shown that the average age Malaysian consumes 160 g/day/person of marine fish daily (FAO 2009). From a human health perspective it is important to assess whether the metals pose a risk, especially in regions where fish can be a cheap and readily available source of protein. Oral reference doses (RfDs) of Pb 2 mg/kg body wt./day, Zn 300 mg/kg body wt./day, Cd 0.5 mg/kg body wt./day, Cu 40 mg/kg body wt./day have been published by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 2003).
The following equation was used to estimate risk:
where HQ is chemical-specific Hazard Quotient (unitless); FIR is the fish ingestion rate (fish: 160 g/day/person) (FAO 2009); C is the metal concentration in the muscle of fishes (edible fish part) (mg/g); RfD is the oral reference dose (JECFA 2003); WAB is the average body weight (64 kg; the reference weights were derived from numerous local Malaysian studies (Lim et al. 2000). If the Hazard Quotient >1.00, there is a potential risk related to the studied metal. Calculations of health risk from fish consumption for the non-carcinogenic metals (Pb, Zn, Cd and Cu) are shown in Table 4. This calculation was made by assuming that the maximum studied fish consumption is seven times per week.
Calculation of the allowable daily consumption (CRlim)
where CRlim is the maximum safe daily consumption rate of fish (kg/day); RfD is reference dose for each heavy metal (mg/kg/day); BW is average consumer body weight kg); and Cm is measured concentration of chemical in the edible part of fish (mg/kg). The RfD is determined by the US EPA and is an estimate of the daily intake of a contaminant over a lifetime that would not be expected to cause adverse health effects (US EPA 2000). The results of the calculation of the allowable studied fish consumption rates (kg/day) showed that the Pb, Zn, Cd and Cu-based allowable consumption rate of Anadara granosa from the Juru River was the lowest (0.47, 1.00, 0.46 and 2.10 kg/day, respectively). The allowable consumption rate of Cd and Cu was on the top in Megalops cyprinoides (3.20 and 8.83 kg/day respectively). Zinc in the Lobotes surinamensis fish species was associated with the highest allowable consumption rate of 3.89 kg/day, as shown in Table 2.
Results and Discussion
Details of 13 species of fish analysed in this study are given in Table 3, showing concentrations of four heavy metals, Pb, Cu, Zn and Cd, in the tissue of thirteen fish species from Juru River during two sampling occasions (December 2009 and April 2010); it reveals that the ranking order of mean concentration of the heavy metals in the fish tissues were Zn (64.86) > Cu (3.03) > Pb (0.72) > Cd (0.21) (mean; µg/g dry weight) respectively. Zinc was detected in the range from 19.4 to 115, Cu from 1.24 to 6.89, Pb from 0.26 to 1.65, and Cd from 0.07 to 0.43 µg/g on a dry wt. basis. Metal accumulation in the tissues of fish varies according to the rates of uptake, storage and elimination (Kalay and Canli 2000). This means that metals which have high uptake and low elimination rates in tissues of fish are expected to be accumulated to higher levels (Kalay and Canli 2000). According to living habitats the mean concentration of metals in shellfish species are (Zn 88.74 µg/g, Cu 4.96 µg/g, Pb 1.22 µg/g, Cd 0.34 µg/g dry wt. basis.), whereas in demercial fish tissues the value are 61.37, 2.80, 0.57 and 0.19 µg/g on a dry wt. basis, respectively. The mean metals concentration in pelagic fish tissues are (Zn 49.70 µg/g, Cu 1.66 µg/g, Pb 0.60 µg/g, Cd 0.13 µg/g on a dry wt. basis.). There are many factors that influence the rate of accumulation such as locality, type of food and living habitats (Boscolo et al. 2007). The Anadara granosa (cockle) accumulated the highest of all metals followed by Perna viridis (clam); this could be due to the fact that the habitat of Anadara granosa (cockles) that are living at the bottom of sea, in close proximity to the sediment where various kinds of hazardous and toxic substances are accumulated (Kamaruzzaman et al. 2011). According to Boscolo et al. (2007), bivalve mollusc species had a high capacity and propensity to concentrate pollutants. All studied metals varied significantly at (p < 0.05) in demersal, pelagic and shellfish species. This is in agreement with Gale et al. (2004) who reported that Cu and Pb concentrations in muscle of bottom-feeding fish were higher than top-feeding fish. Sediment is well known to act as a sink for metal deposition and these metals are easily taken by demersal and shellfish fishes compared to the pelagic species. Similar results were obtained by Romeo et al. (1999) who pointed out that Cd concentration in tissues of pelagic fish species are lower than benthic fish species. All species of fish studied in this study were commercially consumed by people. Concentration of the metals in the reference site was 0.09 µg/g dry weight for Pb, 0.92 µg/g dry weight for Zn, 0.05 µg/g dry weight for Cd, 0.09 µg/g dry weight for Cu which were much lower than the results of this study. The metal concentrations in fish and clam tissues in this study were found to be lower than the maximum allowable concentration as listed in the Malaysian Food Act and is considered safe for local consumption. The Hazard Quotient (HQ) values of Pb, Zn, Cd and Cu in Anadara granosa (cockle) were the highest at 1.25, 0.76, 1.80 and 0.36 respectively, whereas the lowest HQ for Pb, Zn, Cd and Cu were observed in Sillago chondropus, Lobotes surinamensis, Platycephalus indicus and Megalops cyprinoides at 0.40, 0.19, 0.40 and 0.08 respectively (Table 4). In all studied fish the Hazard Quotient is lower than 1.00 except for Pb and Cd in some fish species which means that consuming fishes from the Juru River could pose a health risk to the natives for these two metals. If the Hazard Quotient >1.00, there is a potential risk related to the studied metal. For maximum allowable fish consumption rates, the studied heavy metals in 13 fish species in the Juru River showed that there would not be expected to be any cause for negative health effects for humans.
Conclusion
This study has identified the level of heavy metals such as Pb, Zn, Cd and Cu in fish and shellfish caught along the Juru River. All metals were present in all samples but values obtained were below permitted limits set by the Food Act 1983. In all studied fish the Hazard Quotient is lower than 1.00 except for Pb and Cd in some fish species which means that consuming fishes from the Juru River could pose a health risk to the natives for these two metals. If the Hazard Quotient >1.00, there is a potential risk related to the studied metal.
References
Agemian H, Starvent DP, Austen KD (1980) Simultaneous acid extraction of six trace metals from fish tissues by hot-block digestion and determination by atomic absorption spectrophotometry. Analyst 55:125–130
Balachandran KK, Lalu Raj CM (2005) Heavy metal accumulation in a flow restricted, tropical estuary. Estuar Coast Shelf Sci 65:361–370
Boscolo R, Cacciatore F, Berto D, Giani M (2007) Polychlorinated biphenyls in clams Tapes philippinarum cultured in the Venice Lagoon (Italy): contamination levels and dietary exposure assessment. Food Chem Toxicol 45:1065–1075
Couture P, Rajott JW (2003) Morphometric and metabolic indicators of metal stress in wild yellow perch (Perca flaveseens) from Sudbury, Ontario: a review. J Environ Monit 5:216–221
DOE (2005) Malaysia environmental quality report 2004. Department of environment, Ministry of Natural Resources and Environment Malaysia, Malaysia
Domingo JL, Bocio A, Falc´o G, Llobet JM (2007) Benefits and risks of fish consumption part I. A quantitative analysis of the intake of omega-3 fatty acids and chemical contaminants. Toxicology 230:219–226
FAO-Food and Agriculture Organization (2009) Fishery and aquaculture statistics. (ftp://fao.org/FI/CDrom/CD_yearbook_2009/navigation/index_content_food_balance_e.htm)
Gale NL, Adams CD, Wixson AB, Loftin KA, Huang Y (2004) Lead, zinc, copper and cadmium in fish and sediment from the Big River and Flat River Creek of Missouri’s Old Lead Belt. Environ Geochem Health 26:37–49
JECFA—Joint Expert Committee for Food Additives (2003) Summary and conclusions of the 61st meeting of the joint FAO/WHO expert committee on food additives (JECFA). JECFA/61/SC. Rome, Italy
Kalay M, Canli M (2000) Elimination of heavy metals (Cu, Zn, Cd and Pb) in tissues of a freshwater fish Tilapia zilli. Turk J Zool 24:429–436
Kamaruzzaman BY, Shuhada NT, Akbar B, Shahbudin S, Jalal KCA, Ong MC, Al-Barwani SM, Goddard JS (2011) Spatial concentrations of lead and copper in bottom sediments of Langkawi coastal area, Malaysia. Res J Environ Sci 5:179–186
Lim PE, Kiu MY (1995) Determination and speciation of heavy metals in sediment of the Juru River, Penang, Malaysia. Environ Monit Assess 35:85–95
Lim TO, Ding LM, Zaki M, Suleiman AB, Fatimah S, Siti S, Tahir A, Maimunah AH (2000) Distribution of body weight, height and body mass index in a national sample of Malaysian adults. Med J Malays 55:108–128
Malaysian Food Act (1983) MDC. Malaysia, 1995
McKim JM, Eaton JG (1978) Metal toxicity to embryos and larvae of eight species of freshwater fish-II: copper. Bull Environ Contam Toxicol 19:608–616
Nussey G, Vuren JHJ, Preez HH (2000) Bioaccumulation of chromium, manganese, nickel and lead in the tissue of the moggel, Labeo, umbratus (Cyprinidae), from Witbank Dam, Mpumalanga. Water SA 26:269–284
Romeo M, Siau Y, Sidoumou Z, Gnassia-Barelli M (1999) Heavy metal distribution in different fish species from the Mauritania Coast. Sci Total Environ 232:169–175
US EPA-US Environmental Protection Agency (2000) Guidance for assessing chemical contaminant data for use in fish advisory, vol. II: risk assessment and fish consumption limits. US Environmental Protection Agency. Office of science and technology. Office of water, Washington (DC), EPA823-B-00-008
Viarengo A (1989) Heavy metals in marine invertebrates: mechanisms of regulation and toxicity at the cellular level. Crit Rev Aquat Sci 1:295–317
Acknowledgments
We would like to acknowledge the Faculty of Science and Technology, National University of Malaysia and the Ministry of High Education in Libya for financial support and also the laboratory assistants for sampling and analysing the fish samples.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Idriss, A.A., Ahmad, A.K. Heavy Metal Concentrations in Fishes from Juru River, Estimation of the Health Risk. Bull Environ Contam Toxicol 94, 204–208 (2015). https://doi.org/10.1007/s00128-014-1452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1452-x