Abstract
This study aims to determine the composition and sources of particulate matter with an aerodynamic diameter of 10 μm or less (PM10) in a semi-urban area. PM10 samples were collected using a high volume sampler. Heavy metals (Fe, Zn, Pb, Mn, Cu, Cd and Ni) and cations (Na+, K+, Ca2+ and Mg2+) were detected using inductively coupled plasma mass spectrometry, while anions (SO4 2−, NO3 −, Cl− and F−) were analysed using Ion Chromatography. Principle component analysis and multiple linear regressions were used to identify the source apportionment of PM10. Results showed the average concentration of PM10 was 29.5 ± 5.1 μg/m3. The heavy metals found were dominated by Fe, followed by Zn, Pb, Cu, Mn, Cd and Ni. Na+ was the dominant cation, followed by Ca2+, K+ and Mg2+, whereas SO4 2− was the dominant anion, followed by NO3 −, Cl− and F−. The main sources of PM10 were the Earth’s crust/road dust, followed by vehicle emissions, industrial emissions/road activity, and construction/biomass burning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The issue of air pollution is widespread throughout the world and has adverse consequences on both human health and the environment. Particulate matter (PM) is one of the most common air pollutants and as such is used to monitor the level of environmental pollution (Viana et al. 2008). Recent findings on the health problems experienced following exposure to PM with an aerodynamic diameter of 10 μm or less (PM10) include a decline in lung function and an increase in respiratory symptoms, chronic pulmonary disease, heart disease and premature death along with a rise in mortality (Mukae et al. 2001; Miller et al. 2007). The concentration of PM10 itself is dependent on several factors which include climate, season, land use and geographical location. A higher traffic density and the use of fossil fuel for combustion (heating purposes) might explain why the concentration of PM10 was found to be higher during the cold season (Harrison et al. 1997; Tsitouridou et al. 2003). Urban background, as a result of a high number of motor vehicles, more industries and a large quantity of road dust are also suspected to contribute to the level of PM10 in the atmosphere (Juneng et al. 2011).
In order to predict the sources of PM10, it is necessary to determine its composition. PM10 usually consists of several elements (Si, Al, Ca, Fe, Ti, V, Cr, Ni, Cu, Zn, Pb), inorganic ions (SO4 2−, NO3 −, Na+, NH4 +, K+) and volatile organic compounds (Sunder-Raman et al. 2008). Previous studies on the composition of PM10 in Malaysia have shown that Fe recorded the highest reading of heavy metals, which indicated that heavy-duty vehicle emissions were the main source of PM10 around an incredibly busy highway (Ismail et al. 2011). However, according to Sulaiman et al. (2005), the concentration of Zn was the highest compared to Fe, Cr and Mn which showed an association between Zn and emissions from the use of car brakes, tyres and also different types of petrol fuel in vehicles (Sternbeck et al. 2002). According to Mohd Tahir et al. (2008), the majority of the trace elements recorded in the city centre were recorded at higher levels than at inner and outer city stations.
This paper presents the composition of PM10 in Bangi, which is a semi-urban area in Malaysia. The analyses focused on selected heavy metals (Fe, Zn, Pb, Cu, Mn, Cd and Ni) and ions (Na+, Ca2+, K+, Mg2+, SO4 2−, NO3 −, Cl− and F−). The source identification of PM10, based on the elements detected from the samples in this area, was evaluated using a combination of principle component analysis (PCA) and multiple linear regression (MLR) techniques. This research may be useful for scientific records and raising public awareness since very few studies on the source tracking of these chemical contaminants have been conducted, particularly in tropical countries.
Materials and Methods
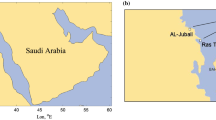
Bangi is situated in the south of Hulu Langat district, in the state of Selangor, Malaysia. It is about 25 km south of Kuala Lumpur city centre. Bangi was previously surrounded by palm oil estates but in the last few decades has grown to become a semi-urban area with many residential and industrial areas. For this study, the sampling station used is located at the main entrance of Universiti Kebangsaan Malaysia, Bangi Campus (N02°55.47′ E101°46.39′). During peak hours, this station experiences a high flow of traffic, particularly on weekdays, due to a high level of traffic from Universiti Kebangsaan Malaysia. Figure 1 shows the sampling location for this study.
The PM10 sampling took place between November 2011 and January 2012 for 21 days. The sampling was undertaken using a high volume sampler (HVS) equipped with fibreglass filter paper (Whatman EPM 2000, size 20.3 cm × 25.4 cm). Before sampling, the filter paper was pre-heated in a furnace oven at a temperature of 500°C for 5 h. The filter paper was stored in a desiccator for 24 h before being weighed using a 5-digit analytical balance (Shimadzu). The sampling was conducted for 24 h at a flow rate of 1.13 m3/min. After sampling, the filter paper was returned to the desiccator for 24 h then reweighed before being stored in the refrigerator at a temperature of ≤4°C until further analysis.
For the metal analysis, a quarter of the PM10 filter paper was cut into smaller pieces (1 cm × 1 cm) and placed into a 100 mL conical flask. Then, 40 mL of nitric acid and 10 mL of perchloric acid (4:1 ratio) were put into the flask and the mixture was heated on a heating plate to digest the filter paper. Digestion was conducted in the fume chamber for about 1 h and 30 min as performed by Latif et al. (2011). After that, the sample solution was left to cool in the fume chamber before being filtered using a vacuum pump equipped with a fibreglass filter paper (Whatman 47 mm diameter, 0.2 μm pore size). The sample was transferred to a volumetric flask and diluted to 250 mL using deionised water. The determination of the heavy metals was undertaken using inductively coupled plasma mass spectrometry (ICP-MS PerkinElmer Elan 9000). Calibration of the ICP-MS instrument was performed with standard multi-element solutions. The detection limits for the analysis of heavy metals using this methodology were 0.07 ng/m3 for Cu, 0.17 ng/m3 for Pb, 0.10 ng/m3 for Zn, 0.10 ng/m3 for Fe, 0.03 ng/m3 for Cd and 0.13 ng/m3 for Ni.
For the ionic composition, a quarter of the PM10 filter paper was cut into smaller pieces (1 cm × 1 cm) and then placed in a 250 mL conical flask. 100 mL of deionised water was added and ultrasonic shakers were used for extraction at 60°C for 1 h. Next, the samples were filtered using a vacuum pump equipped with a 47 mm fibreglass filter paper (0.2 μm pore size). The samples were then transferred to a 250 mL volumetric flask and diluted using deionised water. The determination of the cations (Na+, Ca2+, K+ and Mg2+) was achieved through the use of an Inductively Coupled Plasma Mass Spectrometer (ICP-MS PerkinElmer Elan 9000), with a detection limit of 0.1 ng/m3 for Na+, 0.07 ng/m3 for Ca2+, 0.07 ng/m3 for K+ and 0.1 ng/m3 for Mg2+. Meanwhile the determination of the anions (SO4 2−, NO3 −, Cl− and F−) was undertaken using Ion Chromatography (IC-Metrohm 850 IC plus) with a detection limit of 0.77 ng/m3 for SO4 2−, 0.27 ng/m3 for NO3 −, 0.40 ng/m3 for Cl− and 0.23 ng/m3 for F−. In order to maintain the quality of the results, all instruments involved in the study were calibrated before use. The field blank sample was also analysed for the control analysis. The results were corrected based on the average blank concentration. A recovery test, which was in the range of 85 %–117 %, was also conducted for each analysis.
The data obtained was analysed using SPSS version 17.0 (Student Version of the Statistical Package for Social Sciences). Several analyses were undertaken, such as: the normality test, one-way ANOVA and Pearson correlation. In addition, the multivariate analyses were used to identify the sources of pollution that contributed to the PM10 concentrations recorded at the study area. PCA was utilised together with MLR using the XLSTAT 2012 software. After the data was found to be normally distributed, PCA was performed using the varimax rotation method. This ensured the variable was maximally correlated with one component but had little correlation with the others (Mohd Tahir et al. 2013; Wahid et al. 2013). The factor loadings after rotation were classified as strong (>0.70), moderate (0.50–0.70) and weak (<0.50). However, in this study, only strong factor loadings were chosen to clarify the sources of PM10 in the study area. MLR was used to calculate the contribution of each parameter to the level of PM10 by predicting the variability between independent and dependant variables (Shi et al. 2011). The results of the factor scores were used as the variables in MLR models. They were compared, based on the modelling performance, by referring to the coefficient of determination (R2). In this study, each variable was independently introduced to a linear regression model with PM10 concentrations as the dependent variable. Once the possible sources of PM10 were clarified, the contribution of each source was defined using the backward stepwise technique based on the R2 value. Using this method, the largest R2 value indicated the best linear model.
Results and Discussion
The average concentration of PM10 recorded in Bangi was 29.5 ± 1.5 μg/m3, with the minimum and maximum values of 24.7 and 34.8 μg/m3, respectively. This level of PM10 was well below the 150 μg/m3 recommended by the Malaysian Government in the Recommended Malaysian Air Quality Guidelines (RMAQG). In this study, the sampling stations only experienced a high volume of traffic during peak hours: in the morning, during the lunch hour and after working hours when the majority of university staff were using the main entrance. This situation may have resulted in the concentration of PM10 recorded here, which is moderate when compared to other studies in Malaysia which focused on urban areas (Mohd Tahir et al. 2008; Mohd Tahir et al. 2013).
The average concentration of heavy metals and ions recorded are shown in Table 1. Significant variations (p < 0.05) were observed between the elements studied, where Fe recorded the highest average concentration (172 ± 35 ng/m3) relative to the other heavy metals. Generally, vehicle exhaust emissions and brake friction from motor vehicles along with road dust were considered to be the most likely contributors to the higher Fe levels in the PM10 samples (Mohd Tahir et al. 2009; Mansha et al. 2012). As the study area experienced a high volume of traffic on the roads, notably during peak hours, the concentration of Fe might also increase. In addition, the occurrence of construction activity around the study area may have led to a higher concentration of Fe (Mazzei et al. 2008). The second highest heavy metal concentration was Zn (84.5 ± 21.2 ng/m3) followed by Pb > Cu > Mn > Cd > Ni. These elements are often associated with traffic emissions, either vehicular exhaust emissions or non-engine combustion sources, such as tyres and clutch wear (Sternbeck et al. 2002; Mohd Tahir et al. 2013).
For the ionic composition, significant variations (p < 0.05) were observed between the elements studied. For cations in PM10, the concentrations of the elements followed the trend of Na+ > Ca2+ > K+ > Mg2+, whereas for anions the trend was SO4 2− > NO3 − > Cl− > F− respectively. For cations, the average concentration of Na+ had the highest level (10.8 ± 9.76 μg/m3) compared with the other cations studied. According to previous studies, the majority of Na+ originates from the Earth’s crust and marine aerosol (Orlic et al. 1999). The Earth’s crust source, also proven as the source of Ca2+ (Viana et al. 2008), gave the second highest cation concentration in PM10 samples (0.204 ± 0.106 μg/m3). For anions, overall, SO4 2− showed the highest concentration (2.52 ± 0.724 μg/m3) followed by NO3 − > Cl− > F−. This most likely occurred as a result of the photochemical process which converts SO2 to SO4 2− (Terzi et al. 2010). The second highest anion concentration was NO3 − (0.358 ± 0.001 μg/m3) which indicates that PM10 might also originate from motor vehicle emissions, particularly those from diesel fuel (Shukla and Sharma 2008).
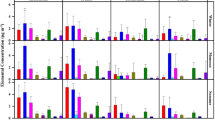
The results from the factor loading after varimax using PCA are shown in Table 2. Four main factors with eigenvalues >1 were identified with a total variance of 78.03 % in the PM10 samples. The first factor (F1) gave a high factor loading for Ni, Na+, Ca2+ and K+ which is thought to be derived primarily from the construction activities occurring in the areas surrounding the campus as well as biomass burning (Tsitouridou et al. 2003). The second factor (F2) showed the dominancy of Zn, followed by Cd and Pb, which are markers for industrial emissions and originate from road activities such wear from rubber tyres, friction from braking and fuel combustion (Mazzei et al. 2008). The third factor (F3) was dominated by Fe, Mn and SO4 2− which demonstrated the Earth’s crust as a source along with the existence of road dust (Viana et al. 2008). The fourth factor (F4) correlated with F− which may well have been derived from vehicle emissions (Mansha et al. 2012). Figure 2 shows the PCA ordination score plots which visualise the distribution of samples on the respective factor axes.
The R2 values and standardised coefficient resulting from the MLR analysis are shown in Table 3, while the equation for the model is presented in Eq. (1).
where F1 = construction/biomass burning, F2 = industrial emissions/road activity, F3 = Earth’s crust/road dust, F4 = vehicle emissions.
Based on the backward stepwise technique, the R2 for each individual variable were determined to predict the major sources of PM10 in this study area. Based on the result, the largest R2 (0.298) was detected from F3 (Earth’s crust/road dust), followed by F4 (vehicle emissions, R2 = 0.085), F2 (industrial emissions/road activity, R2 = 0.007) and F1 (construction/biomass burning, R2 = 0.002). It appears that the Earth’s crust/road dust has contributed significantly to the PM10 concentrations recorded in this semi-urban area probably due to the effects of wind velocity and wind direction. This result concurs with those from several previous studies (Tsitouridou et al. 2003; Viana et al. 2008; Mohd Tahir et al. 2013).
By way of a conclusion, the average concentration of PM10 was found to be well below the Recommended Malaysian Air Quality Guidelines (RMAQG) of 150 μg/m3 in 24 h. Overall, the trend of heavy metals recorded in the study area was Fe > Zn > Pb > Cu > Mn > Cd > Ni. A decreasing trend of cations in PM10 samples was shown to be Na+ > Ca2+ > K+ > Mg2+, while for anions it was SO4 2− > NO3 − > Cl− > F−, respectively. The PCA–MLR analysis clearly showed that the possible sources of PM10 in Bangi were dominated by the Earth’s crust and road dust, followed by vehicle emissions. This study suggests that both natural and anthropogenic sources have contributed to the amount of PM10 in the ambient air. Due to the effects of PM10, which may incur health risks, the level and the composition of PM10 should be monitored regularly in order to maintain a healthy environment, particularly within the university campus in question.
References
Harrison MR, Deacon RA, Jones RM, Appleby RS (1997) Sources and processes affecting concentrations of PM10 and PM2.5 particulate matter in Birmingham (UK). Atmos Environ 31:4103–4117
Ismail I, Laiman R, Ahmad H (2011) Study of particulate matter (PM10) concentration and elemental composition at Damansara–Puchong highway. 2011 International Conference on Biology, Environment and Chemistry. Singapore
Juneng L, Latif MT, Tangang F (2011) Factors influencing the variations of PM10 aerosol dust in Klang Valley, Malaysia during the summer. Atmos Environ 45(26):4370–4378
Latif MT, Baharudin NH, Velayutham P, Awang N, Hamdan H, Mohamad R, Mokhtar MB (2011) Composition of heavy metals and airborne fibers in the indoor environment of a building during renovation. Environ Monit Assess 181(1–4):479–489
Mansha M, Ghauri B, Rahman S, Amman A (2012) Characterization and source apportionment of ambient air particulate matter (PM2.5). Sci Total Environ 425:176–183
Mazzei F, D’Alessandro A, Lucarelli F, Nava S, Prati P, Valli G, Vecchi R (2008) Characterization of particulate matter sources in an urban environment. Sci Total Environ 401(1–3):81–89
Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD (2007) Long-term exposure to air pollution and incidence of cardiovascular events in women. N Eng J Med 356(5):447–458
Mohd Tahir N, Poh SC, Hamzah S, Wood KH, Rahman SA, Wee BS, Elias S, Salim NAA (2008) Analysis of PM10 in Kuala Terengganu by instrumental neutron activation analysis. Malays J Anal Sci 12(1):187–194
Mohd Tahir N, Poh SC, Suratman S, Ariffin MM, Shazali NAM, Yunus K (2009) Determination of trace metals in airborne particulate matter of Kuala Terengganu, Malaysia. Bull Environ Contam Toxicol 83:199–203
Mohd Tahir N, Suratman S, Fong FT, Hamzah MS, Latif MT (2013) Temporal distribution and chemical characterization of atmospheric particulate matter in the eastern coast of Peninsular Malaysia. Aerosol Air Qual Res 13(2):584–595
Mukae H, Vincent R, Quinlan K, English D, Hards J, Hogg JC, Van Eeden SF (2001) The effect of repeated exposure to particulate air pollution (PM10) on the bone marrow. Am J Respir Crit Care Med 163(1):201–209
Orlic I, Wen X, Ng TH, Tang SM (1999) Two years of aerosol pollution monitoring in Singapore: a review. Nucl Inst B 150(1–4):457–464
Shi G-L, Zeng F, Li X, Feng Y-C, Wang Y-Q, Liu G-X, Zhu T (2011) Estimated contributions and uncertainties of PCA/MLReCMB results: source apportionment for synthetic and ambient datasets. Atmos Environ 45(17):2811–2819
Shukla SP, Sharma M (2008) Source apportionment of atmospheric PM10 in Kanpur, India. Environ Eng Sci 25(6):849–862
Sternbeck J, Sjodin A, Andreasson K (2002) Metal emissions from road traffic and the influence of resuspension: results from two tunnel studies. Atmos Environ 36(30):4735–4744
Sulaiman N, Abdullah M, Chieu PLP (2005) Concentration and composition of PM10 in outdoor and indoor air in industrial area of Balakong Selangor, Malaysia. Sains Malays 34(2):43–47
Sunder-Raman R, Hopke P, Holsen T (2008) Characterization of fine aerosol and its inorganic components at two rural locations in New York State. Environ Monit Assess 144(1–3):351–366
Terzi E, Argyropoulos G, Bougatioti A, Mihalopoulos N, Nikolaou K, Samara C- (2010) Chemical composition and mass closure of ambient PM10 at urban sites. Atmos Environ 44(18):2231–2239
Tsitouridou R, Voutsa D, Kouimtzis T (2003) Ionic composition of PM10 in the area of Thessaloniki, Greece. Chemosphere 52(5):883–891
Viana M, Kuhlbusch TAJ, Querola X, Alastueya A, Harrison RM, Hopke PK, Winiwarter W, Vallius M, Szidat S, Prévôt ASH, Hueglin C, Bloemen H, Wåhlin P, Vecchi R, Miranda AI, Kasper-Giebl A, Maenhaut W, Hitzenberger R (2008) Source apportionment of particulate matter in Europe: a review of methods and results. J Aerosol Sci 39(10):827–849
Wahid NBA, Latif MT, Suratman S (2013) Composition and source apportionment of surfactants in atmospheric aerosols of urban and semi-urban areas in Malaysia. Chemosphere 91(11):1508–1516
Acknowledgments
This study was funded by research Grant (FRGS/1/2013/STWN01/UKM/02/2) and industrial Grant (INDUSTRI-2011-013). Special thanks to K. Alexander and Dr. Rose Norman for assistance with the proofreading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wahid, N.B.A., Latif, M.T., Suan, L.S. et al. Source Identification of Particulate Matter in a Semi-urban Area of Malaysia Using Multivariate Techniques. Bull Environ Contam Toxicol 92, 317–322 (2014). https://doi.org/10.1007/s00128-014-1201-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1201-1