Abstract
Black carbons (BC) which result from the incomplete combustion of farm waste [man-made (burned) BC] are highly absorbent. In Taiwan, the burning of farm waste known as slash and burn is common. The BCs from the burning may present an environmental challenge. Little is known about the effect of BCs on the transport of hydrophobic organic contaminants (HOC). This study investigates the sorption of anthracene and naphthalene to BCs in soil and efficiency of the surfactants Tween 80 and Triton X-100 in their removal. Both surfactants demonstrated 2–6 times increased solubility in the soils with the addiction of BC. Column experiments were performed to imitate the transportation of these contaminants in groundwater through soils before and after adding BC produced by burning farm waste in the lab. We found significantly increased sorption of anthracene in soil added with BCs produced in the lab, suggesting that fraction of organic carbon (foc) can contribute to sorption of such HOCs. Sorption of naphthalene was increased but not significantly. Comparing the concentrations of contaminants, we found the soil containing BC from burned farm waste absorbed HOC more efficiently than the organic BC (naturally-occurring) in the original soil. Therefore, sorption capacity and influence on the transport of HOC cannot be estimated simply by the foc of the soil because the two BCs differ greatly in their sorption ability. BC from farm waste absorbs more contaminants than naturally occurring BC in the soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polycyclic aromatic hydrocarbons (PAHs) pose a serious threat to human health and the environment. PAHs are hydrophobic organic compounds (HOCs) that are not easily biodigested. PAHs generated by natural and anthropogenic processes during the incomplete combustion of organic matter are often emitted to ambient in gaseous form or sorbed to particles. Atmospheric PAHs are deposited on land surfaces and tend to accumulate in soil over long periods of time. It is also known that PAHs released from industrial wastewater and soil runoff may heavily pollute sediment (Wang et al. 2009).
Black carbon (BC), commonly found in soil, has a high specific surface area, so its influence on sorption and the distribution of nutrients as well as HOCs, including pesticides and PAHs, cannot be ignored (Ghosh et al. 2000). There is a high affinity between BCs and PAHs, increasing the likelihood of high concentration of PAH in the soil from farm waste combustion. Intensely cultivated farmlands become increasing deficient of organic matter especially when only chemical fertilizers are used. As the naturally occurring carbon is depleted, the effect of BC resulting from the combustion of farm waste will grow in importance as it accumulates in the soil and exerts a greater effect on the sorption of HOC. Soil remediation is dependent on how contaminants react with the soil (Chi et al. 2011). Although the interaction of natural organic matter with pollutants (Chi and Amy 2004) has been studied, little is known about the influence of man-made carbon, such as that produced by farmers who seasonally use slash and burn farming techniques in Taiwan, on the sorption and transport of HOCs.
Surfactants, which compete with soil organic matter for hydrophobic compounds, have been successfully applied in the remediation of HOC contaminated soils (Bernardez et al. 2009). The nonionic surfactant TX-100 can increase the apparent solubility of PAHs, as it has been found that increasing the concentration of TX-100 in the flushing solution can enhance the flushing efficiency of the solution (Chi 2011). TX-100 is reported to have a better effect on the remediation of PAHs, PCE, and heavy metals in soils than ionic surfactants. Another nonionic surfactant, Tween 80 (T80) has been found able to remove 4,4′-dichlorobiphenyl effectively by the formation of micelle in solution, which results in the increased solubility and mobility of the contaminants (Damrongsiri et al. 2010).
This study performed column experiments to investigate the sorption and transport of two pollutants, anthracene and naphthalene, in soils with naturally occurring BC and manmade BC. The efficacy of the surfactants Tween 80 and Triton X-100 in the removal of these pollutes was also evaluated.
The effect of adsorbents on the transport of naphthalene (Na) and anthracene (An) was studied using different amounts of adsorbent as the dependent variable. Like many other PAHs, anthracene is a carcinogenic substance listed by the occupational safety and health administration and naphthalene is listed as possibly carcinogenic to humans and animals (Group 2B) by the International Agency for Research on Cancer. PAHs with three benzene rings are often found in the surface water and in sediment, and those with less than three are often found in the vapor phase or sorbed in the colloids. We used naphthalene (2 benzene rings) and anthracene (3 benzene rings) as model PAH compounds for this study.
Materials and Methods
Soils samples obtained from the campus of Kun Shan University (KS) and from its vicinity around Yu-Non road (YN) were wind dried and sieved through mesh #140 (0.1 mm) and #200 (0.074 mm). The method of measuring PAH concentrations in solution was adopted from Chi and Amy (2004) using fluorescence detector (Jasco FP 6200). The dissolved organic carbon in solution and in soils was measured by spectrophotometer (Skalar TOC Analyzer). The characteristics of soils, PAHs, surfactants, and the conditions of fluorescence spectroscopy used in this study are listed in Table 1. Sodium nitrate was used to measure the pore volume of soil in the column experiment. In order to mimic the groundwater, we used calcium chloride to adjust the ionic strength (0.015 M) in the solutions in our experiments. The excitation and emission wavelengths (nm) selected for the measurement of anthracene were 251 and 403, and 264 and 335 for naphthalene, respectively. Two or three replicates were done in most of the experiments. After being packed, the soil columns stood overnight before the experiment.
Both the batch and column experiments were conducted to understand the optimal conditions of equilibrium and kinetics. In batch experiments, PAHs were added directly to the soils to prepare synthetic contaminated soils for the investigation of the desorption efficiency. The burned BC was added and mixed thoroughly with soil in the weight percentage of 0.1 %, 0.5 % and 1.0 % accordingly. Then, flushing solution was added into 20 g of 500 mg/kg of PAH contaminated soils in a flask (W:V = 1:10) and shaken for 24 h. The supernatant was centrifuged in 4,500 rpm for five minutes and measured using fluorescence spectroscopy.
A column 5 cm long and 1.9 cm in diameter was packed with soils for continuous flow column experiments. Stainless steel frits (pore size 2 micron at the ends of the column) were used to spread flow evenly across the diameter of the column. A Darcy velocity 5.1 m/day controlled by a HPLC pump used to simulate the high flow rate of ground water. Different flushing solutions were introduced during the sorption and desorption experiments. The operational conditions in column experiments were: pH of 6.0, ionic strength of 0.015 M, room temperature. The pore volume (pv) was estimated from the breakthrough curve of sodium nitrate in the column (Chi and Amy 2004). The pore volumes of the two soils in packed columns are 7.5 and 6.0 mL for KS and YN soils, respectively. We experimented with three different flushing solutions—calcium chloride (0.005 M), Tween 80, and Triton X-100 at concentrations 0.1 % (v/v) to test the desorption efficiencies. BC was prepared by burning the crop waste collected from the field in Tainan (cultivar Tainan 11) for 30 s, covering it to stop the combustion and collecting the residual BC. The BET surface area of the burned BC is 24 m2/g).
An extraction method used by Johnson (2000) was used to measure PAH concentrations in solutions. After discrete PAH solution samples were collected from the soil column, hexane was used to extract the PAHs. A mixture of hexane to sample (1:1 by volume) was shaken vigorously for 2 min with a vibrator. The hexane layer was removed, and PAH concentrations were measured by fluorescence intensity using a spectrofluorometer, according to Gauthier et al. (1986). Linear relationships exist between fluorescence intensity and the PAH concentrations used in this study.
Results and Discussion
The sorption of naphthalene onto the soils was low due to its low hydrophobicity. It took less than 26 pore volumes to breakthrough the soil with 1 % of burned BC compared to the anthracene of 260 pore volumes in only 0.1 % of burned BC. The organic content (foc) and clay content of KS soil was higher than that of YN soil. Therefore, when the same ratio of adsorbent was added, naphthalene showed a higher adsorption capacity onto KS than to YN. During the experiments using different concentrations of adsorbent in samples with no addition of BC, it took 7.5 pore volumes to breakthrough YN soil and 14 pore volumes to breakthrough KS soil. When PAHs solution infiltrated the packed soil column, the solution traveled through the pore space from inlet to outlet. The breakthrough curve is a plot of the concentration measured at the outlet versus time. In samples made up of 0.5 % BC it required 14.1 and 23.8 pore volumes of naphthalene to break through YN and KS soil, and in those made up of 1.0 % BC it took 16 and 26.3 pore volumes, respectively (Fig. 1). For both soils, the increase from 0.5 % to 1.0 % only increased breakthrough by two pore volumes. Sorption is influenced by the characteristics of solid phase and chemicals. In this case, low sorption was caused by the low hydrophobicity of naphthalene.
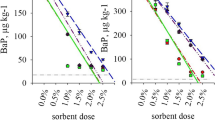
Because anthracene has a higher hydrophobicity than naphthalene, it has greater affinity to soils. In the untreated soils, it took 146 and 180 pore volumes of anthracene to breakthrough in the YN and KS soils, respectively (Fig. 1). However, when 0.1 % of the BC was added, it took 219 and 259 pore volumes breakthrough in YN and KS soils, respectively. The adsorption of anthracene in the soils with the addition of only 0.1 % of BC increased 1.5 times compared to its adsorption in soil without the added BC. The high adsorption capacity of the BC can be attributed to its high surface area. The soil with a higher concentration of the BC was found to have a higher adsorbed anthracene. Anthracene took about 10 times longer than naphthalene to breakthrough in both soils, suggesting a higher affinity between the soil and anthracene than that of naphthalene.
The organic carbon contents (foc) of the original untreated of YN and KS soils were 0.10 % and 0.32 % respectively. The foc in the adsorbent (BC) from burned farm waste was 7.13 %. The foc of YN and KS soils increased to 0.11 % and 0.33 %, respectively, when the adsorbent was added to a ratio of 0.1 %. This suggested that even a small amount of the BC added had a strong impact on foc.
For our desorption experiments, we tested three solutions, CaCl2 solution 0.05 mol, Tween 80 solution 0.1 %, and Triton X-100 solution 0.1 %. The result showed following the broke through of naphthalene in YN soil and then eluted the soil with 10 pore volumes of CaCl2 solution, most of the adsorbed naphthalene (98 %) had been desorbed (Fig. 2). When Tween 80 solution was used, there was no difference on desorption efficiency compared to that of CaCl2. When Triton X-100 solution was used, there were peaks shown at 4–7 pore volumes, indicating a high solubility of naphthalene and a shorter desorption time needed for remediation. When 0.5 % and 1.0 % of adsorbent was added in YN soil (Fig. 2), the desorption efficiency conducted by CaCl2 solution reached 90 % after 20 pore volumes, which was twice the time needed in soil with no added adsorbent. Desorption efficiency was also decreased. Tween 80 did not increase the desorption efficiency. Triton X-100 increased desorption 1.6 and 1.1 times in 0.5 % and 1 % adsorbent added soils, respectively, compared to CaCl2 solution. In the KS soil, after naphthalene had broken through the packed KS soils in the column, three flushing solutions were eluted to study their desorption efficiency in the soils containing 0 %–1 % adsorbents (figure not shown). We found no obvious difference in the desorption efficiencies between Tween 80 and CaCl2 solution. Tween 80 solution had desorption efficiencies that were 1.0 and 1.6 times in 0.5 % and 1 % BC-added soils, respectively, compared to CaCl2. Triton X-100 had desorption efficiencies 1.6 and 1.8 times higher in the same BC added soils, respectively.
After anthracene had broken through in the column packed with the untreated YN soil, the results showed that 90 % of the sorbed anthracene was removed after 100 pore volumes of CaCl2 solution moved through the column (figure not shown); however, once 0.1 % of adsorbent was added to the soil, only 75 % of sorbed anthracene was desorbed with the use of 150 pore volumes of CaCl2 solution. Tween 80, on the other hand, increased solubility by 5 and 6.8 times of higher apparent solubilities in the presence 0 % and 0.1 % adsorbent, respectively. Triton X-100 increased solubilities by 2.8 and 4 times, compared to CaCl2 solutions.
After anthracene broke through KS soils, desorption of anthracene by 150 pore volumes of CaCl2 was found to be 87 % in samples untreated with burned BC and 65 % in samples with 0.1 % burned BC (Fig. 3). When Tween 80 solution was used, the desorption efficiencies were 2.0 and 1.7 times higher than that achieved by CaCl2 in the two soils, respectively. When Triton X-100 solution was used, the desorption efficiencies increased 1.9 and 1.7 times than that achieve by the CaCl2 in the two soils, respectively. The concentrations of Tween 80 solution and Triton X-100 solution were 68 CMC and 10 CMC, respectively. Tween 80 was expected to have a higher desorption ability than that of Triton X-100 due to its lower HLB and higher CMC values. However, no significant difference was found. In YN soil, Tween 80 did show a higher desorption capacity than did Triton X-100. However, in this study of anthracene desorbed from KS soil, the surfactant concentration investigated was only 0.1 % in both surfactants, the differences in HLB and CMC did not show a significant difference between the two surfactants. It needs more investigation in the future.
When the concentrations of sorbed anthracene and naphthalene in the two soils were measured, the partition coefficients (Kpm) could be calculated by the concentration in solid phase (mg/kg) divided by the concentration in solution (mg/L) (Table 2). The theoretical partition coefficient (Kpt) could be derived by multiplying the partition coefficient in organic carbon (Koc) times the fraction of organic carbon in soil (foc). For naphthalene, the ratio of Kpt to Kpm was less than 3 in YN soil and less than 5.5 in KS soil. For anthracene, they were less than 1.8 in YN soil and less than 3.1 in KS soil. This theoretical value can be used predict the sorption of hydrophobic organic compound in soils. We found the higher the hydrophobicity of the compounds, the smaller the ratio of Kpt to Kpm. Based on the weight of the organic carbons in soil, we found that the original naturally occurring organic carbons in the soil had lower sorption capacity than that of the added organic burned carbons. The organic BCs produced by combustion of crop residuals have higher sorption capacity for HOCs.
In summary, the BC produced by burning farm waste has much higher sorption capacity for PAHs than original soil organic carbon. Calculating PAHs sorption capacity simply by the foc of the soil might lead to underestimates of the soil’s sorption capacity. The surfactants, Tween 80 and Triton X-100, show higher desorption efficiencies of PAHs from KS and YN soils, especially with the higher hydrophobic anthracene. Additionally, the theoretical partition coefficient is close to the real partition coefficient in the field especially at high hydrophobic compounds. It can provide useful estimation on the sorption of PAHs in soils.
References
Bernardez LA, Therrien R, Lefebvre R, Martel R (2009) Simulating the injection of micellar solutions to recover diesel in a sand column. J Contam Hydrol 103:99–108
Chi F-H (2011) Remediation of polycyclic aromatic hydrocarbon-contaminated soils by nonionic surfactants: column experiments. Environ Eng Sci 28(2):139–145
Chi F-H, Amy GL (2004) Kinetic study on the sorption of dissolved natural organic matter onto different aquifer materials: the effects of hydrophobicity and functional groups. J Colloid Interface Sci 274(2):380–391
Chi F-H, Leu M-H, Tsao C-W, Shiu G-C (2011) Removal of anthracene contaminated soil using micro-emulsified solvent and mixed surfactant. Sustain Environ Res 21(3):181–186
Damrongsiri S, Tongcumpou C, Weschayanwiwat P, Sabatini DA (2010) Solubilization of dibutyltin dichloride with surfactant solutions in single and mixed oil systems. J Hazard Mater 181:1109–1114
Gauthier TD, Shane EC, Gurein WF, Seltz WR, Grant CL (1986) Fluorescence quenching method for determining equilibrium constants for polycyclic aromatic hydrocarbons binding to dissolved humic materials. Environ Sci Technol 20(11):1162–1166
Ghosh U, Gillette JS, Luthy RG, Zare RN (2000) Microscale location, characterization, and association of polycyclic aromatic hydrocarbons on harbor sediment particles. Environ Sci Technol 34:1729–1736
Johnson WP (2000) Sediment control of facilitated transport and enhanced desorption. J Environ Eng 126(1):47–57
Wang D-G, Yang M, Jia H-L, Zhou L, Li Y-F (2009) Polycyclic aromatic hydrocarbons in urban street dust and surface soil: comparisons of concentration, profile, and source. Arch Environ Contam Toxicol 56:173–180
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chi, FH. The Influence of Black Carbon on the Sorption and Desorption of Two Model PAHs in Natural Soils. Bull Environ Contam Toxicol 92, 44–49 (2014). https://doi.org/10.1007/s00128-013-1127-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-013-1127-z