Abstract
Multixenobiotic resistance (MXR) represents an important cellular detoxification mechanism in aquatic organisms as it provides them robustness toward natural and man-made contaminants. Several ABC transporters have major roles in the MXR phenotype – P-gp/ABCB1, MRP1–3/ABCC1–3 and BCRP/ABCG2. In this study, we identified the presence of ABC transporters involved in the MXR mechanism of Arbacia lixula and Paracentrotus lividus. AlABCB1/P-gp, AlABCC3/MRP3, AlABCC9/SUR-like and AlABCG-like transcripts were identified in A. lixula; and PlABCC1/P-gp, PlABCC3/MRP3, PlABCC5/MRP5, and PlABCC9/SUR-like transcripts in P. lividus. For each of the new partial sequences, we performed detailed phylogenetic and identity analysis as a first step toward full characterization and understanding of the ecotoxicological role of these ABC transporters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Organisms are constantly exposed to a vast variety of substances present in their surrounding aquatic environment, including natural and anthropogenic contaminants. In order to survive, aquatic organisms use a highly efficient cellular protection system mediated by complementary activity of the efflux transport proteins and detoxification enzymes (Goldstone et al. 2006; Epel et al. 2008). Efflux transporters are members of the large ATP binding cassette (ABC) transporter superfamily and have the ability to actively efflux various endogenous and exogenous substrates, including xenobiotics, across cell membranes against their concentration gradients (Szakács et al. 2008). Numerous studies indicated that similar ABC transporters represent integral parts of the cellular detoxification machinery for aquatic organisms living in contaminated environments, mediating the so-called multixenobiotic resistance (MXR) mechanism (Kurelec 1992; Bard 2000). Key ABC efflux transporters involved in the MXR mechanism are: P-glycoprotein (MDR1/ABCB1/P-gp); multidrug resistance-associated proteins 1–3 (MRP1–3/ABCC1–3); and the breast cancer resistance protein (BCRP/ABCG2) (Szakács et al. 2008). The MXR mechanism has also an important role in resistance of very sensitive early stage embryos (Hamdoun and Epel 2007).
Sea urchins represent an ecologically relevant animal group and a valuable model frequently used for assessing toxicity of contaminants (Cesar et al. 2004; Bošnjak et al. 2009). A fully sequenced genome of Strongylocentrotus purpuratus is available, and characterization of chemical defense genes and related activities were reported for this species (Goldstone et al. 2006; Sea Urchin Genome Sequencing Consortium 2006). Among defense genes, there are 48 annotated ABC transporter, including orthologues, involved in the MXR mechanism – P-gp (ABCB1), MRP (ABCC), BCRP-like (ABCG) efflux transporters, and also the sulfonylurea receptor (ABCC9/SUR) from the ABCC subfamily (Goldstone et al. 2006). In humans, SUR receptor is responsible for mediating closure of the ATP-sensitive potassium channel, and thereby stimulates insulin release in the β-cell plasma membrane (Panten et al. 1996). But in S. purpuratus genome, the SUR receptor is reported to be also involved in the MXR mechanism (Goldstone et al. 2006). The main goal of this study was the identification of various ABC transporters in two Mediterranean sea urchin species: black, Arbacia lixula and rocky, Paracentrotus lividus. Targeted tissue for detection of ABC transporter mRNA transcripts was gonad tissue for locating ABC transporter proteins that are present in egg cells, which would contribute to the MXR mechanism mediated protection of future embryos. The main aim of the study was identification of all possible ABC transporters involved in MXR activity.
Materials and Methods
Adult sea urchins – A. lixula and P. lividus, were collected in a coastal region (1–5 m depth) at two locations: Crikvenica harbor (Crikvenica, Croatia, 45°10′11″N 14°41′44″E) located at the northern part and Marijan Peninsula (Split, Croatia, 43°30′13″N 16°24′30″E) located at the southern part of the Adriatic coast. Both chosen sampling locations are in areas of excellent sea water quality. Adult animals were transported in tanks to laboratory where they were either dissected immediately for collection of gonad tissue samples, or kept for 1–3 days in flow-through thanks (18 ± 2°C) until dissection. Only mature female gonad tissue samples were collected. Samples were obtained in the spring (April) when both species are at the peak of the reproduction cycle.
Primer pairs (Table 1) were designed based on the highly conserved regions of Homo sapiens and S. purpuratus Abcb1, Abcc1, Abcc3, Abcc5, Abcc9 and Abcg2 gene sequences. All primers were obtained from Sigma to Aldrich (St. Louis, MO, USA).
Total RNA was isolated from ~30 mg of gonad tissue pooled from 5 to 8 A. lixula and P. lividus females, respectively. RNA was immediately placed into RNA later Stabilization Reagent (Applied Biosystems, Foster City, CA, USA) and kept at 4°C until RNA extraction was performed with the RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. cDNA was synthesized from 4 μg of RNA using H Minus M-MulV reverse transcriptase (Fermentas, Burlington, Canada).
ABC transporter orthologs were amplified in the PCR reactions using either a Biometra thermal personal cycler (Göttingen, Germany) or Mastercycler Personal (Hamburg, Germany). PCR was performed for 35 cycles at 94°C (30 s), 55°C (30 s) and 72°C (2 min) or in a touchdown mode (from 60 to 45°C). Aliquots of each reaction were resolved by electrophoresis on 1.2 % agarose gel in tris–acetate-EDTA (TAE) buffer. The gels were stained with ethidium bromide, and PCR products visualized under UV light. The expected products were gel purified (MiniElute PCR Purification Kit (Qiagen, Germany), cloned into the pGEM-T vector (Promega, Madison, WI, USA) and sequenced using an ABI PRISM® 3100-Avant Genetic Analyzer (Ruđer Bošković Institute DNA Service, Zagreb, Croatia) using standard cycle sequencing protocols.
Obtained partial cDNA sequences were analyzed using the National Center for Biotechnology Information (NCBI, Bethesda, MD, USA) basic alignment search tool (tblastx and protein blast). Multiple sequence alignments and determinations of identity rates between amino acid sequences of ABC transporters from different species were performed using BioEdit software and Clustal X version 2.0 implemented in the MEGA 5 software, with default parameters. MEGA 5 software was also used to perform phylogenetic analysis (Neighbor-Joining (NJ) analysis). Reliabilities of phylogenetic relationships were evaluated using a non-parametric bootstrap analysis with 1,000 replicates for NJ analysis. Bootstrap values exceeding 70 were considered well supported.
Results and Discussion
Both A. lixula and P. lividus are frequently used as model organisms in ecotoxicological studies (Cesar et al. 2004; Arslan and Parlak 2007). Their rapid development and transparent embryo and larval stages enable monitoring of any morphological abnormalities and/or the developmental arrest caused by exposure to toxic compounds. A low specificity for substrates, a typical feature of ABC efflux transporters as key components of the MXR mechanism, certainly represents an enormous advantage for aquatic organisms (Epel et al. 2008). However, in highly complex mixtures of pollutants, certain compounds may act as inhibitors of ABC transporters and cause the chemosensitization and/or oversaturation of ABC transporter efflux (Smital et al. 2004; Epel et al. 2008). In an attempt to develop ecologically relevant and technically feasible high throughput screening methods for identification of potential environmental MXR inhibitors (chemosensitizers), sea urchin embryos represent a promising model system. An embryotoxicity test utilizing the cell cycle arrest as an end-point caused by the enhanced accumulation of inorganic compounds (e.g. mercuric chloride, tributyl tin) due to the inhibition of the ABCC/MRP-like transport has already been established for both A. lixula and P. lividus embryos (Bošnjak et al. 2011).
ABC transporters are present but not active in sea urchin eggs, and their activation during fertilization is needed for protection during embryonic development (Hamdoun et al. 2004; Shipp and Hamdoun 2012). Therefore, by sampling only mature female gonad tissue, we focused on identification of ABC transporters present in egg cells. Specific pairs of primers (Table 1) generated fragments of desired ABC transporter amplicons. Cloning, sequencing and finally identification resulted with 8 partial ABC transporter sequences. Respective translated amino acid sequences of these partial PCR products showed a high degree of identity with P-gp, MRP3, MRP5, SUR-like and ABCG2/BCRP-like proteins, respectively, from various other organisms, and this homology was confirmed with NCBI Blast2 protein database query (data not shown). All of the obtained sequence data were registered at the GenBank (Table 2).
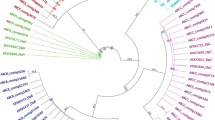
Relationships of eight identified A. lixula and P. lividus partial ABC transporter sequences with ABC transporter orthologs from human, mouse, chicken, fish, frog, fruit fly, mussel and purple sea urchin species are shown in the presented phylogenetic trees (Fig. 1a–c). GenBank accession numbers of sequences of all compared orthologs are shown in Table S1 I–III and all of the obtained identities are shown in Fig. S1a–c. Identity of the PlABCB1 sequence is 77 % congruent with the egg permeability glycoprotein from S. purpuratus (Fig. S1a) – the main protein from the ABCB subfamily involved in efflux of a broad range of hydrophobic xenobiotics (Hamdoun et al. 2004). Identity of the AlABCB1 sequence is 41 % congruent with the ABCB4 from S. purpuratus and 21 %–27 % with ABCB1, ABCB4 and ABCB11 proteins from various orthologs, respectively (Fig. S1a). The ABCB4 member is also similar to mammalian P-gp/ABCB1 member (Szakács et al. 2008). The phylogenetic tree shows clustering of partial AlABCB-like and PlABCB1 with respective ABCB1 and ABCB4 orthologs from S. purpuratus and with ABCB1 orthologs from mussels (Fig. 1a). The identified AlABCC3 sequence is 85 % identical with partial PlABCC3 sequence, and 54 % congruent with the ABCC3A protein from S. purpuratus (Fig. S1b). Identities of PlABCC3 sequence are 57 % congruent with ABCC3A protein from S. purpuratus. Overall, both of these sequences were more identical with MRP proteins than with SUR proteins from various orthologs (Fig. S1b). In mammalian species, MRP3 is involved in the efflux of organic and inorganic anions that are direct products of phase I and II metabolism and present in the form of glutathione, glucuronic or sulphate water soluble conjugates (Cole and Deeley 2006). Therefore, this transporter most likely has an important role in the MXR mechanism in sea urchins.
Phylogenetic tree based on multiple alignments (Clustal X) of different ABC transporter subtype sequences from various vertebrates and invertebrates. a ABCB, b ABCC, c ABCG. Tree was inferred by the NJ method implemented in MEGA software. Bootstrap support values for the NJ tree are shown at the nodes (out of 1,000 replicates). The accession numbers retrieved in this study for ABCB and ABCC transporters are listed in Table S1 I–III
Identities of the AlABCC9 sequence are 60 % congruent with the partial PlABCC9 sequence and 32 % with the ABCC9 protein from S. purpuratus. Identities of the PlABCC9 sequence are 47 % congruent with the ABCC9 protein from S. purpuratus. Overall, both of these sequences showed more identity with SUR proteins than with MRP proteins from various orthologs (Fig. S1b). In S. purpuratus, a high expression of ABCC9 mRNA was determined during the first 58 h of development (Shipp and Hamdoun 2012). It is more likely that this transporter acts as a part of the MRP-like rather than SUR-like efflux activity in sea urchins (Goldstone et al. 2006). The partial PlABCC5 sequence was the shortest of all, and was 16 % congruent with the ABCC5 protein from H. sapiens and 9 %–17 % with SUR and MRP proteins from various orthologs (Fig. S1b). The phylogenetic tree shows clustering of partial AlABCC3, PlABCC3 and PlABCC5 sequences within the MRP protein group, and partial AlABCC9 and PlABCC9 sequences with the SUR protein group (Fig. 1b). The last identified ABC transporter was an ABCG/BCRP-like ortholog obtained from A. lixula gonad tissue. Identities of the AlABCG-like sequence are 56 % congruent with ABCG11 from S. purpuratus and 28 %–34 % with other protein members of the ABCG subfamily (Fig. S1c). The phylogenetic tree shows clustering of the partial AlABCG-like protein with ABCG11 ortholog from S. purpuratus (Fig. 1c). The identified ortholog has higher similarity (56 %) with the ABCG11 from S. purpuratus than with the ABCG2 (29 %) member.
Although the same primer pairs were used in PCR reactions for detection of ABC transporters in both sea urchin species, the ABCG-like partial sequence was detected only in A. lixula, and the ABCC5-like partial sequence was detected only in P. lividus. Some ABC efflux transporters could be identified only in one species, possibly because of some differences in expression of ABC transporters in otherwise closely related sea urchin species. The ABCC1 sequence was not detected in gonad tissue of either sea urchin species which could also indicate that this transporter is not expressed. Further research is needed in order to clarify these results.
The presented identification of ABCB1, ABCC3, ABCC9 and ABCG-like partial sequences in A. lixula and ABCB1, ABCC3, ABCC5 and ABCC9 partial sequences in P. lividus represents the first and necessary step in characterization of ABC transporters in these two urchin species. There is no doubt, however, that a full understanding of the role and ecological relevance of ABC efflux transporters in these species can be obtained only by identification and subsequent in-depth molecular characterization of individual proteins. Therefore, we are directing our research efforts toward completion of the characterization of all proteins potentially involved in the MXR defense in Mediterranean sea urchins.
References
Arslan O, Parlak H (2007) Embryotoxic effects of nonylphenol and octylphenol in sea urchin Arbacia lixula. Ecotoxicology 16:439–444
Bard SM (2000) Multixenobiotic resistance as a cellular defense mechanism in aquatic organisms. Aquat Toxicol 48:357–389
Bošnjak I, Uhlinger KR, Heim W, Smital T, Franekić Čolić J, Coale K, Epel D, Hamdoun A (2009) Multidrug efflux transporters limit accumulation of inorganic, but not organic, mercury in sea urchin embryos. Environ Sci Technol 43:8374–8380
Bošnjak I, Šegvić T, Smital T, Franekić J, Mladineo I (2011) Sea urchin embryotoxicity test for environmental contaminants – potential role of the MRP proteins. Water Air Soil Pollut 217:627–636
Cesar A, Marín A, Marín-Guirao L, Vita R (2004) Amphipod and sea urchin tests to assess the toxicity of Mediterranean sediments: the case of Portmán Bay. Sci Mar 68:205–213
Cole SP, Deeley RG (2006) Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol Sci 27:438–446
Epel D, Luckenbach T, Stevenson CN, MacManus-Spencer LA, Hamdoun A, Smital T (2008) Efflux transporters: newly appreciated roles in protection against pollutants. Environ Sci Technol 42:3914–3920
Goldstone JV, Hamdoun A, Cole BJ, Howard-Ashby M, Nebert DW, Scally M, Dean M, Epel D, Hahn ME, Stegeman JJ (2006) The chemical defensome: environmental sensing and response genes in the Strongylocentrotus purpuratus genome. Dev Biol 300:366–384
Hamdoun A, Epel D (2007) Embryo stability and vulnerability in an always changing world. Proc Natl Acad Sci USA 104:1745–1750
Hamdoun AM, Cherr GN, Roepke TA, Epel D (2004) Activation of multidrug efflux transporter activity at fertilization in sea urchin embryos (Strongylocentrotus purpuratus). Dev Biol 276:452–462
Kurelec B (1992) The multixenobiotic resistance mechanism in aquatic organisms. Crit Rev Toxicol 22:23–43
Panten U, Schwanstecher M, Schwanstecher C (1996) Sulfonylurea receptors and mechanism of sulfonylurea action. Exp Clin Endocrinol Diabetes 104:1–9
Sea Urchin Genome Sequencing Consortium (2006) The genome of the sea urchin Strongylocentrotus purpuratus. Science 314:941–952
Shipp LE, Hamdoun A (2012) ATP-binding cassette (ABC) transporter expression and localization in sea urchin development. Dev Dyn 241:1111–1124
Smital T, Luckenbach T, Sauerborn R, Hamdoun AM, Vega RL, Epel D (2004) Emerging contaminants – pesticides, PPCPs, microbial degradation products and natural substances as inhibitors of multixenobiotic defense in aquatic organisms. Mutat Res 552:101–117
Szakács G, Váradi A, Özvegy-Laczka C, Sarkadi B (2008) The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME–Tox). Drug Discov Today 13:379–393
Acknowledgments
This work has been supported by the Ministry of Science, Education and Sports of the Republic of Croatia, Project Nos. 058-0582261-2246 and 098-0982934-2745.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bošnjak, I., Zaja, R., Klobučar, R.S. et al. Identification of ABC Transporter Genes in Gonad Tissue of Two Mediterranean Sea Urchin Species: Black, Arbacia lixula L., and Rocky, Paracentrotus lividus L.. Bull Environ Contam Toxicol 91, 415–419 (2013). https://doi.org/10.1007/s00128-013-1021-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-013-1021-8




