Abstract
In this study, the ranges of pollutants found in the soft tissues of Perna viridis collected from Kg. Masai and Kg. Sg. Melayu, both located in the Straits of Johore, were 0.85–1.58 μg/g dry weight (dw) for Cd, 5.52–12.2 μg/g dw for Cu, 5.66–8.93 μg/g dw for Ni and 63.4–72.3 μg/g dw for Zn, and 36.4–244 ng/g dry weight for ∑PAHs. Significantly (p < 0.05) higher concentrations of Cd, Cu, Ni, Zn and ∑PAHs in the mussels were found in the water of a seaport site at Kg. Masai than a non-seaport site at Kg. Sg. Melayu population. The ratios of low molecular weight/high molecular weight hydrocarbons (2.94–3.42) and fluoranthene/pyrene (0.43–0.45) in mussels from both sites indicated the origin of the PAHs to be mainly petrogenic. This study has demonstrated the utility of using the soft tissues of P. viridis as a biomonitor of PAH contamination and bioavailability in the coastal waters of Peninsular Malaysia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The Straits of Malacca, including the Straits of Johore, receive a broad range of anthropogenic micropollutants, both inorganic and organic, from various local land- and marine-based sources (Law 1994; Amin et al. 2009). Organic micropollutants have also been transported from other regions via the atmosphere and global ocean transport systems (Iwata et al. 1993). Therefore, environmental monitoring studies are important in order to know the pollutant levels in the aquatic ecosystem.
Polycyclic aromatic hydrocarbons (PAHs) are widespread contaminants of the marine environment. PAHs have carcinogenic and mutagenic properties (Lehr and Jerina 1977), and are generated by three main processes, namely: (1) combustion of organic matter at very high temperatures; (2) release of petroleum; or (3) digenetic processes (degradation of organic matter) (Neff 1979). The mixture of PAHs that is generated by each process can serve as a fingerprint. Therefore, by studying the composition of the PAH mixture, it is possible to determine the process that generated the PAHs. PAHs in general are characterized by the occurrence of organic compounds having a wide range of molecular weights, while petroleum hydrocarbons are dominated by PAHs with lower molecular weights (Neff 1979).
Marine mussels have been proposed as being useful for biomonitoring trace levels of toxic contaminants in coastal waters due to their wide distribution, sessile lifestyle, ease of sampling, tolerance to a considerable range of salinities, resistance to stress and high accumulation of a wide range of chemicals (Goldberg et al. 1978). In particular, the green-lipped mussel, Perna viridis has been utilized as a biomonitor throughout the Indo-Pacific region under the Asia-Pacific Mussel Watch Program (Tanabe et al. 2000).
Several studies on heavy metals (Yap et al. 2003a; 2004) and PAHs (Zakaria et al. 2000; Tsutsumi et al. 2002; Isobe et al. 2007) have been conducted on P. viridis in Malaysia. However, a comparison of inorganic and organic pollutants between seaport and non-seaport coastal waters has not yet been reported in the literature. Therefore, the objectives of this study were to analyze and compare the concentrations of heavy metals and PAHs in seaport and non-seaport waters in the Straits of Johore.
Materials and Methods
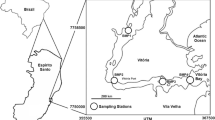
Mussels with shell lengths of about 110 mm were collected from Kg. Masai (seaport site) and Kg. Sg. Melayu (non-seaport site) (Fig. 1, Table 1) on 11 August 2004. The samples were kept in clean plastic bags and put into an ice compartment at <10°C until transported to Universiti Putra Malaysia (UPM). After measuring the shell of each mussel, the soft tissues were dissected by removing the byssus and the shell.
For metal analysis, 20 individuals from each population were selected and analyzed individually. After reaching constant dry weight in an oven (105°C), the dried tissues were digested in 10 mL concentrated HNO3 (Analar grade, BDH 69 % purity). They were initially placed in a hot-block digester first at a low temperature for 1 h (h) and then were fully digested at a high temperature (140°C) for at least 3 h (Yap et al. 2003b). The digested samples were diluted to 40 mL with double-distilled water (DDW). After filtration, the heavy metal levels were determined by using an air-acetylene flame atomic absorption spectrophotometer (Perkin-Elmer Model Analyst 800, Bridgeport Avenue Shelton, USA). The data are presented in μg/g dry weight.
For PAH analysis, the soft tissues of 25 individuals from each population were homogenized and stored at −10°C until analysis. The procedures for homogenization, extraction and column chromatography followed those described by Takada et al. (1994) and Tsutsumi et al. (2002). The identification and quantification of 18 PAH compounds were achieved using the ChemStation software (Texas, USA) based on matching their retention times with those of a mixture of PAH standards. The 18 individual PAH compounds identified in this study were: dibenzothiophene (Dibenz), phenanthrene (Phen), anthracene (Anth), 3-methylphenanthrene (3-MP), 2-methylphenanthrene (2-MP), 2-methylanthracene (2-MA), 9-methylphenanthrene (9-MP) 1-methylphenanthrene (1-MP), fluoranthene (Fluo), pyrene (Pyr), 1-methylpyrene (1-MePy) chrysene (Chy), benzo[a]anthracene B(a)A, benzo[k]fluoranthene B(k)F, benzo[e]acephenanthrylene B(e)A, benzo[e]pyrene B(e)P, benzo[a]pyrene B(a)P and dibenzo[a,h]anthracene D(a,h)A.
An aliquot of 1 g of each of the homogenized soft tissue categories was weighed on a pre-weighed aluminum pan and dried for at least 24 h at 65°C to a constant dry weight. The percentage of dry weight was determined by calculating the ratio of tissue weights after and before placement in the oven.
For lipid determination, the method described by Bligh and Dyer (1959) was used whereby the lipid content was gravimetrically determined. A total of 25 individuals of mussels from each population was used to determine the lipid content. In short, 5 g of sample were mixed with 15 mL of chloroform:methanol (1:2, v/v). The mixture was homogenized for 2 min in a Sorvall Omnimixer homogenizer, centrifuged (10 min, 3000 rpm) and filtered. The residue was rehomogenized with 5 mL of chloroform, centrifuged (10 min, 3000 rpm), filtered and collected together with the previous filtrate. This filtrate was mixed with 5 mL of distilled water and shaken vigorously. The final biphasic system was allowed to separate by centrifugation (10 min, 3000 rpm). The upper aqueous phase was eliminated. The lower chloroformic phase was filtered through anhydrous sodium sulphate and collected. Lipid content was then determined gravimetrically after chloroform was evaporated using a rotary evaporator under vacuum followed by further drying under nitrogen.
For PAHs, quality control samples made previously from the standard solution of five deuterated PAH compounds were used to avoid possible contamination. Surrogate standards were used to examine the recovery of each sample and for quantifying the analytes. The range of the surrogate standard recovery was between 40 % and 120 %. P-Terphenyl-d14 acted as the Internal Injection Standard (IIS) for quantifying and recognizing errors of injection. In addition, a procedural blank was performed for every batch of samples as a contamination control.
For the quality control of heavy metal analysis, all glassware and equipment used were acid-washed. Procedural blanks and quality control samples made from standard solutions of Cd, Cu, Ni, and Zn were analyzed after every 5–10 samples in order to check for sample accuracy. The percentages of recoveries for the heavy metal analyses were acceptable at 80 %–110 %. In addition, the quality of the analytical method was checked with the Certified Reference Material for Dogfish (DOLT-3, National Research Council Canada) and the metal recoveries were satisfactory (80 %–100 %). The detection limits for Cd, Cu, Ni, and Zn were 0.009, 0.01, 0.01, and 0.007 mg/L, respectively.
The data obtained were analyzed statistically by using the Statistical Package for the Social Sciences (SPSS for Windows, Release 10.0.5, Standard Version, Copyright© SPSS Inc., 1989–1999). The independent sample t test was applied to determine the significance of the difference at p < 0.05 between the two sampling sites.
Results and Discussion
The concentrations of heavy metals (Cd, Cu, Ni and Zn) in P. viridis from Kg. Masai were higher than those from Kg. Sg. Melayu (Table 2). Heavy metal concentrations in the total soft tissues of P. viridis from this study were compared with data reported in the literature. The present findings are within the range of or lower than those reported for polluted mussels from Kennedy Bay of Hong Kong (Nicholson and Szefer 2003), Thailand (Sukasem and Tabucanon 1993) and south east coastal waters of India (Senthilnathan et al. 1998), and comparable to the concentrations of Cu, Cd and Zn reported for P. viridis collected from throughout Peninsular Malaysia (Yap et al. 2003a).
The lipid content was significantly lower (0.22 %) in Kg. Masai than Kg. Sg. Melayu (Table 2). The large difference of lipid contents between Kg. Masai and Kg. Sg. Melayu could be attributable to multiple factors such as lifecycle, sex, variation of plankton in different seasons and temperature, which could influence physiological activities and metabolism (Li et al. 2007). The difference could be due to higher values of temperature, water salinity, conductivity and pH at Kg. Masai when compared to Kg. Sg. Melayu (Table 1). However, only further studies can confirm the direct relationship between lower lipid contents and differences of the above water parameters between the two sites.
Mussels from the Kg. Masai site had significantly (p < 0.05) higher concentrations of total PAHs (summation of 18 PAHs) than the mussels from the Kg. Sg. Melayu site (Table 2). It is unlikely that the large difference in lipid content be the main reason for the observed differences in PAHs levels between the two sites because a significant positive relationship (0.79, p < 0.01) between PAHs and lipid contents was reported for P. viridis (Yap et al. 2010). Since a low lipid content (0.22 %) was found in Kg. Masai with elevated PAHs concentrations, the PAHs was most possibly related the closer proximity of the Kg. Masai mussels to local sources of pollution. For a better characterization of the PAH mixture and possible sources, several PAH ratios were calculated, including LMW/HMW, MP/P, Fluo/Py and Phen/Anth, as presented in Table 2. Since the ratios of LMW/HMW are higher than 1.0, the PAHs identified in the populations of Kg. Sg. Melayu and Kg. Masai were dominated by the presence of LMW PAHs, suggesting petrogenic and petroleum related compounds as the main PAH sources (Kennish 1977). These results are consistent with those of Tsutsumi et al. (2002) who reported that the sources of PAHs in the sediments in Malaysia were mainly petrogenic. Therefore, although the Kg. Masai population had significantly (p < 0.05) higher ∑ PAH levels than the Kg. Sg. Melayu population, in both populations the PAHs originated mainly from petrogenic sources.
The presence of LMW PAHs in both mussel populations could be due to the higher bioavailability of LMW PAHs in the coastal waters of the two sampling sites. This could be due to the LMW PAHs in the coastal waters were being more susceptible to microbial degradation and volatilization and dissolution into the water column (Zakaria et al. 2000), thus, becoming more bioavailable to mussels. Consequently, mussels were more enriched with LMW PAHs in relation to their environments. The present finding agreed with those reported by Isobe et al. (2007) in which they reported >1.0 ratios of LMW/HMW in 33 out of 34 mussel populations from seven Asian countries. Skarpheðinsdottir et al. (2007) also reported that blue mussels collected close to harbours had relatively higher levels of LMW PAHs. Baumard et al. (1999) found that the blue mussels sampled in the western Baltic Sea were enriched in LMW PAHs. Thus, the presence of higher LMW PAHs in marine mussels is due to the marine mussels are known to be selectively uptake LMW PAHs (Farrington et al. 1983; O’Connor 1991).
Mussels are known to be filter-feeding organisms which they can filter large volumes of water (Yap et al. 2003c) and absorb xenobiotics by two pathways: direct absorption of compounds present in the water phase through the gills, and; indirect absorption of compounds adsorbed onto small-grained particles passing through the digestive system (Baumard et al. 1998). Mussels are exposed to both dissolved and particulate forms of hydrocarbons present in the water column. The partitioning of PAHs into either dissolved or particulate forms is due to their specific water solubilities. Hence, the mussels can directly absorb lower weight PAHs from the water that they filter. Heavier weight hydrocarbons (four or more rings) are mainly obtained by ingestion of particulate matter into the digestive system (Broman et al. 1990, 1999; Widdows and Donkin 1992; Piccardo et al. 2001).
The concentrations of the sum of four methyl-phenanthrene compounds to phenanthrene (∑ MP/P) was greater than 2 for petroleum while it was lower than 1 for pyrolytic sources of PAHs in individual mussels (Garrigues et al. 1987). Based on the guideline for MP/P, the MP/P ratio of 1.74 for the Kg. Masai population fell between petrogenic and pyrolytic origins. The Kg. Sg. Melayu population had a MP/P ratio of 2.8, suggesting petrogenic origins. Indeed, the ratio of fluoranthene to pyrene (Fluo/Py) <1 for both mussel populations, indicated petrogenic sources too (Table 2). However, individuals which exhibited phenanthrene/anthracene (Phen/Ant) ratios <10 for both mussel populations indicated a pyrogenic origin. It should be noted that pyrolytic PAHs are mainly anthropogenic, which could be due to industrial activities. Since there was no petroleum industry observed here, with the only industrial activities being mussel processing, PAH transport via atmospheric aerosols and particles could be considered as one of the pyrogenic inputs.
The PAH levels in the total soft tissues of P. viridis from this study are comparable with those of earlier studies on polluted and clean sites using other marine mussel species. For example, Broman et al. (1990) reported the mean level of a total of 19 PAHs in the tissues of Mytilus edulis sampled from the Swedish coast of the Baltic Sea to be 443 ng/g dry wt. (40–1330 ng/g dry wt.). Baumard et al (1998) analyzed blue mussels sampled near the German coast in the southwest Baltic Sea near point sources of pollution. The sum of 18 PAHs ranged between 90 and 3,900 ng/g dry wt., a much wider range than found in our study (36.4–244 ng/g dry wt.). This indicates that the PAH contamination in the Straits of Johore is not as great as in those sites from the Baltic Sea. Skarpheðinsdottir et al. (2007) reported total 32 PAH levels as 10–11,670 ng/g dry wt. in Mytilus spp. from Nordic coastal waters. Baumard et al. (1999) studied levels of 14 PAHs in M. galloprovincialis from 10 remote and polluted sites of the Mediterranian coasts of Spain and France, and found them to range from 25 to 337 ng/g dry wt. Hence, in general, our PAH levels (20.4–169 ng/g) seem to correspond to the less polluted areas of Europe. However, it should be noted that a comparison of the concentrations of heavy metals and PAHs in P. viridis between studies should be done with caution. Many factors could affect the tissue pollution levels, such as intertidal impacts, seasonal variations, spawning conditions and lipid levels (Gosling 1992).
The higher concentrations of heavy metals and PAHs that were found in Kg. Masai than in Kg. Sg. Melayu could be related to its vicinity to a port, Pasir Gudang, and hence, industrial effluents (Yap et al. 2006). Besides, the high levels of pollutants found in the mussels from Kg. Masai could also be due to domestic wastes through riverine inputs such as from the Tebrau, Skudai and Segget rivers, which all empty into the narrow Straits of Johore (Yap et al. 2006). These inputs would carry heavy metals and PAHs, which could put the local marine ecosystem at risk.
Based on ratios of LMW/HMW and Fluo/Pyr, it was found that the PAH in the Kg. Masai and Kg. Sg. Melayu mussel populations mainly originated from a petrogenic source. However, there were significantly (p < 0.05) higher concentrations of heavy metals and ∑PAHs in the total soft tissues of P. viridis collected from the coastal water of Kg. Masai than from Kg. Sg. Melayu. In general, the results also indicated that the soft tissues of P. viridis were useful for biomonitoring heavy metal and PAH contamination and bioavailability in the coastal waters of Peninsular Malaysia.
References
Amin B, Ismail A, Arshad A, Yap CK, Kamarudin MS (2009) Anthropogenic impacts on heavy metal concentrations in the coastal sediments of Dumai, Indonesia. Environ Monitor Assess 148(1–4):291–305
Baumard P, Budzinski H, Garrigues P (1998) Polycyclic aromatic hydrocarbons (PAHs) in sediments and mussels of the western Mediterranean Sea. Environ Toxicol Chem 17:765–776
Baumard P, Budzinski H, Garrigues P, Dizer H, Hansen PD (1999) Polycyclic aromatic hydrocarbons in recent sediments and mussels (Mytilus edulis) from the Western Batlic Sea: occurrence, bioavailability, and seasonal variations. Mar Environ Res 47:17–47
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Broman C, Näf C, Lundberg I, Zebuhr Y (1990) An in situ study on the distribution, biotransformation and flux of polycyclic aromatic hydrocarbons in an aquatic food chain (seston-Mytilus edulis L- Somateria mollissima) from the Batlic: an ecotoxicological perspective. Environ Toxicol Chem 9:429–442
Farrington JW, Goldberg ED, Risebrough RW, Martin JH, Bowen VT (1983) U.S. “Mussel Watch” 1976–1978: an overview of the trace-metal, DDE, PCB, hydrocarbons, and artificial radionuclide data. Environ Sci Tech 17:490–496
Garrigues P, Soclo H, Marniesse MP (1987) Origin of pyrolytic aromatic hydrocarbons (PAHs) in recent sediments from the continental shelf of the “Golfe de Gascogne” (Atlantic Ocean) and in the Gironde Estuary. Environ Anal Chem 28:121–131
Goldberg ED, Bowen VT, Farrington JW, Harvey G, Martin JH, Parker PL, Risebrough RW, Robertson W, Schneider W, Camble E (1978) The mussel watch. Environ Conserv 5:101–125
Gosling E (1992) The mussel Mytilus: ecology physiology, genetics and culture. Elsevier, Amsterdam
Isobe T, Takada H, Kanai M, Tsutsumi S, Isobe KO, Boonyatumanond R, Zakaria MP (2007) Distribution of polycyclic aromatic hydrocarbons (PAHs) and phenolic endocrine disrupting chemicals in South and Southeast Asian mussels. Environ Monitor Assess 135:423–440
Iwata H, Tanabe S, Sakai N, Tatsukawa R (1993) Distribution of persistent organochlorines in the oceanic air and surface sea water and the role of ocean on their global transport and fate. Environ Sci Tech 27:1080–1098
Kennish MJ (1977) Practical handbook of estuarine and marine pollution. CRC Press Boca Raton, Florida
Law AT (1994) Oil pollution in the Malaysia seas. Dewan Persidangan, Pusat Pengembangan dan Pendidikan Lanjutan, Universiti Pertanian Malaysia
Lehr RE, Jerina DM (1977) Metabolic activation of polycyclic hydrocarbons. Arch Toxicol 39:1–6
Li D, Zhang Y, Sinclair A (2007) Seasonal variations of lipid content and composition in Perna viridis. Lipids 42(8):1–9
Neff JM (1979) Polycyclic aromatic hydrocarbons in the aquatic environment: source, fates and biological effects. Appl Sci Publisher LTD, London
Nicholson S, Szefer P (2003) Accumulation of metals in the soft tissues, byssus and shell of the mytilid mussel Perna viridis (Bivalvia: Mytilidea) from polluted and uncontaminated locations in Hong Kong coastal waters. Mar Pollut Bull 46:1035–1048
O’Connor TP (1991) Concentration of organic contaminants in mollusks and sediments at NOAA national status and trend sites in the coastal and estuarine United States. Environ Health Perspect 90:69–73
Piccardo MT, Coradeghini R, Valerio F (2001) Polycyclic aromatic hydrocarbon pollution in native and caged mussels. Mar Pollut Bull 42(10):951–956
Senthilnathan S, Balasubramaniam T, Venugopalan VK (1998) Metal concentrations in mussel Perna viridis (Bivalvia/Anisomyaria) from some parts in the southeast coast of India. Indian J Mar Sci 27:206–210
Skarpheðinsdottir H, Ericson G, Svavarsson J, Næs K (2007) DNA adducts and polycyclic aromatic hydrocarbon (PAH) tissue levels in blue mussels (Mytilus spp.) from Nordic coastal sites. Mar Environ Res 64:479–491
Sukasem P, Tabucanon MS (1993) Monitoring heavy metals in the Gulf of Thailand using mussel watch approach. Sci Total Environ 139(140):297–305
Takada H, Farrington JW, Bothner MH, Johnson CH, Tripp BW (1994) Transport of sludge-derived organic pollutants to deep sea sediment at deep water Dump Site 106. Environ Sci Technol 28:162–1072
Tanabe S, Prudente MS, Kan-atireklap S, Subramanian A (2000) Mussel watch: marine pollution monitoring of butyltins and organochlorines in coastal waters of Thailand, Philippines and India. Ocean Coast Manage 43:819–839
Tsutsumi S, Yamaguchi Y, Nishida I, Akiyama K-I, Zakaria MP, Takada H (2002) Alkylbenzenes in mussels from South and South East Asian coasts as a molecular tool to assess sewage impact. Mar Pollut Bull 45:325–331
Widdows J, Donkin P (1992) Mussels and environmental contaminants: bioaccumulation and physiological aspects. In: Gosling EM (ed) The mussel Mytilus: ecology, physiology, genetics and culture. Elsevier, Amsterdam, pp 383–417
Yap CK, Ismail A, Tan SG (2003a) Background concentrations of Cd, Cu, Pb and Zn in the green-lipped mussel Perna viridis (Linnaeus) from Peninsular Malaysia. Mar Pollut Bull 46:1043–1048
Yap CK, Ismail A, Tan SG (2003b) Cd and Zn concentrations in the straits of Malacca and intertidal sediments of the west coast of Peninsular Malaysia. Mar Pollut Bull 46:1341–1358
Yap CK, Ismail A, Tan SG, Omar H (2003c) Effects of heavy metals (Cd, Cu, Pb and Zn) on the filtration rate of the green-lipped mussel Perna viridis (Linnaeus). Malays Appl Biol 32(1):7–13
Yap CK, Ismail A, Tan SG, Rahim Ismail A (2004) Assessment of different soft tissues of the green-lipped mussel Perna viridis (Linnaeus) as biomonitoring agents of Pb: field and laboratory studies. Water Air Soil Pollut 153:253–268
Yap CK, Ismail A, Edward FB, Tan SG, Siraj SS (2006) Use of different soft tissues of Perna viridis as biomonitors of bioavailability and contamination by heavy metals (Cd, Cu, Fe, Pb, Ni and Zn) in a semi-enclosed intertidal water, the Johore Straits. Toxicol Environ Chem 88(4):683–695
Yap CK, Mashinshian MA, Ismail A, Pauzi Zakaria M (2010) Relationships and comparative studies of heavy metals and organic PAH compounds in the soft tissues Perna viridis. Res J Chem Environ 14(1):56–61
Zakaria MP, Takada H, Horinouchi A, Tanabe S, Ismail A (2000) Application of biomarkers for source identification of oil pollution in the Straits of Malacca, Malaysia. Environ Sci Technol 34:1189–1196
Acknowledgments
The authors wish to acknowledge the financial support provided through the Research University Grant Scheme (RUGS) [vote no.: 9316800], provided by Universiti Putra Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yap, C.K., Shahbazi, A. & Zakaria, M.P. Concentrations of Heavy Metals (Cu, Cd, Zn and Ni) and PAHs in Perna viridis Collected from Seaport and Non-seaport Waters in the Straits of Johore. Bull Environ Contam Toxicol 89, 1205–1210 (2012). https://doi.org/10.1007/s00128-012-0838-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0838-x