Abstract
Our purpose was to detect, isolate and characterize tensioactive agents with or without emulsifying activity from marine bacterial strains present in seawater and sediment samples from the Venezuelan Atlantic Front. Biosurfactants found in cell-free supernatants from all cultures presented high surface activity as they were able to reduce the water surface tension from 72 dynes cm−1 to values between 41.7 and 33.9 dynes cm−1. However, high indirect CMC values were registered for the most of these compounds (51.4 %–56.1 % v/v). Culture supernatants from S3 and S29 strains showed highest emulsifying activity in the dispersion assay with diesel oil (absorbance 1.06 and 1.18, respectively), while supernatant from the S10 strain induced the most stable oil-in-water emulsions with 16° and 25°API crude oils. Only culture supernatant from S3 strain was able to produce stable oil-in water emulsions with diesel oil and both type of crude oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
It has been estimated that oil-rig platforms may lose some 50,000 tons of crude oil per year worldwide. This has led to an increase of research on microorganisms capable to degrade oil and its subproducts (Lizarraga-Partida et al. 1991). The growth of such microorganisms, particularly hydrocarbonoclastic bacteria (Pseudomonas aeruginosa, P. fluorescens, Bacillus subtilis, B. brevis, Mycobacterium sp., Serratia mascescens, Rhodococcus sp., Nocardia sp.) and fungi (Torulopsis apicola, T. petrophilum, Candida tropicalis, C. lipolytica), are usually associated to their production of amphipathic molecules, which are secreted directly into the surrounding medium (Yateem et al. 2002; Satpute et al. 2010a). These compounds, called biosurfactants, comprise hydrophilic and hydrophobic portions, which allow them to become concentrated in the oil–water interface, decreasing the surface tension (ST) of the aqueous phase (Cameotra and Bollang 2003).
Biosurfactants show advantages over synthetic surfactants, such as low environmental toxicity, easy biodegradation, biocompatibility, large-scale low-cost production and specific actions determined by their functional groups (Torres and Sánchez 2001). Most studied bacterial genera respect to biosurfactant production are Nocardia, Rhodococcus, Mycobacterium, Brevibacterium and Arthrobacter that produce glycolipids when cultivated in n-alkanes, waste lubricant oil or polycyclic aromatic hydrocarbons (Churchill et al. 1999; Zeinali et al. 2007; Satpute et al. 2010b). Also, the genus Pseudomonas that uses glycerol, mannitol, glucose, n-alkanes and vegetable oils, from which produced glycolipidic biosurfactants, mainly rhamnolipids (Abdel-Mawgoud et al. 2010; Thavasi et al. 2011); the genus Bacillus that uses vegetable oils thus like waste lubricating oil and crude oil producing several types of cyclic lipopeptide biosurfactants (Haba et al. 2000; Satpute et al. 2010b), and Corynebacterium that grows in the same renewable substrates that Bacillus spp. but producing glycolipopeptides and phospholipids (Satpute et al. 2010b). In view of the importance of biosurfactants at the industrial and environmental levels, the search for new tensioactive agents and the evaluation of their potential uses become quite relevant. Also, it is known that in South America (North–East), important natural reservoir have been impacted for relevant amounts of oil waste generated from the oil cleaning of ships, submarine oil spills and oil exploitation from the oil industry activities in Venezuela and its neighbor countries (Agarrad et al. 1988; Conde 2001). In this sense, we propose that tensioactive and/or emulsifying compounds can be obtained from isolated bacteria from water and sediments in the Venezuelan Atlantic Coastline.
Materials and Methods
For this study, six strains were selected for being β-hemolytic, since many bacteria having this characteristic produce biosurfactants. The bacterial strains were isolated from the water column and sediments of the Venezuelan Atlantic Coastline and grown in enrichment media containing minimal medium and a mineral oil (Malavé et al. 2005). Initially, strains were individually diluted on marine nutrient agar (MNA) by the streak plate method and the pour plate method to grant pure cultures. MNA was prepared as follows (g L−1): antibiotic agar 8, casitone 10, peptone 10, glucose 5, triptose 10, meat extract 1.2 and agar–agar 20, at pH 7.2 all dissolved in 900 mL of distilled water and 100 mL of artificial seawater containing (NH4)2SO4 (12 g L−1), CaCl2 (1 g L−1), MgSO4 (1 g L−1), ferric citrate (0.02 g L−1), MgCl2 (1 g L−1), KCl (0.55 g L−1) and NaCl (194.5 g L−1). Selected strains identified as S3, S10, S19, S21, S24 and S29, were grown at 30°C for 48 h in a LAB-LINE® incubator. Cultures were later transferred to 250 mL Erlemeyer flasks containing 45 mL of PYG (Peptone-Yeast extract-Glucose) broth and 5 mL of artificial seawater; the PYG broth composition was (g L−1): peptone 5, yeast extract 5 and glucose 15.
Pseudomonas aeruginosa ATCC55925 was used as reference strain for the detection and characterization of tensioactive and/or emulsifying agents, in view of its capability to emulsify oils ranging from light to heavy crude. Biosurfactant production has been followed in relation to the growing phase for this strain. It has been shown that this bacterial strain initiates secretion of the rhamnolipidic biosurfactant just between the end of exponential phase and the beginning of the stationary phase, that is, after 9 h of growth in PYG medium at 30°C, 120 rpm. In addition we have detected that the biosurfactant reaches its critical micellar concentration (CMC) value after 5 h of growth in the stationary phase, that is, after 14 h of cell growth (Rocha and Infante 1997; Rocha et al. 2000, 2011). The cultures obtained from the previous procedure were incubated in a shaker (LAB-LINE® ORBITE ENVIRONM SHAKER) at 150 rpm, 30°C, for 48 h. For the tests, each aliquot was standardized to an absorbance of 0.6 at 620 nm on a Bausch & Lomb spectrophotometer. This was taken as the 1 % v/v starter for the homogeneous growth of all the strains, under the previously described conditions. After the incubation period the cultures were sterilized on a vertical autoclave (FANEM® mod. 415, Brasil). The sterile samples were centrifuged at 10,000g for 20 min at 4°C (SORVALL® RC-5B Refrigerated Superspeed) with a SS34 rotor. Supernatants obtained were filtered through 0.22 μm pore-size nitrocellulose (Millipore) membranes and stored at 4°C.
Surface tension (ST) was determined in each supernatant, by means of Du Nouy ring and a ST analyzer (CSC-DuNOUY®, USA) (Torres and Sánchez 2001). Briefly, 14 mL of sterile supernatant from each strain were placed in a Petri dish of 50 mm diameter and the superficial activity was determined. ST measurements of distilled water and PYG broth were carried out as controls. The relative concentrations of biosurfactant from each strain were determined by the indirect CMC in the respective supernatants, adding 500 μL aliquots to 9 mL of distilled water in Petri dishes and recording the minimum ST values in 10 consecutive readings at room temperature.
The emulsifying properties of the tensioactive compounds were determined by dispersing 100 μL of diesel oil in 7.5 mL Tris-MgSO4 buffer at pH 7.0 in presence of 500 μL of each supernatant. These mixtures of diesel/buffer/supernatant were vigorously shaken for 30 s at room temperature and left to settle for 2 min. Afterwards, the degree of dispersion of the diesel oil was estimated from the absorbance values at 620 nm. Similar mixtures of diesel/buffer and PYG medium were prepared as blanks. For further analyses, 15 mL of each supernatant of the strains were mixed with 15 g of 16° or 25°API crude oil in capped plastic tubes for 60 s. Oil-in water emulsion quality was determined visually. Additionally, emulsions were left undisturbed for 24 h, in order to ascertain the static stability of these emulsions. These mixtures which yielded stable emulsions were used for viscosity analyses. Each emulsion was manually shaken for 60 s and its viscosity was determined for 30 s with a Cannon LV2000 viscometer and compared with that of distilled water. All essays were run in duplicate at room temperature.
Results and Discussion
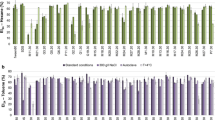
Surface tension measurements of distilled water and PYG broth, as well as supernatants extracted from various strains, are shown in Fig. 1. The ST values exhibited by the samples from strains S3, S10, S19 and S29 were from 7 % to 16 % lower than that of broth alone and only slightly less than that shown by the reference strain. One of the typical features of surfactants is their capability to decrease the ST of aqueous solutions and this constitutes a common descriptor of the effectiveness of a biosurfactant (Margaritis et al. 1979; Bodour et al. 2003). Those with the highest tensioactive capacity can reduce the ST of aqueous phases to 25 ± 5 dynes cm−1 depending on the concentration and type of surfactant (Zhang and Miller 1992). Comparison of the ST value of the samples from the reference strain “P.a.” with the values from other isolates (Fig. 1) suggests that strains S3, S10, S19 and S29 produced such tensioactive compounds. Even though the strains were grown under the same overall conditions, ST values varied to each other. These results suggest that their respective biosurfactants could be of different chemical nature (Boudour et al. 2003) or they were at different concentrations.
The determination of the CMC of a substance is one of the chief tests to evaluate surfactant activity in aqueous phases, as it expresses the minimum amount required to obtain the lowest ST value (Margaritis et al. 1979; Cooper and Zajic 1980). For this test we added consecutive aliquots of 500 μL of the supernatant samples from various strains to distilled water. The results are shown in Fig. 2. The highest capabilities to reduce the ST of distilled water on addition of the first aliquot were detected in the samples from strains S3 and S10, with initial values of 47.1 and 46.8 dynes cm−1, respectively. These strains also gave the lowest final ST values, together with strains S19 and S29 (at 35.2, 34.6, 36.0 and 36.6 dynes cm−1, respectively), and the highest percentages of indirect CMC (Fig. 2; Table 1). These results indicate that high tensioactive activity and the low micellar concentration of the experimental strains were similar to those obtained from the reference strain (ST: 38.5 dynes cm−1; 56.1 % CMC), suggesting that isolated biosurfactants were of good quality (Margaritis et al. 1979; Satpute et al. 2010a, b) and of similar chemical nature.
It has been stated that one of the most outstanding features of microorganisms which grow in presence of hydrocarbons is their ability to secrete tensioactive substances, which emulsify or solubilize the organic phase, rendering it available for cellular absorption and hence, susceptibility to biodegradation (Margaritis et al. 1979; Cooper and Zajic 1980). This characteristic was noted specially in strains S3 and S29 by the quantitative emulsion test, which depicted higher turbidity than the others strains, reaching values of 1.06 and 1.18, respectively (Fig. 3). These values even surpassed that of the reference strain (0.80). On the other hand, strains S21, S10, S19 and S24 only attained turbidity values between 0 and 0.77 absorbance units. Considering that isolated biosurfactants had similar ST and CMC values, but different emulsifying properties, we propose the presence of stabilizing agents associated with tensioactive agents obtained from S3 and S29. Sample S21 depicted surface activity, and showed 0 % turbidity which means that S21 biosurfactant-mediated emulsion was neither produced nor maintained to a measurable level. This probably occur by its chemical nature or because no emulsion stabilizer was present in the sample. This is known to occur for biosurfactant surfactin produced by B. subtilis, which is one of the most potent tensoactive agents with little emulsifying properties in contrast to biosurfactant emulsan, produced by Acinetobacter calcoaceticus, which is a potent emulsifying agent with little surface activity (Haba et al. 2000).
Biosurfactants comprise a wide spectrum of molecules which produce oil in-water and water in-oil emulsions by lowering of interfacial tension along with the action of stabilizing agents (Yateem et al. 2002). This feature was observed in samples from S10 and reference strains, which formed very stable emulsions in both kind of crude oil (Table 2). In addition, during qualitative emulsion test with the same strains we perceived formation of foam, less adsorption of oil to the test-tube walls and a restoration of the emulsion state after 24 h, which required less mechanical agitation than initially, this implying a higher degree of static stability. Contrasting with these findings, tensioactive agent from strain S29 induced unstable emulsions after shaking with both crude oils, as did strains S19 and S24 with the 16°API oil.
Like S10, strains S3, S21 and S24 appeared to produce biosurfactants able to induce very stable emulsions of 25°API oil in aqueous media. Whereas S3 strain registered good emulsifying activity for all types of substrates (diesel oil, 16° and 25°API crude oil), S29 strain emulsified diesel oil but not crude oil (Fig. 3; Table 2). These results indicated that biosurfactants depicted different substrate specificity, probably based on slight differences in terms of chemical nature. Most common biosurfactants reported in the literature have been glycolipid, mainly lipids associated with disaccharides (such as trehalose and sophorose), monosacharides (such as rhamnose) and polysaccharides. In addition, lipopeptids, lipids joint to a single aminoacid (such as ornithine), fosfolipids, corinomycolic acid, polymers (lipid/protein/carbohydrate) and different mixtures of fatty acids and neutral lipids are part of the chemical compounds with tensioactive properties that have been isolated from terrestrial and marine bacteria and fungi (Haba et al. 2000; Satpute et al. 2010b). Considering our data, particularly the ST values and the CMC values obtained from all biosurfactants detected in this study, we speculate that glycolipids and polymers are strong candidates for the tensio-active agents produced by the strains S3, S10, S19, S21, S24 and S29 as other biosurfactants of different chemical nature usually depict either more moderate ST values in relation to water (>45 dynes cm−1) and/or higher CMC values (>20), with few exceptions. As it was shown in this study, all biosurfactants registered a combination of good ST activity and CMC properties. In summary, we propose that biosurfactants probably had chemical structures close enough each other as to depict similar ST and CMC values but different enough as to show diverse emulsifying properties.
Emulsions induced by the biosurfactants from all bacterial strains depicted different viscosity values in relation to the reference strain (Fig. 4). In relation to 25°API crude oil, it was observed that the biosurfactant obtained from S24 strain was able to induce oil-in water emulsions with the lowest viscosity values. Contrary to S24 strain, S3 and S19 produced biosurfactant-mediated oil-in water emulsions with high viscosity values. Oil-in water emulsions with intermediate viscosity values were registered for biosurfactans produced from strains S10 and S21. In contrast to 25°API crude oil-in water emulsions, 16°API crude oil-in water emulsions from strains S3, S10 and S21 had poor viscosity reduction. These results suggest that viscosity depends on the dispersion grade of the oil phase into the continuous phase. This is why P.a.-mediated emulsions, which depicted the best emulsifying activity and emulsion stability also showed a high viscosity reduction for both type of oils, suggesting that in this case a higher proportion of both oils were dispersed into the micelles in relation to the other strains.
To conclude, different types of biosurfactants were obtained from the marine strains of the Venezuelan Atlantic coastline as compared to that isolated from P. aeruginosa ATCC 55925, a biosurfactant-producing strain obtained from soil. Biosurfactant activity was followed by tensio-active properties (ST and CMC values), emulsifying activity and viscosity. According to these parameters, biosurfactants isolated in this study could have been of different chemical nature. It was also shown that biosurfactant-producing strains are common in other environment not necessarily exposed to oil spills and could be used as potential tools in bioremediation.
References
Abdel-Mawgoud AM, Lépine F, Déziel E (2010) Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336
Agarrad J, Boodoosing M, Gobin J (1988) Petroleum residues in superficial sediments from the Gulf of Paria, Trinidad. Mar Poll Bull 219:231–233
Bodour AA, Drees KP, Maier RM (2003) Distribution of biosurfactant-producing bacteria in undisturbed and contaminated arid southwestern soils. Appl Environ Microbiol 69:3280–3287
Cameotra SS, Bollang JM (2003) Biosurfactant-enhanced bioremediation of polycyclic aromatic hydrocarbons. Crit Rev Environ Sci Technol 30:111–126
Churchill SA, Harper JP, Churchill PF (1999) Isolation and characterization of a Mycobacterium species capable of degrading three and four-ring aromatic and aliphatic hydrocarbons. Appl Environ Microbiol 65:549–552
Conde JE (2001) The Orinoco River Delta, Venezuela. In: Seeliger U, Kjerfve B (eds) Ecological studies coastal marine ecosystems of Latin America, vol 144. Springer, Berlin, pp 61–69
Cooper DG, Zajic JE (1980) Surface-active compounds from microorganisms. Adv Appl Microbiol 26:229–253
Haba E, Espuny MJ, Busquets M, Manresa A (2000) Screening and production of rhamnolipids by Pseudomonas aeruginosa 47T2 NCIB 40044 from waste frying oils. J Appl Microbiol 88:379–387
Lizarraga-Partida ML, Izquierdo-Vicuna FB, Wong-Chang I (1991) Marine bacteria on the Campeche Bank oil field. Mar Poll Bull 22:401–405
Malavé R, Barbosa M, Suárez P (2005) Detección de bacterias tolerantes a metales en el agua y los sedimentos del frente Atlántico Venezolano. In: Capaldo M, Yanes C, Martín A, Gómez MG (eds) Frente Atlántico Venezolano. Investigaciones geoambientales: ciencias ambientales. Tomo I, Petróleos de Venezuela, SA (PDVSA)-Fondo Editorial Fundambiente, Caracas, pp 22–30
Margaritis A, Zajic JE, Gerson DF (1979) Production and surface-active properties of microbial surfactants. Biotechnol Bioeng 21:1151–1162
Rocha CA, Infante C (1997) Enhanced oily sludge biodegradation by a tensio-active agent isolated from Pseudomonas aeruginosa USB-CS1. Appl Biotechnol Bioeng 47:615–619
Rocha CA, González D, Iturralde L, Lacoa U, Morales F (2000) Production of oily emulsions mediated by a microbial tensio-active agent (Process). U.S. Pat. 6.060 287.r
Rocha CA, Pedregosa AM, Laborda F (2011) Biosurfactant-mediated biodegradation of straight and methyl-branched alkanes by Pseudomonas aeruginosa ATCC 55925. AMB Express 1:9–18
Satpute SK, Banpurkar AG, Dhakephalkar PK, Banat IM, Chopade BA (2010a) Methods for investigating biosurfactants and bioemulsifiers: a review. Crit Rev Biotechnol 30:127–144
Satpute SK, Banat IM, Dhakephalkar PK, Banpurkar AG, Chopade BA (2010b) Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol Adv 28:436–450
Thavasi R, Subramanyam VRM, Jayalakshmi S, Balasubramanian T, Banat IM (2011) Biosurfactant production by Pseudomonas aeruginosa from renewable resources. Indian J Microbiol 51:30–36
Torres JM, Sánchez JA (2001) Producción de un biosurfactante microbiano por Torulopsis magnoliae en cultivos sumergidos por carga. Ciencia 9:305–312
Yateem A, Balba MT, Al-Shayji Y, Al-Awadhi N (2002) Isolation and characterization of biosurfactant-producing bacteria from oil-contaminated soil. Soil Sediment Contam 11:41–55
Zeinali M, Vossoughi M, Ardestani SK (2007) Characterization of a moderate thermophilic Nocardia species able to grow on polycyclic aromatic hydrocarbons. Lett Appl Microbiol 45:622–628
Zhang Y, Miller RM (1992) Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant). Appl Environ Microbiol 58:3276–3282
Acknowledgments
We gratefully acknowledge the financial support from Projects of INTEVEP-PDVSA and the Decanato de Investigación y Desarrollo of the Universidad Simón Bolívar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bozo-Hurtado, L., Rocha, C.A., Malavé, R. et al. Biosurfactant Production by Marine Bacterial Isolates from the Venezuelan Atlantic Front. Bull Environ Contam Toxicol 89, 1068–1072 (2012). https://doi.org/10.1007/s00128-012-0820-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0820-7