Abstract
A new analytical method for the determination of 14 pesticide residues in fresh peppermint was developed based on the QuEChERS sample preparation technique followed by gas chromatography coupled to electron capture and nitrogen phosphorus detectors (GC/ECD/NPD). The validation study clearly demonstrated suitability of the method for its intended application. The overall recoveries of the pesticides from peppermint, at the three spiking levels of 0.01, 0.1 and 1.0 mg kg−1, were 100 % ± 10 % with relative standard deviations of 6 % ± 5 % on average. The limit of quantification was 0.01 mg kg−1 for all the pesticides. The expanded uncertainties were in the range between 7 % and 30 % (14 % on average), which was distinctively less than a maximum default value of ±50 %. Compared with our previous method, that entailed dichloromethane/acetone extraction and florisil column cleanup with collection of four fractions, the new method was more straightforward, less time and labour intensive as well as more sensitive, selective and accurate, simultaneously.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Peppermint (Mentha piperita L.) a herbaceous plant which is indigenous to Europe but it is widespread cultivated throughout the world. Peppermint is one of the most widely consumed single ingredient herbal teas as well as it is an important row material for the food, cosmetic and farmaceutical industries. Due to a high menthol content, peppermint is often used for flavouring purposes. Apart of menthol, other major constituents include menthone and the phenolic compounds such as rosmarinic acid and several flavonoids. As peppermint is known for the bioactivity and potential health benefits, it is widely used in traditional medicines (McKay and Blumberg 2006). Under cultivation, peppermint is prone to fungal diseases such as mint rust and mint powdery mildew. Unwanted plants (weeds) can interfere with cultivated plants and diminish crop yields. Also, the crop can be damaged by harmful insects pests such as flea beetles, leafhoppers, lygus bugs, mint leaves beetles, tortoise beetles and tortrix moths. Pesticides are applied for preventing crop losses to harmful insects and other pests but each use of pesticides carries some associated risk due to the possibility of leaving residues on harvested crops. Due to the potential consumers’ health concerns, it is necessary to investigate the pesticides in peppermint in order to identify the residues present and quantify their concentrations. Therefore, there is an increasing demand for reliable pesticide analytical methods that are adequately sensitive, selective and accurate to detect and quantify pesticide.
For the purpose of pesticide residue analysis numerous methods have been developed. In the past few years, the miniaturized acetonitrile-base extraction technique know under the acronym name QuEChERS (quick, easy, cheap, effective, rugged and safe) has become one of the main analytical tools in many pesticide residue analysis applications (Lehotay et al. 2010). Although the QuEChERS approach is often considered as the method of choice for sample preparation before analysis by gas and liquid chromatography coupled to mass spectrometry, e.g. GC/MS, GC/MS/MS, LC/MS/MS, GC/TOF MS, LC/TOF MS, there is also an increasing interest in the adaptation of the QuEChERS with gas chromatography combined with element-specific detection such as electron capture (GC/ECD), nitrogen phosphorus (GC/NPD) and pulsed flame photometric (GC/PFPD) as a cost-effective alternative. The QuEChERS approach in conjunction with GC/ECD, GC/NPD or GC/PFPD has already been applied to analysis of pesticide residues in fruits and vegetables (Liu et al. 2011; Srivastava et al. 2011), toasted barley and chickpea flours (González-Curbelo et al. 2012a), American ginseng (Wu et al. 2011), banana leaves (González-Curbelo et al. 2011), traditional Chinese medicine (Xu et al. 2011), choi sum, yardlong beans and aubergines (Chai et al. 2012), cereal samples (González-Curbelo et al. 2012b), cereal-based baby foods (Anagnostopoulos et al. 2010) and low-fatty baby foods (Georgakopoulos et al. 2011). In this work, we report the application of QuEChERS to analysis of pesticide residues in peppermint. The main objective was to study the feasibility of a QuEChERS-based method for the extraction, cleanup and preconcentration of a group of 14 pesticides including eight insecticides, four herbicides and two fungicides from fresh peppermint prior to their determination by GC/ECD/NPD techniques. Detailed validation study was carried out in order to assess fitness for purpose of the proposed method.
Materials and Methods
Certified pesticide analytical standards were obtained from Witko (Łódź, Poland) except for fluazifop-P-butyl which was obtained from Institute of Industrial Organic Chemistry (Warszawa, Poland), and were of the highest available purity. Individual pesticide stock solutions were prepared at approximate concentrations of 1,000 μg mL−1 in residue grade acetone. A single composite pesticides mixture at approximately 10 μg mL−1 was prepared from the stock solutions by dilution with residue grade acetone. Subsequent working standards were prepared by dilutions of the appropriate volumes of the pesticide mixture with acetone. Matrix-matched calibration standards were prepared in peppermint matrix extract in petroleum ether. Pre-weighted mixtures of 4 g anhydrous magnesium sulphate, 1 g sodium chloride, 0.5 g di-sodium hydrogen citrate sesquihydrate and 1 g sodium citrate dehydrate sodium, and pre-weighted mixtures of 150 mg PSA, 45 mg GCB and 900 mg anhydrous magnesium sulfate were purchased from Perlan Technologies (Warszawa, Poland). Acetonitrile (POCH, Gliwice, Poland) and petroleum ether (Chempur, Krupski Młyn, Poland) were distilled from glass before use.
An Agilent Technologies 6890 gas chromatograph was employed for all sample analyses. The system was equipped with electronic pressure control (EPC) and an autosampler. Analytes were separated on a DB-1701 30 m × 0.25 mm × 0.25 μm column (Agilent Technologies, Folsom, USA. The carrier gas was nitrogen (purity 6.0). The column was connected to NPD and ECD using a universal Y-splitter. The ECD and NPD were maintained at 270 and 300°C, respectively. Nitrogen was the make up gas for the ECD (30 mL min−1). For the NPD, hydrogen and air flows were kept at 3 and 30 mL min−1, respectively. The make up gas was nitrogen at 30 mL min−1. The column temperature was held at 100°C for 1 min, increased at 20°C min−1 to 180°C which was held for 4 min, increased at 20°C min−1 to 260°C held for 30 min. Injector temperature was held at 250°C. Sample extract volumes of 2 μL were injected in the splitless mode. HP ChemStation, Rev. A 10.02 chromatography software was used for the instrument control, data acquisition and processing.
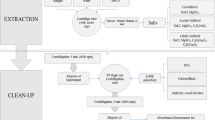
For sample extract preparation, the citrate buffered QuEChERS procedure was followed (Lehotay et al. 2010). However certain changes were made to adapt the method to our needs and laboratory resources. The sample size was reduced to 5 g, 10 mL water was added and the final extract was diluted with petroleum ether. To prepare sample extracts for GC/ECD/NPD analysis, 5 g of previously comminuted sample of fresh peppermint was weighted into a 50 mL disposable polypropylene centrifuge tube then 10 mL acetonitrile and 10 mL distilled water were added. The contents were shaken by hand for 1 min then a mixture of 1 g sodium chloride, 0.5 g di-sodium hydrogen citrate sesquihydrate, 1 g sodium citrate dehydrate and 4 g magnesium sulfate was added. Again, the contents were shaken by hand for 1 min and centrifuged at 3000 rpm for 5 min. A 6 mL aliquot of the upper layer was transferred into a 15 mL disposable polypropylene centrifuge tube with pre-weighted 150 mg PSA, 45 mg GCB and 900 mg anhydrous magnesium sulfate. The contents were shaken by hand for 2 min and centrifuged at 3000 rpm for 5 min. For the analysis by GC/ECD/NPD, a 2 mL aliquot of the supernatant was evaporated under a gentle stream of nitrogen and reconstituted in 2 mL of petroleum ether.
Results and Discussion
Determination of residual pesticides in peppermint can be considered as a difficult task since peppermint is known to contain large amounts of chlorophylls, other coloured compounds and peppermint oil. Crude extract of this kind of matrix can give rise to massive interferences in the GC determination and result in ambiguity of identification or complete suppression of chromatographic peaks of certain pesticides. Therefore, adequate cleanup of the extract is needed for reduction of co-extractives in order to enhance sensitivity (with less noise) and facilitate identification and quantification of low concentration of pesticides. For this purpose, a cleanup step was carried out by the combined use of primary secondary amine (PSA), a sorbent which exhibits retaining activity for sugars, fatty acids and other organic acids and graphitized carbon black (GCB), a sorbent which exhibits retaining activity for planar compounds such as natural pigments and sterols. Analytical performance of the selected approach was subjected to validation study in terms of trueness, precision, selectivity, limits of quantification and measurement uncertainty in order to assess fitness for purpose of the method.
Selectivity of the method was evaluated considering the absence of interfering peaks from co-extractives at the retention time of each target pesticide. For this purpose extracts of organically grown peppermint were analyzed (with no detectable pesticides). Linearity of calibration curves was investigated over the concentration range between 0.01 and 1.00 μg mL−1 by GC/ECD/NPD analysis of matrix-matched calibration standards (five levels). Over the studied concentration range, the linearity of GC/ECD/NPD responses was highly satisfactory with coefficients of determination (R2) higher than 0.99 (Table 1).
Accuracy was evaluated by means of spiking recovery determinations at three concentration levels of 0.01, 0.1 and 1.0 mg kg−1 (six replicates per level). Precision was calculated from the recovery experiments and it was expressed in terms of relative standard deviation (RSD; n = 6) at each spiking level. Overall recovery and RSD for each individual pesticide was also determined. The spiking experiments were carried out on organically grown peppermint. Figure 1 shows example chromatograms of peppermint sample spiked with the pesticides at 0.1 mg kg−1. The recovery results are shown in Table 2 indicating that the recoveries were in the acceptance range of 70 %–120 % with RSD ≤ 20 % for all the analyzed pesticides. The limit of quantification (LOQ) was established as the lowest spiking level at which the method fulfilled the aforementioned validation criteria, which was 0.01 mg kg−1 for all the pesticides. The LOQ values were less or equal to the MRLs required by the European legislation which were in the range from 0.01 mg kg−1 (diazinon and fenithrotion) to 2 mg kg−1 (cypermethrin). Uncertainty was estimated following the “top-down” experimental model using overall recovery and precision data. The expanded uncertainty was calculated individually for each pesticide as twice the value of the uncertainty (k = 2, confidence level 95 %), and ranged between 7 % and 30 %, with an average of 14 % (Table 2). These uncertainty values comply with the European Union requirement of a maximum default value of 50 %. As evident from the obtained method performance characteristics, the method is suitable for the intended application.
Our previous method for analysis of pesticide residues in peppermint entailed extraction of pesticides with dichloromethane/acetone mixture followed by cleanup on column packed with florisil and sodium sulfate and collection of eluates in four fractions which were then analysed by GC/ECD/NPD separately. Using the former method, the total organic solvents consumption was over 350 mL (Sadło et al. 2006). The current method is greatly improved compared with the previous one as it is more straightforward, less time and labour intensive, and more sensitive, selective and accurate, simultaneously. The consumption of organic solvent is approximately 30-fold less, and the usage of environmentally concerned chlorinated solvents is eliminated. The current analytical scope includes 14 pesticides but clearly the method can be expanded to include more pesticides unless limited by GC capillary column resolution or excessive presence of matrix interferents.
References
Anagnostopoulos CJ, Aplada Sarli P, Miliadis GE, Haroutounian CA (2010) Validation of the QuEChERS method for the determination of 25 priority pesticide residues in cereal-based baby foods by gas chromatography with electron capture and nitrogen phosphorous detection. Hell Plant Prot J 3:71–80
Chai L-K, Zaidel N-D, Hansen HCB (2012) A rapid multi-residue method for the determination of pesticide residues in choi sum, yardlong beans and aubergines. Food Chem 131:611–616
Georgakopoulos P, Zachari R, Mataragas M, Athanasopoulos P, Drosinos EH, Skandamis PN (2011) Optimisation of octadecyl (C18) sorbent amount in QuEChERS analytical method for the accurate organophosphorus pesticide residues determination in low-fatty baby foods with response surface methodology. Food Chem 128:536–542
González-Curbelo MÁ, Hernández-Borges J, Ravelo-Perez LM, Dionis-Delgado MA (2011) Insecticides extraction from banana leaves using a modified QuEChERS method. Food Chem 125:1083–1090
González-Curbelo MÁ, Dionis-Delgado S, Asensio-Ramos M, Hernández-Borges J (2012a) Pesticide analysis in toasted barley and chickpea flours. J Sep Sci 35:299–307
González-Curbelo MÁ, Hernández-Borges J, Borges-Miquel TM, Rodriguez-Delgado MÁ (2012b) Determination of pesticides and their metabolites in processed cereal samples. Food Addit Contam A 1:104–116
Lehotay SJ, Son KA, Kwon H, Koesukwiwat U, Fu W, Mastovska K, Hoh E, Leepipatpiboon N (2010) Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromatogr A 1217:2548–2560
Liu X, Wang X, Xu J, Dong F, Song W, Zheng Y (2011) Determination of tebuconazole, trifloxystrobin and its metabolite in fruit and vegetables by a quick, easy, cheap, effective, rugged and safe (QuEChERS) method using gas chromatography with a nitrogen-phosphorus detector and ion trap mass spectrometry. Biomed Chromatogr 65:406–409
McKay DL, Blumberg JB (2006) A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother Res 20:619–633
Sadło S, Szpyrka E, Rogozińska K, Rupar J (2006) Occurrence of some pesticides residues in peppermint Mentha piperita L. in 2003–2005. Rocz Panstw Zakl Hig 57:211–216 [Article in Polish]
Srivastava AK, Trivedi P, Srivastava MK, Lohani M, Srivastava LP (2011) Monitoring of pesticide residues in market basket samples of vegetable from Lucknow City, India: QuEChERS method. Environ Monit Assess 176:465–472
Wu J, Liu Y, Zhao R, Xu R (2011) Fast pesticide multiresidue analysis in American ginseng (Panax quinquefolium L.) by gas chromatography with electron capture detection. J Nat Med 65:406–409
Xu R, Jianwei Wu, Liu Y, Zhao R, Chen B, Yang M, Chen J (2011) Analysis of pesticide residues using the quick easy cheap effective rugged and safe (QuEChERS pesticide multiresidue method in traditional Chinese medicine by gas chromatography with electron capture detection. Chemosphere 84:908–912
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Słowik-Borowiec, M., Szpyrka, E. & Walorczyk, S. Analysis of Pesticide Residues in Fresh Peppermint, Mentha piperita L., Using the Quick Easy Cheap Effective Rugged and Safe Method (QuEChERS) Followed by Gas Chromatography with Electron Capture and Nitrogen Phosphorus Detection. Bull Environ Contam Toxicol 89, 633–637 (2012). https://doi.org/10.1007/s00128-012-0717-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0717-5