Abstract
A laboratory study was conducted with four pesticides, viz. a fungicide (carbendazim), two insecticides (chlorpyrifos and cartap hydrochloride) and an herbicide (pretilachlor) applied to a sandy clay loam soil at a field rate to determine their effect on microbial biomass carbon (MBC) and carbon mineralization (Cmin). The MBC content of soil increased with time up to 30 days in cartap hydrochloride as well as chlorpyrifos treated soil. Thereafter, it decreased and reached close to the initial level by 90th day. However, in carbendazim treated soil, the MBC showed a decreasing trend up to 45 days and subsequently increased up to 90 days. In pretilachlor treated soil, MBC increased through the first 15 days, and thereafter decreased to the initial level. Application of carbendazim, chlorpyrifos and cartap hydrochloride decreased Cmin for the first 30 days and then increased afterwards, while pretilachlor treated soil showed an increasing trend.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The use of synthetic pesticides as crop protection chemicals has become the most accepted weapon for assured crop production. Continuous use of pesticides may accumulate appreciable quantities of pesticides and their degradation products in the soil ecosystem. Pesticides that disrupt the activities of soil microorganisms may affect the nutritional quality of soils, resulting in serious ecological consequences (Handa et al. 1999). Hence, it is important to avoid serious injury to the soil microflora, whose functions are vital in maintaining the soil fertility. Microbial biomass carbon (MBC) in soil is considered as an important attribute of soil quality (Doran and Parkin 1994), and acts as a strong predictor of the pesticide degradation capacity of a particular soil (Voos and Groffman 1997). Carbon mineralization (Cmin) is an age-old reliable method for studying microbial activities in soil. Changes in carbon mineralization have also been used as criteria for pesticide toxicity (Torstenssen and Stenstorm 1986).
Owing to the ever increasing use of pesticides in the agricultural economy, this investigation was carried out to study the effects of pesticide application on soil MBC and Cmin. The compounds studied were two representative insecticides (chlorpyrifos and cartap hydrochloride), a fungicide (carbendazim), and an herbicide (pretilachlor), all of which are used extensively worldwide.
Materials and Methods
Soil samples were collected in polythene bags from the surface layer (0–15 cm) from Central Rice Research Institute, Cuttack, India (85°55′E, 20°25′N; elevation 24 m above mean sea level). Mean annual maximum and minimum temperatures of the area are 39.2 and 22.5°C, respectively, and the mean annual temperature is 27.7°C. The annual precipitation is about 1,500 mm year−1. The difference between mean summer soil temperature and mean winter soil temperature is more than 5°C, thus qualifying for the hyperthermic temperature class. The soil of the farm area has been developed from the deltaic sediments of Mahanadi River in recent times. The experimental soil is an Aeric Endoaquept with sandy clay loam texture (25.9% clay, 21.6% silt and 52.5% sand), bulk density 1.40 Mg m−3, cation-exchange capacity (CEC) 15.6 cmol(P+) kg−1, pH 6.7 (using 1:2.5, soil: water suspension), organic carbon 6.8 g kg−1, total nitrogen 0.9 g kg−1, and electrical conductivity 0.5 dS m−1. The soil sample was processed and sieved using a 2 mm sieve; 50 g of processed soil sample was taken in each Schott bottles (500 mL), and a calculated quantity of water was added to bring the soil samples to 60% of maximum water holding capacity. They were then acclimatized at 25 (±1)°C in the dark for 1 week.
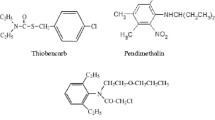
Calculated amounts of pesticides at the field rate, viz. cartap hydrochloride @ 1 kg a.i./ha, chlorpyrifos @ 0.5 kg a.i./ha, carbendazim @ 0.1% and pretilachlor @ 0.75 kg a.i./ha were applied to 50 g soil in individual Schott bottles in solution form. The conversion of the field application to milligrams of pesticide per kilogram of soil was calculated assuming an even distribution of pesticides in the 0–15 cm layer and soil density of 1.4 Mg m−3. The flasks were incubated at 28°C for periodic observation. Eight sets of each pesticide treated soil, along with control were maintained under similar conditions and replicated thrice. Some details of the pesticides used for the study are presented in Table 1.
The treated soils in Schott bottles were incubated at 60% water holding capacity (WHC) and 28°C temperature. The bottles contained 0.1 N NaOH in a vial to trap evolved CO2. The vials were removed at 0 (2 h after application), 1, 7, 15, 30, 45, 60 and 90 days of incubation for estimation of Cmin. Three sets of soil samples, 10 g each, were taken from the Schott bottle for estimation of MBC. One set of soil was fumigated using ethanol-free chloroform (25 mL) placed in a vacuum desiccator (Joergensen 1996). The chloroform was allowed to boil under reduced pressure for 2 min followed by incubation at 25°C for 24 h. The second set of soil was kept under similar conditions in the desiccator without chloroform (unfumigated). The third set of soil was kept in an oven for estimation of the moisture content. The fumigated and unfumigated soils were extracted separately with 40 mL of 0.5 (M) K2SO4 for 30 min in an oscillating shaker at 200 rpm. An aliquot of the filtered extract (8 mL) was refluxed with 0.4 (N) K2Cr2O7 (2 mL) for 30 min, and the residual dichromate was measured by back titration with 0.04 (N) ferrous ammonium sulfate using ferroin indicator (Vance et al. 1987) to estimate the extracted carbon. MBC was calculated by subtracting the extracted carbon in unfumigated samples from that measured in fumigated samples, and dividing it by a Kc value (extraction efficiency of microbial biomass carbon) of 0.45 (Vance et al. 1987). The CO2–C evolved from soil was measured by back titrating the unspent alkali in the vial with standard HCl for estimating potential carbon mineralization (Zibilski 1994). Periodic observations were taken for Cmin and MBC on 0 (2 h after application), 1, 7, 15, 30, 45, 60 and 90 days of incubation.
The data were subjected to analysis of variance (ANOVA) using SAS 9.2 software, followed by Duncan’s Multiple Range Test for comparison of means for all possible pairs.
Results and Discussion
Microbial biomass is the part of organic matter in soil that constitutes living microorganisms smaller than 5–10 cubic micrometers, and it is the fraction of soil organic matter that is sensitive to management practices and pollution (Powlson 1994). Microbial biomass is an important attribute of soil quality (Doran and Parkin 1994) as well as crop ecology (Beelen and Doelman 1997). The effects of the studied pesticides on soil MBC are presented in Table 2. The MBC increased significantly up to 30 days in chlorpyrifos as well as cartap hydrochloride treated soils; but thereafter decreased progressively with time. In both chlorpyrifos and cartap hydrochloride treated soil, the MBC was statistically higher compared to the control and carbendazim treated soil throughout the incubation period. Application of carbendazim showed a decreasing trend of soil MBC with time; however, the decrease was not significant up to 7th day of incubation. In comparison to the control, the soil MBC was significantly lower from day 7 onwards during the period of incubation. In pretilachlor treated soil, the soil MBC remained steady throughout the incubation period except for the first 24 h wherein it showed a significant increase. Pretilachlor treated soil showed significantly higher soil MBC over control up to 7th day of incubation.
Handa et al. (1999) showed that an increase in soil microbial biomass during incubation might be due to increased availability of energy and / or nutrient source required for cell proliferation with the progressive mineralization of insecticides. The higher MBC in the control soil and soils treated with cartap and chlorpyrifos during the first 30 days might be due to a proliferation of fungal populations (Pandey and Singh 2004). Sivasithamparam (1969, 1970) reported similar increases in soil actinomycetes and other bacterial populations with the application of chlorpyrifos. In general, actinomycetes and saprophytic fungi appear to be more resistant to the action of pesticides, and for many pesticides, an increase in their numbers was observed (Sylvestre and Fournier 1979). The decrease in soil MBC during the initial period of incubation in carbendazim treated soil might be due to the inhibitory effect of carbendazim on soil mycoflora. The fungi-toxic effect of carbendazim decreased with increased time, and resulted in the reappearance of certain fungi after a particular time. A reduction in various species of soil fungi was more extensive in the first 20 days than in the latter half of the experimental period with the fungicide, carbendazim (Aggarwal et al. 2005).
Carbon mineralization is an important parameter for assessing the side effects of pesticides (Sommerville 1987; Alef 1995). Cmin depends not only on the intensity of the stress but also on the period of exposure of the microbes to the stress. The Cmin values recorded under the present experimentation are shown in Table 3. In cartap and carbendazim treated soil, CO2 evolution decreased up to 15 days, remained steady between 15 and 30 days, followed by an increasing trend up to 90 days. At this time, they had regained CO2 evolution to be on par with the initial period of incubation. However, in chlorpyrifos treated soil, the evolution decreased up to 30 days, and thereafter showed an increasing trend. A similar trend was also observed in control. In cartap hydrochloride treated soil Cmin was significantly lower compared to control throughout the incubation period except at 90 days. There were no significant differences in Cmin among control, chlorpyrifos and carbendazim on most of the observation days during the incubation period. This indicates that the applied doses of pesticides, i.e. chlorpyrifos and carbendazim, are not high enough to suppress soil microbial activities. In contrast to the above results, an increasing trend of CO2 evolution was observed in pretilachlor treated soils, which was significant up to the 15th day. It remained stable thereafter, indicating that the pretilachlor treatment stimulated soil microbial activity.
The decline in carbon mineralization for up to 30 days in insecticide treated soils might be due to the utilization of the available substrates by the soil microorganisms for cell proliferation, or the development of a competitive environment because of the increased biomass with limited O2 (Sengupta et al. 2009). Zelles et al. (1985) reported a minor effect of atrazine, lindane and captan on Cmin, but the chloracetamide group of herbicides induced measurable changes in the behavior of the microorganisms. Several of these herbicides at various concentrations stimulated the production of CO2. Tu (1992) through laboratory tests indicated that Cmin increased significantly after 96 h of incubation with atrazine. He also opined that the herbicidial treatments at the recommended dose/concentrations are not drastic enough to be considered deleterious to soil microbial activities involved in maintaining soil fertility. Further, Haney and Senseman (2000) observed that herbicide treatment significantly stimulated soil microbial activity as measured by C and N mineralization, but did not affect soil microbial biomass.
Therefore, it can be concluded from our study that chlorpyrifos and carbendazim at recommended field doses have only marginal effects on soil microbial activities, viz, MBC and Cmin; and that microorganisms are capable of recovering rapidly. In cartap treated soil, the suppressing effect of the treatment remained for a period of 2 months, and thereafter regained its original level. The application of pretilachlor stimulated microbial activity. However, laboratory results and field results may differ considerably, as in the field many factors could mask or reduce the potential toxicity of pesticides. Therefore, before arriving at a general conclusion for the effect of these pesticides on microbial biomass and carbon mineralization activities of soil microbial populations, further detailed studies under field conditions would be useful.
References
Aggarwal A, Sharma D, Parkash V, Sharma S, Gupta A (2005) Effect of bavistin and dithane M-45 on the mycorrhizae and rhizosphere microbes of sunflower. Helia 28(42):75–88
Alef K (1995) Estimation of soil respiration. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press, London
Beelen PV, Doelman P (1997) Significance and application of microbial toxicity tests in assessing ecotoxicological risks of contaminants in soil and sediment. Chemosphere 34:455–499
Berteau PE, Deen WA (1978) A comparison of oral and inhalation toxicities of four insecticides to mice and rats. Bull Environ Contam Toxicol 19(1):113–120
Doran JW, Parkin TB (1994) Defining and assessing soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (eds) Defining soil quality for sustainable environment. Special Pub. 35. Soil Science Society of America, Inc., Madison
Fabro L, Varca LM (2011) Pesticide usage by farmers in Pagsanjan-Lumban catchment of Laguna de Bay. Philipp Agric Water Manag. doi:10.1016/j.agwat.2011.08.011
Gosselin RE, Smith RP, Hodge HC (1984) Clinical toxicology of commercial products, 5th edn. Williams and Wilkins, Baltimore
Handa SK, Agnihotri NP, Kulshreshtha G (1999) Pesticide residues; significance, management and analysis. Research Periodicals and Book Publishing House, Texas
Haney RL, Senseman SA (2000) Effect of glyphosate on soil microbial activity and biomass. Weed Sci 48:89–93
Joergensen RG (1996) The fumigation–extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol Biochem 28:25–31
Pandey S, Singh DK (2004) Total bacterial and fungal population after chlorpyrifos and quinalphos treatments in groundnut (Arachis hypogaea L.) soils. Chemosphere 55:283–290
Powlson DS (1994) The soil microbial biomass: before, beyond and back. In: Dighton J, Giller KE, Ritz K (eds) Beyond the biomass. Wiley, Chichester
Sengupta D, Aktar W, Purkait S, Ganguly M (2009) Impact of quinalphos on microbial biomass and activities in tropical clay loam soil. EJEAFChe 8(11):1127–1135
Sivasithamparam K (1969) Some effects of an insecticide (Dursban) and a weedicide (Linuron) on the microflora of a submerged soil. Proc Ceylon Ass Advmt Sci 25:1–8
Sivasithamparam K (1970) Some effects of an insecticide (Dursban) and a weedkiller (Linuron) on the microflora of a submerged soil. Riso 19:339–346
Sommerville L (1987) Perspective on side effect testing. In: Sommerville L, Greaves MP (eds) Pesticide effects in soil microflora. Taylor and Francis, London
Sudhakar P, Chaitopadhyay GN, Gangwar SK, Ghosh JK, Saratchandra B (2000) Effect of common pesticides on nitrogen fixing bacteria of mulberry (morus alba L.). Indian J Agric Res 34(4):211–216
Sylvestre GS, Fournier JC (1979) Effect of pesticides on the soil microflora. In: Brady NC (ed) Advances in agronomy, USA. Academic Press, USA
Tomlin CDS (ed) (1997) The pesticide manual—a world compendium. British Crop Protection Council, Surrey
Torstenssen L, Stenstorm J (1986) Basic respiration rate as a tool for prediction of pesticide persistence in soil. Toxic Assess 1:57–72
Tu CM (1992) Effect of some herbicides on activities of microorganisms and enzymes in soil. J Environ Sci Health 27:695–709
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass. Soil Biol Biochem 19:703–707
Voos G, Groffman PM (1997) Relationship between microbial biomass and dissipation of 2, 4-D and Dicamba in soil. Biol Fertil Soils 24:106–110
Zelles L, Scheunert I, Korte F (1985) Side effects of some pesticides on non-target soil microorganisms. J Environ Sci Health 20:457–488
Zibilski LM (1994) Carbon mineralization. In: Bingham JM, Mickelson SH (eds) ‘Methods of soil analysis’, Part 2. Microbiological and biochemical properties. SSSA, Book Series No. 5. ASA, SSSA, Madison
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A., Nayak, A.K., Shukla, A.K. et al. Microbial Biomass and Carbon Mineralization in Agricultural Soils as Affected by Pesticide Addition. Bull Environ Contam Toxicol 88, 538–542 (2012). https://doi.org/10.1007/s00128-012-0538-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0538-6