Abstract
The purpose of this study was to determine the leaching characteristics of heavy metals from artificial soils composed of sewage sludge and fly ash. A leaching experiment was carried out over a period of 90 days. The leachates from artificial soils were collected every 15 days, and the concentrations of cadmium, lead, copper, zinc, chromium, and nickel in leachates were determined. Results showed that pH values of the artificial soils leachate were stable, ranging from 6.71 to 7.62 at the end of the experiment. Except of the cadmium, the concentrations of chromium, nickel, and copper in leachates of the artificial soils reached a stable level at the end of the experiment. The final concentrations of copper in leachates varied from 27.1 to 127.5 μg L−1, which was lower than European threshold value for drinking water, (1,000 μg L−1), while final nickel and chromium concentrations in leachates exceeded the European threshold ones. Amorpha fruticosa and Robina pseudoacacia grown in the artificial soils had different effects on cadmium, nickel, chromium, and copper leaching behavior. Amorpha fruticosa resulted in higher Ni and Cu concentrations in leachates, while Robina pseudoacacia enhanced leaching concentration of Cr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Despite a principal contributor to the rapid economic growth, the mining industry in China produced a large amount of wasteland, damaged native vegetations and caused water pollution and soil erosion as well as other environmental damages (Shen et al. 2004). Remediation of mineland is one of the pressing needs to be addressed for social and economic development to be healthy and sustainable. However, mines always locate in the mountainous areas, where the natural soil resources is lack.
Fly ash (FA) is a by-product of combustion of coal. It is a ferro-alumino-silicate mineral containing most of the essential elements (calcium, potassium, sodium, magnesium) for the growth and development of the plant, but is deficient in nitrogen and occasionally in available phosphorus (Carlson and Adriano 1993). Sewage sludge (SS) is a source of organic matter, a pool of slow-release essential nutrients (nitrogen, phosphorus, sulphur and magnesium) and microorganisms (Abbott et al. 2001). In view of high cost of disposal and environmental management, utilization of FA/SS mixtures as artificial soil in mine eco-remediation may be a viable option. Additionally, previous studies demonstrated that nutrients content of the artificial soil composed of FA and SS were up to a high fertilizer level with available potassium being 762.66 mg kg−1, available phosphorus being 375.83 mg kg−1 and available nitrogen being 109.7 mg kg−1 (Zhang et al. 2008). Field trails utilizing FA/organic waste mixtures as fertilizers for maize (Zea mays L.) produced comparable yields to conventional fertilization techniques (Schumann 1997).
However, fly ash and sewage sludge also contain high concentrations of potentially toxic elements such as Cd, Ni, Cr, Pb, Cu and Zn (Brown et al. 2003). During disposal, storage and land application phases, the residues from sewage sludge and fly ash are subjected to leaching effects of rain and part of the undesirable components in the wastes may pollute both ground and surface waters (Egiarte et al. 2006). These solid residues such as municipal sewage sludge and fly ash may be leached in high concentrations of heavy metals surpassing the threshold for drinking water standards, and in turn cause contamination in drinking water sources. Different elements have different leaching behaviors owing to their properties, pH of the solution and leaching time. Egiarte et al. (2006) reported that concentrations of Zn, Cd, Ni and Pb in leachates from municipal sludge amended-soil never surpassed the European threshold values for drinking water, while Cr concentration in leachates was higher than the threshold values. There is some evidence that surface application of neutralized fly ash may reduce the concentrations of contaminants in leachate (Abbott et al. 2001; Brown et al. 2003).
Leaching characteristics of fly ash and sewage sludge individual are well documented. However, little information is available on the leachability of heavy metals from FA/SS mixtures. The specific aims of this study were to investigate the leaching behavior of Ni, Cu, Zn, Cd, Pb, and Cr from artificial soils used for remediation of mine tailings, and analyze the potential influence of the artificial soils on ground water quality.
Materials and Methods
The mine spoil material was collected from an abandoned iron mine (Dagushan mine). The exact location of the site is 123°03′36.6″E and 41°03′03″N. Fly ash (FA) used in this study was collected from a Power Plant of Anshan Steel Company, and sewage sludge (digested sludge) (SS) came from the Anshan North Waste Water Treatment and Haicheng Sewage Treatment Works. Fly ash and mine spoil material samples were air-dried before mixed with sewage sludge. The sewage sludge used in this experiment was composted outdoors for several days after being collected. When the moisture of SS was 55–60%, it was well mixed with dry fly ash. Then the mixture materials were air-dried, placed to pass through a 2-mm sieve and determined for the basic physicochemical properties. Three artificial soil treatments signed T1, T2 and T3 were selected for the leaching experiment based on previous experiments (Zhang et al. 2007).
The leaching experiment was conducted in the laboratory. Five thousand grams of mine waste rocks was first placed in each of the eighteen polypropylene containers with leaching ports in the bottom (40 × 35 cm). Then three thousand grams of the artificial soil samples were placed on the mine tailing rocks. Plants of Robina pseudoacacia and Amorpha fruticosa were planted in the artificial soil, and the treatments planted with Robina pseudoacacia were signed T1r, T2r, T3r, and treatments planted with Amorpha fruticosa were signed T1a, T2a, T3a on 2 April, 2009, respectively. Three individual plants were planted in each artificial soil treatment, and three replicate were designed.
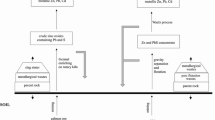
The experiment was carried out at room temperature (23–25°C). Every 15 days, the treatments were irrigated with 80 L deionized water according to the annual rainfall in Anshan city (700–800 mm), and pH of the deionized water was adjusted to 6.5–7.5 by HCl and NaOH prior to irrigate. Leaching collection device used in the experiment to collect leachate samples for trace elements analysis was plastic bottle. The plastic bottle was washed, brushed, rinsed with distilled water, and soaked in 20% nitric acid (Merck) for 24 h; then it was rinsed several times with de-ionized water and sealed into double black plastic bags. Then the leachate was collected to determine. The experimental design is shown in Table 1 and Fig. 1.
After researchers collected the leachates of artificial soils, all the samples were filtered through a 0.45-μm polycarbonate micropore membrane filter prior to analyses and stored at 4°C.
Results and Discussion
The leachate pH of artificial soil treatments showed slight change during the experimental period of 90 days (Fig. 2a). However, the pH of FA/SS mixtures increased with an increase application ratio of FA, and it was observed that FA has amelioration effect on the artificial soil acidifying nature. The leachates pH of artificial soil T1r,a (FA:SS = 2:1) remained the highest value, whereas T3r,a (FA:SS = 0.5:1) remained the lowest during the 90 days leaching experiment. By the end of the experiment, the leachates pH value of the 6 artificial soil treatments occurred in the order: T1r (7.62) > T1a (7.50) > T2a (7.11) > T2r (6.83) > T3a (6.80) > T3r (6.71). The final pH values of these artificial soils were close to an idea value for most crops growth (Feng et al. 2006). Fly ash contains a high level of lime and has a high pH value ranging from 10 to 12, occasionally even higher, and can substitute lime in reducing sewage sludge acidity when applied on the eco-remediation at mining areas (Wang et al. 2007). However, the influence of different plants on the leachate pH value was not distinct from this paper.
In Table 2 the analytical results of total content determination of mine tailing background levels were summarized. It showed that Cd background level of mine spoil area was only 0.074 mg kg−1. The municipal sludge and fly ash used in the study contained 3.71 and 0.66 mg kg−1 of Cd (Zhang et al. 2008), respectively, which were lower than China quality guideline values (10 mg kg−1) used as standards for urban waste application. It is commonly accepted that the toxicity and mobility of heavy metals in soils depend not only on the total concentration, but also on their specific chemical form, the metal properties, environmental factors, soil properties like pH, organic matter content and type, redox conditions, and root exudates acting as chelates (Rodríguez et al. 2009).
As shown in Fig. 3, leachates Cd concentrations remained relatively constant at first, but then declined. After the third leaching cycle, there was an order of magnitude increase in Cd concentrations, resulting in a pulse that abated after cycle 4, with the highest leachates Cd concentrations ranging from 21 to 27 μg L−1. Subsequently, leaching Cd from artificial soil-amended mine tailing rapidly decreased. It was reported that from pH range 4–6 and up to pH 11, the cadmium solutions were composed from positive charged ions (Cd2+, CdOH+, Cd2(OH)3+) as well as neutral species (Cd(OH) 02 ) while the high acidic solution was composed from single Cu2+ and Cd2+ cations (Peng et al. 2009). Similarly, Leviminzi and Petruzzelli (1984) noted that when pH decreased from 6.4 to 4.8, more cadmium was released. In our studies, the pH value for all leachates ranged from 6.26 to 7.62. Under this condition, the cadmium was easily precipitated as their insoluble hydroxides. In addition, the final leachates Cd concentrations from artificial soil treatments varied from 2.99 to 12.69 μg L−1, which were near to the EU drinking water threshold of 10 μg L−1 (Schumann 1997).
From Fig. 4, it was found that leachates Cr concentrations had a small increase at first. Then they declined towards the lowest point at the third leaching cycle. However, after cycle 3, there was an order of magnitude increase in Cr concentrations, suggesting that the soluble Cr in artificial soil or mine tailing rocks was first combined with some complexing ligands, but this combination was transient and unstable. Long time kinetic adsorption–desorption experiments also showed that metal freshly associated with particles presents less stability and higher potential transformation than those associated for a long time (Peng et al. 2009).
During the leaching experiment, leachates Cr concentrations from treatments T(1–3)r with Robina pseudoacacia growth were always higher than those for the treatments T(1–3)a with Amorpha fruticosa growth, with values for T3r treatment being the highest (342.0 μg L−1) at the end of the experiment. Therefore, it seems that plant species might influence the leaching characteristic of heavy metals from artificial soil due to rhizosphere environment, phytoextraction, accumulation and other elements. Yang et al. (2005) reported that the metal contents, pH, CEC, water content, organic substances and other elements in the rhizosphere affected the bioavailability and plant uptake of heavy metals from the soils; in turn, it might affect the leaching concentration of Cr.
Chromium may exist as either cationic Cr3+ or the anion chromate, Cro 2−4 . While chromate (Cr(VI)) is the more mobile Cr species, it is also a strong oxidizing agent, and is likely to be reduced to Cr(III) in the presence of soil organic matter (Lake et al. 1984; Egiarte et al. 2006). Solubility of Cr(III) is pH dependent, and at pH > 5.5 complete precipitation of this species is expected; however, complexing organic ligands may enhance Cr(III) solubility at pH values when precipitation would otherwise be predicted. Thus, changes in the Eh/pH of a soil system can affect redox transformations of Cr species and adsorption to certain solid phases can also affect such redox reactions (Jackson and Miller 2000; Peng et al. 2009).
After the fifth leaching circle, the concentrations of Cr in leachates tended to be constant, and the balance concentrations were in the following order: T3r (342.0 μg L−1) > T2r (313.0 μg L−1) > T1a (301.5 μg L−1) > T1r (297.5 μg L−1) > T2a (295.0 μg L−1) > T3a (288.0 μg L−1). The high concentrations of Cr in leachates will also be transported to the surface and ground water during the application to mine areas.
Leachates Ni concentrations from all artificial soils significantly increased during the first several days (Fig. 5). This is probably due to the OM degradation and the acid volatile sulfide oxidation. The pH of the leachates decreased from 6.67–7.61 to 6.26–7.18 (Fig. 2a), which resulted in Ni being released from artificial soil or/and mine tailing rock into leachates (Bonnissel-Gissinger et al. 1998; Peng et al. 2009). Thereafter, the concentrations of Ni in leachates sharply declined and reached the lowest point at the fourth leaching circle. Then they slightly increased and became a stable level at the end of the leaching experiment. The lowest Ni concentrations from artificial soil treatments ranged from 11.3 ± 0.12 to 34.3 ± 1.64 μg L−1, which were near to the EU drinking water threshold of 20 μg L−1 (Schumann 1997).
The amounts of Ni leached from artificial soils Ta(1–3), with the exception of the second leaching circle, were consistently greater than those from artificial soils Tr(1–3). The balance Ni concentrations in leachates were found in the order of T3a (183 μg L−1) > T2a (175.3 μg L−1) > T1a (157.3 μg L−1) > T3r (150.3 μg L−1) > T2r (130 μg L−1) > T1r (90 μg L−1) at the end of the leaching experiment. This suggested that compared to Robina pseudoacacia, Amorpha fruticosa grown in artificial soil, it may result in higher Ni concentration in leachates. The influence of plant comprises of two tiers, one by plants themselves and the other by the root colonizing microbes, which degrades the toxic compounds to further non-toxic metabolites. Ni concentrations in leachates increased generally with increased SS rate. This is similar to the results reported by Lake et al. (1984), who noted that Ni shifted to more available forms in sludge-amended soils. Many documents have focused on the ability of plants to uptake and accumulate of heavy metals (Rodríguez et al. 2009), but few studies were carried out to investigate the influence of different plants on the leaching characteristic of heavy metals. This study revealed that plants affected the leachates concentration for heavy metals from artificial soil. But the mechanism would be complicated, and we will go further in the study of leachates characteristic about plants and other factors.
As shown in Fig. 6, the leachates Cu concentrations from artificial soil treatments reached the peak at the second leaching circle. Treatment T3a exhibited the largest leachate Cu concentration (597 μg L−1), which was much higher than those from other treatments, while treatment T3r exhibited the lowest leachates Cu concentration (57.6 μg L−1). Choi et al. (2002) have reported that the element in the ash particles was mainly associated with the surface and these surface-associated fractions might dominate the leachate chemistry at the early stages. Complexation with dissolved organic carbon is acknowledged as a mechanism that enhances Cu solubility and in the absence of complexation, Cu is strongly bound by variably charged surfaces in soils at pH > 5.5 (Jackson and Miller 2000).
After the second leaching circle, the leachates Cu concentrations from artificial soil-amend mine rocks sharply declined and rapidly reached a steady state. During the experiment, leachates Cu concentrations ranged from 1.6 to 597.0 μg L−1, and the balance Cu concentration in leachates was decreased in the order of T2a (127.5 μg L−1) > T3a (77.3 μg L−1) > T3r (69.15 μg L−1) > T2r (56.95 μg L−1) > T1a (30.8 μg L−1) > T1r (27.1 μg L−1). In spite of the municipal sludge and fly ash used in the study contained 192.33 and 77.42 mg kg−1 of Cu, respectively (Zhang et al. 2008), and local mine background Cu content was 38.64 mg kg−1 (Table 2), the leachates Cu concentrations were far below the European drinking water threshold of 2,000 μg L−1 and the China drinking water threshold of 1,000 μg L−1 (Schumann 1997; GB5749-85 1986). This suggests poor correlation between Cu leachability and total Cu concentrations in the artificial soils. Little Zn was leached from the artificial soils (<0.001 mg L−1). This is probably because the precipitation may occur at high Zn concentrations and at high pH values.
The leachate pH of all artificial soil treatments showed slight change during the 90 days leaching experiment, with the final pH values ranging from 6.71 to 7.62, which were suitable for plant growth. The concentrations of Cr, Ni, and Cu in leachates from artificial soils got to a steady state by the end of the experiment. With the exception of Cu, the concentrations of Cd, Ni and Cr were near to or surpassed the European drinking water threshold. The plants Amorpha fruticosa and Robina pseudoacacia grown in the artificial soils had different effects on heavy metal leaching concentrations: Amorpha fruticosa might resulted in higher Ni and Cu concentrations in leachates, while Robina pseudoacacia enhanced leaching Cr concentration. In sum, high Cr, Ni and Cd concentrations in leachates will also be transported to the surface and ground water. Some measures should be taken to reduce the concentrations of heavy metals in leachates before these artificial soils are used for remediation of mine tailings. More research is recommended to investigate the overall effects of plant growth on heavy metal movement from mine tailing.
References
Abbott DE, Essington ME, Mullen MD, Ammons JT (2001) Fly ash and lime-stabilized biosolid mixtures in mine spoil reclamation: simulated weathering. J Environ Qual 30:608–616
Bonnissel-Gissinger P, Alnot M, Ehrhardt JJ (1998) Surface oxidation of pyrite as a function of pH. Environ Sci Technol 32:28–39
Brown SL, Henry CL, Chaney R, Compton H, DeVolder PS (2003) Using municipal biosolids in combination with other residuals to restore metal-contaminated mining areas. Plant Soil 249:203–215
Carlson CL, Adriano DC (1993) Environmental impacts of coal combustion residues. J Environ Qual 22:227–247
Choi SK, Lee S, Song YK, Moon HS (2002) Leaching characteristics of selected Korean fly ashes and its implications for the groundwater composition near the ash mound. Fuel 81:1080–1090
Egiarte G, Camps Arbestain M, Ruíz-Romera E, Pinto M (2006) Study of the chemistry of an acid soil column and of the corresponding leachates after the addition of an anaerobic municipal sludge. Chemosphere 65:2456–2467
Feng YJ, Li F, Wang XL, Liu XM, Zhang LN (2006) Principal chemical properties of artificial soil composed of fly ash and furfural residue. Pedosphere 16(5):668–672
GB 5749-85 (1986) Sanitary standards for drinking water. People’s Republic of China
Jackson BP, Miller WP (2000) Soil solution chemistry of a fly ash, poultry litter, and sewage sludge-amended soil. J Environ Qual 29:2430–2436
Lake DL, Kirk WW, Lester JN (1984) Fractionation, characterization, and speciation of heavy metals in sewage sludge and sludge-amended soils. J Environ Qual 13(2):175–183
Leviminzi R, Petruzzelli G (1984) The influence of phosphate fertilizers on Cd solubility in soil. Water Air Soil Pollut 23:423–429
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Meter 161:633–640
Rodríguez L, Ruiz E, Alonso-Azcárate J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. J Environ Manage 90:1106–1116
Schumann AW (1997) Plant nutrient supply from fly ash-biosolid mixtures. Ph.D. University of Georgia, Athens
Shen WS, Cao XZ, Jin Y (2004) The ecological damage and ecologicla reconstruction of mining areas. Environmental Science of China Press, Beijing, pp 1–8
Wang T, Liu TF, Sun C (2007) Application of MSWI fly ash on acid soil and its effect on the environment. Waste Manage. doi:10.1016/j.wasman.06.025
Yang X, Feng Y, He Z, Stoffella PJ (2005) Molecular mechanism of heavy metal hyperaccumulation and phytoremediation. J Trace Elem Med Biol 18:339–353
Zhang HL, Sun LN, Sun TH, Ma GF (2007) Principal physicochemical properties of artificial soil composed of fly ash, sewage sludge and mine tailing. Bull Environ Contam Toxicol 79:562–565
Zhang HL, Sun LN, Sun TH (2008) Heavy metals and trace elements of sewage sludge stabilized by coal fly ash. J Liaoning Techni Univer Natura Sci 27(6):944–946
Acknowledgments
The project was supported by National Natural Science Foundation of China (No. 40901282, 41071304), the High Technology Research and Development Program (863) of China (No. 2009AA06Z320), Excellent Person Program of Liaoning Province (2009R44) and Doctor Startup Fund Program (20212336).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Sun, L. & Sun, T. Leaching of Heavy Metals from Artificial Soils Composed of Sewage Sludge and Fly Ash. Bull Environ Contam Toxicol 88, 406–412 (2012). https://doi.org/10.1007/s00128-011-0507-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0507-5