Abstract
Naïve grass shrimp Palaemonetes pugio were pulse-fed cadmium-contaminated meals containing carbon-14, fluorescent or near-infrared markers and analyzed for carbon assimilation efficiency, gut residence time, feces elimination rate, extracellular digestive protease activity or gut pH. Carbon assimilation efficiency (~83%), minimum gut residence time (~435 min) and proventriculus pH (~5.29 to ~6.01) were not impacted significantly by cadmium ingestion. A dose-dependent decrease in feces elimination rate (from ~14.4 to ~6.4 mm h−1) was observed for shrimp for 2 h following minimum gut residence time. Protease activities increased ~2.4-fold over the range of dietary cadmium exposures, however, this variation was not dose-dependent. Differential impacts of cadmium exposure on carbon and cadmium assimilation reported previously are consistent with work involving shrimp subjected to chronic field exposure. The influence of ingested cadmium on feces elimination rate may be related to pre-assimilatory impacts on packaging, intestinal transport or release of feces. Protease activities may have been influenced by pre-assimilatory interactions between available cadmium ions in gut fluid and enzyme-secreting cells of the hepatopancreatic epithelium or direct impacts on active enzymes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The assimilation of elements by aquatic invertebrates can be influenced by many factors including diet, ingestion rate, bioavailability of elements in gut fluid and digestive physiology (Mayer et al. 1996; Ahrens et al. 2001). Exposure to pollutants (e.g., non-essential metals) through the diet may have implications for digestion and impact the assimilation of nutrients as well as ingested pollutants (Campbell et al. 2005). It is, therefore, necessary to understand the influence of dietary pollutant exposure on key parameters related to digestive physiology, particularly for important nutrient-cycling organisms. Impacts on digestive function may depend upon the manner in which components of the digestive milieu or tissues are subjected to exposure. Pre-assimilatory toxicity may be induced by a pollutant circulating in gut fluid during a feeding cycle (Campbell et al. 2005). Post-assimilatory toxicity may be related to tissue pollutant concentrations accumulated during chronic exposure (Seebaugh et al. 2011). Since impacts of pre- and post-assimilatory toxicity may be interactive for organisms that feed in contaminated conditions, it is important to assess the independent effects of each mode of internal exposure on digestion. In previous work with the grass shrimp Palaemonetes pugio, cadmium (Cd) assimilation efficiencies (AE) were influenced by tissue Cd concentrations in laboratory-exposed prey, suggesting that ingested metal can impact digestion (Seebaugh et al. 2006). Cd AE was not dose-dependent, indicating that metal impacts on digestive parameters may be variable or that interactivity between physiological processes may be important in determining assimilation. For the present study, we investigate carbon AE, gut residence time (GRT), feces elimination rate (FER), extracellular digestive protease activities and gut pH in naïve grass shrimp following pulse-feeding on Cd-contaminated meals.
Materials and Methods
Meals used to assess carbon AE and parameters related to digestive physiology in shrimp were prepared with carbon-14 (14C), fluorescent or near-infrared markers as described in detail in previous studies (Seebaugh and Wallace 2009; Seebaugh et al. 2011). Oligochaetes Tubifex tubifex were exposed to Cd (control, 1.76, 3.52 or 7.04 μM) (2.5 ppt, 18–19°C) through solution for 96 h with renewal of exposure media at 48 h (~0.18 worms mL−1). Exposed worms were stored frozen prior to incorporation into experimental meals. Samples of worms from each treatment were digested in 70% HNO3, dried, resuspended in 2% HNO3, filtered and analyzed for whole tissue Cd concentrations via atomic absorption spectrometry (Khoury et al. 2009). Carbon AE was estimated following ingestion of meals containing 14C labeled diatoms Thalassiosira weissflogii, Cd-contaminated worm homogenate, cod liver oil and melted gelatin crystals. For GRT and FER analyses, the gelatin-worm matrix contained unlabeled diatoms and was amended with 0.5 μm diameter Fluoresbrite microspheres (Polysciences). Digestive protease activities were assessed using meals containing IRDye 800RS casein protease substrate (Li-Cor). Meals used for gut pH analysis included Zymosan A BioParticles fluorescein conjugate (Molecular Probes). Diatoms and cod liver oil were excluded from IRDye and fluorescein meals to reduce potential autofluorescence signal interference. Proportions of Cd-contaminated worm homogenate in prepared meals were equal across treatment groups for each digestive toxicity assay. Oligochaete tissues were diluted ~7-fold after they were homogenized and combined with other meal components.
Adult P. pugio (~3 cm in length) were collected from Great Kills Harbor (GK), Staten Island, New York and acclimated to laboratory conditions for 5–7 days in clean, aerated seawater filtered by an aquarium filter (10 ppt, 18–19°C). This location served as a reference site in previous studies of assimilation, digestion and behavioral toxicity in this species (Perez and Wallace 2004; Seebaugh and Wallace 2009; Seebaugh et al. 2011). Shrimp were fed fish food during acclimation and allowed to clear their guts for ~2 days prior to experiments. A brief overview of the methods used to evaluate carbon AE and digestive toxicity is provided as detailed descriptions are presented elsewhere (Seebaugh and Wallace 2009; Seebaugh et al. 2011 and references therein). Shrimp were maintained in clean seawater while feeding on Cd-contaminated meals containing task-specific markers and mortality during feeding was 0%. To estimate carbon AE, shrimp were fed 14C-labeled meals in 1,000 mL beakers (3–4 shrimp per beaker) for 45 min and transferred to defecation chambers. 14C activities in shrimp tissues and cumulative feces at 24 h were determined by liquid scintillation counting and AE-14C% calculated using the mass balance method. For GRT analysis, 15 shrimp were fed meals containing fluorescent microspheres in a 38 L aquarium for 30 min. Shrimp that consumed meals were housed in defecation chambers and feces collected 1.5 h after feeding and every 30 min thereafter for up to 11.5 h. Dried feces were scanned for microspheres using a microscope equipped with a fluorescent light source. Minimum GRT for individual shrimp was estimated as time between introduction of meals and first detection of microspheres in feces, to the nearest 30 min. Dried feces were measured using a dissecting microscope equipped with a digital imaging system. FER was calculated for 2 h following GRT. To estimate protease activities, 15 shrimp were fed IRDye-labeled casein meals in a 38 L aquarium for 14 min, immobilized in 30 mm diameter glass tubes and scanned with an near-infrared imager every 90 s, from time (t) = 20 min to 33.5 min following introduction of food. Casein hydrolysis rates within the proventriculus and hepatopancreas were measured as increases in integrated intensity (i.e., the initial rate period prior to substrate exhaustion). A regression was fit to the linear portion of each plot (to t = 32 min) representing the mean increase in integrated intensity and the corresponding slope was used to compare rates among treatments. To estimate gut pH, Zymosan A-labeled meals were provided to 15 shrimp housed within a 9 L aquarium. Shrimp that acquired meals were immobilized in 30 mm diameter glass tubes and scanned with a confocal microscope equipped with an argon/krypton laser. pH within anterior and posterior regions of the cardiac proventriculus was estimated using 496 nm:458 nm intensity ratios (emissions at 530 ± 25 nm), calibrated to standards prepared by adding Zymosan A to pH buffers. Shrimp did not exhibit visible signs of stress during assays for digestive toxicity.
Tissue Cd concentrations in oligochaetes were log10 transformed and analyzed using ANOVA. Homogeneity of 14C-labeled food ingestion (counts per min; cpm) by shrimp was tested using Kruskal–Wallis ANOVA (K–W ANOVA). Arcsine transformed AE-14C% data were analyzed using K–W ANOVA. Correlations between AE-14C% and ingested 14C were tested with Spearman rank correlation (r s). Minimum GRT data were tested for treatment effects using K–W ANOVA. FER data and homogeneity of IRDye-labeled food ingestion (integrated intensity at t = 20 min) were tested for treatment effects using ANOVA. Protease activities were analyzed through planned testing of equality of slopes (Sokal and Rohlf 1995). Correlations between rates of casein hydrolysis and initial IRDye signals were analyzed using r s. pH data were converted to H+ concentrations and analyzed using K–W ANOVA (Murphy 1981). pH of anterior and posterior regions of the cardiac proventriculus was compared using the Mann–Whitney U test. Multiple comparisons of Cd concentrations in worms as well as FER and protease activities in shrimp were performed using the t test with Bonferroni correction (Sokal and Rohlf 1995).
Results and Discussion
Whole tissue Cd concentrations in oligochaetes used to prepare experimental meals increased over the range of dissolved Cd exposures from ~0.011 μg g−1 in controls to ~120.3 μg g−1 Cd (wet wt) in worms from the 7.04 μM Cd treatment (~11,000-fold increase) (Fig. 1). Tissue concentrations reported for oligochaetes Limnodrilus hoffmeisteri collected from Foundry Cove, New York were as high as 169 μg g−1 (wet wt) (Wallace et al. 2000). Consumption of prey (including Foundry Cove oligochaetes) with tissue Cd concentrations comparable to those estimated for meals in the present study resulted in impaired prey capture by grass shrimp (Wallace et al. 2000). The fraction of Cd associated with a subcellular compartment presumed to contain trophically-available metal was not characterized for oligochaetes as for invertebrate prey in previous work since Cd in homogenized tissues may have bound to other components during meal preparation (Seebaugh et al. 2006).
Elapsed time between Cd-contaminated food ingestion and data collection was subject to methodological constraints and varied from minutes (protease activities and gut pH) to 24 h (carbon AE). Previous work on metal assimilation by grass shrimp indicates that biphasic digestion and assimilation are completed by 48 h following pulse-feeding (Seebaugh and Wallace 2009). It may, however, be possible that toxicity from a pulse of ingested metal can result from assimilation prior to completion of a digestive cycle.
Homogeneity of 14C-labeled food ingestion was observed (Table 1). AE-14C% did not exhibit variation and was ~83% across Cd treatments, which is consistent with values reported elsewhere (Morgan 1980; Seebaugh and Wallace 2009) (Fig. 2). In previous work, Cd assimilation by shrimp was impacted by tissue concentrations in amphipod prey exposed to dissolved Cd concentrations as high as 4.54 μM (Seebaugh et al. 2006). Following extracellular digestion within the hepatopancreas, nutrients may be transported from the lumen across brush border surfaces of resorptive cells through diffusion or active transport or into blister cells via active transport or endocytosis (Al-Mohanna and Nott 1986; Verri et al. 2001; Vilella et al. 2003; Sousa et al. 2005). Available Cd2+ in gut fluid may be transported across apical surfaces via different pathways, including an electrogenic Na+/1H+ antiporter and transmembrane Ca2+ channels (Ahearn et al. 1994; Zilli et al. 2000). Differential impacts on carbon vs Cd assimilation were also observed in shrimp collected along a pollution gradient (Seebaugh and Wallace 2009).
Minimum GRT in grass shrimp was not influenced by ingestion of a pulse of Cd with food (Fig. 3). In other work, GRT was influenced by pre-exposure to dietary Cd for 15 days followed by 2 days depuration (Seebaugh 2010). These results suggest that impacts of Cd on transit time may necessitate metal incorporation into the hepatopancreatic epithelium, musculature, or perhaps tissues that regulate proventricular peristalsis (Maynard and Dando 1974; Al-Mohanna and Nott 1986). It is usually assumed that increased assimilation is associated with prolonged exposure of ingested materials to gut fluid as well as increased contact time between nutrients and absorptive surfaces within the gut (Lopez 2005). Relationships between assimilation and GRT may, however, be species- or element-dependent. In marine fish, Se and Zn AE were correlated with increasing GRT, but this relationship did not hold for ingested Cd (Zhang and Wang 2006). Cd assimilation from amphipod prey exposed to 0.025 μM Cd through solution was positively correlated with GRT in shrimp collected along an impact gradient, but relationships were not established for carbon or Hg (Seebaugh and Wallace 2009; Seebaugh et al. 2011).
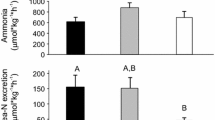
A dose-dependent decrease in FER was observed over the range of Cd exposures (Fig. 4). FER for shrimp that ingested meals containing tissues from worms exposed to 7.04 μM Cd (~6.4 mm h−1) was less than half the value for controls (~14.4 mm h−1). FER may be related to ingestion rate, although homogeneity of 14C- and IRDye-labeled casein meal consumption suggests that Cd-induced variability in this endpoint may be due to interference with feces packaging and transport. Materials rejected by particle sorting within the proventriculus as well as residual wastes and blister cells extruded from the hepatopancreas late in the digestive cycle are packaged in a chitinous peritrophic membrane secreted by the midgut epithelium (Forster 1953; Al-Mohanna and Nott 1986). This membrane may protect the gut from abrasion and provide separation between water entering the gut from anal drinking and soluble materials or feces (Lovett and Felder 1990a). Intestinal muscles generate peristaltic and antiperistaltic waves that transport materials within the midgut may also maintain hydraulic pressure required for expansion of the hepatopancreatic tubules as well as feces compaction (Lovett and Felder 1990a; Sousa and Petriella 2006). Mucopolysaccharides secreted into the midgut may also facilitate movement of feces toward the hindgut (Lovett and Felder 1990b; Sousa and Petriella 2006). Nunez-Nogueira et al. (2006) reported that assimilated Cd and Zn was incorporated into abdominal muscles of penaeid shrimp ~2–5.6 days after ingestion. To influence FER, any impacts of Cd on intestinal musculature or associated abdominal ganglia in grass shrimp would have presumably required incorporation of metal into tissues before digestion of Cd-contaminated meals was complete (Shuranova et al. 2006). In other work, FER was not impacted by pre-exposure to dietary Cd for 15 days (Seebaugh 2010). It seems, therefore, plausible that feces production or elimination by shrimp in the present study was impacted by direct exposure of mechanisms of peritrophic membrane or mucopolysaccharide secretion to Cd within the gut and not the result of post-assimilatory toxicity. Al-Mohanna and Nott (1986) noted that rates of feces production by decapods were not related to timing of the digestive cycle, which is dictated by processes within the hepatopancreas. FER for controls in the present study was up to 4.4× higher than for control shrimp in studies of metal pre-exposure (15 days feeding on oligochaetes) or shrimp collected from GK (a reference site), fed once on fish food and allowed to clear their guts for 3 days in site water (Seebaugh 2010; Seebaugh et al. 2011). Shrimp in the present study were maintained in clean, continuously filtered seawater and fed fish food for 3–5 days prior to clearing their guts for 2 d. This disparity in FER among controls and reference shrimp suggests that previous diet or acclimation conditions may impact feces packaging or transport.
Homogeneity of IRDye-labeled food ingestion by shrimp was observed among Cd treatments (Table 1). Mean changes in integrated intensity in the proventriculus and hepatopancreas were approximately linear from t = 20 and substrate exhaustion in controls was reached by 32 min (plots not shown). Protease activities increased ~2.4-fold over the range of dietary Cd treatments, but did not exhibit dose-dependency (Fig. 5). Casein hydrolysis rates were correlated with initial IRDye signals in shrimp that consumed meals containing tissues from the 1.76 μM Cd worm exposure (Table 1). Hydrolysis rates were not correlated with initial signals for other treatments or over the range of exposures. Chen and Mayer (1998) reported Cu inhibition of protease activities in polychaete gut fluid incubated with contaminated sediments. Pre-assimilatory impacts on proteases in live shrimp may have resulted from interactions between Cd2+ in gut fluid and apical fibrillar cell machinery involved in secretion or through direct impacts on enzymes (e.g., conformation or displacement of active site metal ions) circulating in gut fluid (Mitane et al. 1987; Stöcker et al. 1988; Vogt et al. 1989; Casalino et al. 2002). Casein hydrolysis rates in control shrimp were up to 4.9× slower than for controls pre-exposed to dietary metal or shrimp collected from GK in other work, indicating that diet or acclimation may also impact digestive enzymes (Seebaugh 2010; Seebaugh et al. 2011).
Gut pH varied between ~5.29 and ~6.01 and was not impacted by Cd ingestion (Table 2). In other work, pH of the posterior region of the cardiac chamber of the proventriculus was influenced by pre-exposure to dietary Cd or chronic exposure to impacted field conditions, while anterior and posterior region pH increased with pre-exposure to dietary Hg (Seebaugh 2010; Seebaugh et al. 2011). Anterior and posterior region pH did not vary for individual dietary Cd treatments (Mann–Whitney U: ns for all comparisons), indicating that mixing of food, enzymes and other components of the digestive milieu was rapid and thorough (Powell 1974; Vogt et al. 1989).
Casein hydrolysis rates (t = 20–32 min) for grass shrimp following consumption of a pulse of Cd in meals containing IRDye-labeled casein (n = 6–8; slopes of regressions on linear portions of curves representing mean increases in integrated intensity; plots not shown). Significant differences (p < 0.05) between dietary treatments (t test with Bonferroni correction) are indicated by different letters
Few studies have investigated impacts of ingested pollutants on decapod digestion in vivo, using non-invasive methods. The present work indicates that pre-assimilatory metal exposure can influence digestive physiology and that the mode of internal exposure should be considered during assessments of dietary pollutant toxicity. Future work will examine pre-assimilatory effects of ingested metal on the functional morphology of the hepatopancreatic epithelium in grass shrimp.
References
Ahearn GA, Zhuang Z, Duerr J, Pennington V (1994) Role of the invertebrate electrogenic 2Na+/1H+ antiporter in monovalent and divalent cation transport. J Exp Biol 196:319–335
Ahrens MJ, Hertz J, Lamoureux EM, Lopez GR, McElroy AE, Brownawell BJ (2001) The effect of body size on digestive chemistry and absorption efficiencies of food and sediment-bound organic contaminants in Nereis succinea (Polychaeta). J Exp Mar Biol Ecol 263:185–209
Al-Mohanna SY, Nott JA (1986) B-cells and digestion in the hepatopancreas of Penaeus semisulcatus (Crustacea: Decapoda). J Mar Biol Assoc UK 66:403–414
Campbell PGC, Clearwater SJ, Brown PB, Fisher NS, Hogstrand C, Lopez GR, Mayer LM, Meyer JS (2005) Digestive physiology, chemistry, and nutrition. In: Meyer JS, Adams WJ, Brix KV, Luoma SN, Mount DR, Stubblefield WA, Wood CM (eds) Toxicity of dietborne metals to aquatic organisms. Society of Environmental Toxicology and Chemistry, Pensacola, FL, p 13
Casalino E, Calzaretti G, Sblano C, Landriscina C (2002) Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology 179:37–50
Chen Z, Mayer LM (1998) Digestive proteases of the lugworm, Arenicola marina, inhibited by Cu from contaminated sediments. Environ Toxicol Chem 17:433–438
Forster GR (1953) Peritrophic membranes in the caridea (Crustacea Decapoda). J Mar Biol Assoc UK 32:315–318
Khoury JN, Powers E, Patniak P, Wallace WG (2009) Relating disparity in competitive foraging behavior between two populations of fiddler crabs to the subcellular partitioning of metals. Arch Environ Contam Toxicol 56:489–499
Lopez GR (2005) Diversity of structure and function of the digestive tract in aquatic invertebrates. In: Meyer JS, Adams WJ, Brix KV, Luoma SN, Mount DR, Stubblefield WA, Wood CM (eds) Toxicity of dietborne metals to aquatic organisms. Society of Environmental Toxicology and Chemistry, Pensacola, p 227
Lovett DL, Felder DL (1990a) Ontogeny of kinematics in the gut of the white shrimp Penaeus setiferus (Decapoda: Penaeidae). J Crustacean Biol 10:53–68
Lovett DL, Felder DL (1990b) Ontogenetic changes in enzyme distribution and midgut function in developmental stages of Penaeus setiferus (Crustacea, Decapoda, Penaeidae). Biol Bull 178:160–174
Mayer LM, Chen Z, Findlay RH, Fang J, Sampson S, Self RFL, Jumars PA, Quetel C, Donard OFX (1996) Bioavailability of sedimentary contaminants subject to deposit-feeder digestion. Environ Sci Technol 30:2641–2645
Maynard DM, Dando MR (1974) The structure of the stomatogastric neuromuscular system in Callinectes sapidus, Homarus americanus and Panulirus argus (Decapod Crustacea). Philos Trans R Soc Lond B Biol Sci 268:161–220
Mitane Y, Aoki Y, Suzuki KT (1987) Cadmium inhibits protein secretion from cultured rat liver parenchymal cells. Biochem Pharmacol 36:2647–2652
Morgan MD (1980) Grazing and predation of the grass shrimp Palaemonetes pugio. Limnol Oceanogr. Limnol Oceanogr 25:896–902
Murphy MR (1981) Analyzing and presenting pH data. J Dairy Sci 65:161–163
Nunez-Nogueira G, Rainbow PS, Smith BD (2006) Assimilation efficiency of zinc and cadmium in the decapod crustacean Penaeus indicus. J Exp Mar Biol Ecol 332:75–83
Perez MH, Wallace WG (2004) Differences in prey capture in grass shrimp, Palaemonetes pugio, collected along an environmental impact gradient. Arch Environ Contam Toxicol 46:81–89
Powell RR (1974) The functional morphology of the foreguts of the thalassinid crustaceans, Callinassa californiensis and Upogebia pugettensis. Univ Calif Pub Zool 102:1–41
Seebaugh DR (2010) Relationships between pollutant-induced digestive toxicity and the assimilation and subcellular partitioning of elements by grass shrimp Palaemonetes pugio. Ph.D. Dissertation. City University of New York, New York, NY
Seebaugh DR, Wallace WG (2009) Assimilation and subcellular partitioning of elements by grass shrimp collected along an impact gradient. Aquat Toxicol 93:107–115
Seebaugh DR, Estephan A, Wallace WG (2006) Relationship between cadmium assimilation by grass shrimp (Palaemonetes pugio) and trophically-available cadmium in amphipod (Gammarus lawrencianus) prey. Bull Environ Contam Toxicol 76:16–23
Seebaugh DR, L’Amoreaux WJ, Wallace WG (2011) Digestive toxicity in grass shrimp collected along an impact gradient. Aquat Toxicol 105:609–617
Shuranova ZP, Burmistrov YM, Strawn JR, Cooper RL (2006) Evidence for an autonomic nervous system in decapod crustaceans. Int J Zool Res 2:242–283
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. WH Freeman and Co, New York
Sousa L, Petriella AM (2006) Morphology and histology of P. argentinus (Crustacea, Decapoda, Caridea) digestive tract. Biocell 30:287–294
Sousa LG, Cuartas EI, Petriella AM (2005) Fine structural analysis of the epithelial cells in the hepatopancreas of Palaemonetes argentinus (Crustacea, Decapoda, Caridea) in intermoult. Biocell 29:25–31
Stöcker W, Wolz R, Zwilling R, Strydom DJ, Auld DS (1988) Astacus protease—a zinc metalloenzyme. Biochemistry 27:5026–5032
Verri T, Mandal A, Zilli L, Bossa D, Mandal PK, Ingrosso L, Zonno V, Vilella S, Ahearn GA, Storelli C (2001) d-glucose transport in decapod crustacean hepatopancreas. Comp Biochem Physiol A 130:585–606
Vilella S, Zilli L, Ingrosso L, Schiavone R, Zonno V, Verri T, Storelli C (2003) Differential expression of Na+/d-glucose cotransport in isolated cells of Marsupenaeus japonicus hepatopancreas. J Comp Physiol B 173:679–686
Vogt G, Stöcker W, Storch V, Zwilling R (1989) Biosynthesis of Astacus protease, a digestive enzyme from crayfish. Histochemistry 91:373–381
Wallace WG, Hoexum Brouwer TM, Brouwer M, Lopez GR (2000) Alterations in prey capture and induction of metallothioneins in grass shrimp fed cadmium-contaminated prey. Environ Toxicol Chem 19:962–971
Zhang L, Wang W-X (2006) Significance of subcellular metal distribution in prey in influencing the trophic transfer of metals in a marine fish. Limnol Oceangr 51:2008–2017
Zilli L, Marsigliante S, Zonno V, Verri T, Ahearn GA, Storelli C, Vilella S (2000) Identification of calcium channels in B cells of Penaeus japonicus hepatopancreas. Comp Biochem Physiol A 126:S154
Acknowledgments
This research was supported by PSC-CUNY Research Award #62888-00 40 to W.G. Wallace and CUNY Collaborative Incentive Research Grant #80209-01 14 to G.M. Stewart and W.G. Wallace. Shrimp were collected from Great Kills Harbor under permit from the Division of Natural Resources, National Park Service. Funding for the confocal microscope was provided by National Science Foundation grant DBI 0421046 to W.J. L’Amoreaux. The authors thank the associate editor and an anonymous reviewer for valuable comments on a previous version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seebaugh, D.R., Wallace, W.G., L’Amoreaux, W.J. et al. Carbon Assimilation and Digestive Toxicity in Naïve Grass Shrimp (Palaemonetes pugio) Exposed to Dietary Cadmium. Bull Environ Contam Toxicol 88, 449–455 (2012). https://doi.org/10.1007/s00128-011-0493-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0493-7