Abstract
A modified method for the analysis of monosultap residue in rice plant and environment was developed and validated. Monosultap residue dynamics and final residues in supervised field trials at GAP conditions were studied. At fortification levels of 0.05, 0.5 and 1 mg kg−1, it was shown that recoveries ranged from 75.0% to 109.2% with RSDs of 1.2–5.1% (n = 5). The dissipation experiments showed the half-lives (T1/2) of monosultap in water, soil and rice plants were 1.1–1.9, 1.4–2.1 and 1.3–2.1 days, respectively. At pre-harvest intervals (PHI) of 21 and 30 days, monosultap residue were 0.01–0.06 mg kg−1 in soil, 0.01–0.19 mg kg−1 in rice plants, and 0.01–0.09 mg kg−1 in husked rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Rice is a widely cultivated crop in China. As rice production around the world is being affected by pests and diseases, many kinds of pesticides were applied to protect it. Monosultap, 2-N, N-dimethylamino-1-thiosulphate-3-(sodium thiosulphate) propane, with CAS number of [52207-48-4], is a nereistoxin (NTX) derivatives insecticidal substance. Nereistoxin is generally stomach poisons with some contact action and often shows some systemic action, and tends to be selectively active on Lepidoptera, Coleoptera and diptera insect pests. Few analytical methods have been reported for the determination of monosultap analysis. The analysis of nereistoxin analogues were based on the conversion of the parent compound to nereistoxin, which was then measured by gas chromatography. A GC-FPD method for determination of monosultap residue in tomato and soil has been reported (Tao et al. 2007). Nereistoxin and some of its metabolites have been analyzed in human serum using headspace solid-phase microextraction and GC–MS (Namera et al. 1999). Some studies used HPLC detection in the analysis of nereistoxin analogues were developed (Fisher et al. 1993), sometimes even without making use of clean-up treatments. Several publications have reported the analysis of thiocyclam in vegetables. A LC–MS/MS method in multiclass vegetables after QuEChERS extraction has been reported (Kmellár et al. 2008). Thiocyclam has also been determined by LC–MS/MS in olive oil for high oil content commodities (Hernando et al. 2007). Mol et al. (2007) used a similar approach ethyl acetate extraction method and LC–MS/MS determination.
There has been no maximum residue limit (MRL) of monosultap set by FAO/WHO yet (Regulation EC 396/2005). However, in China, the MRL of monosultap is set at 0.5 mg kg−1 in rice. There has been increased awareness in recent years about the residues pesticide in crops and environment matrices where risk they may pose has to be controlled. Little work has been found in the literature on the residue analysis of monosultap in rice and the environmental samples. In this work, a modified GC–MS method was established to detect the residue of monosultap. A field study was designed to investigate the dissipation dynamics and final residues of monosultap in rice plant and environment. The present work aimed at ensuring the scientific application of monosultap formulation in rice and contributing these residue data to the risk assessment and to provide guidance on the proper and safe use.
Materials and Methods
Monosultap standards (99.0%) and the formulations (20% Monosultap Abamectin ME) were supplied from China Agricultural University. Toluene of HPLC grade was procured from Fisher Chemicals (USA). Deionized water was obtained from a Milli-Q water purification system (Millipore, USA). Hydrochloric acid (37%), sodium hydroxide, sodium sulfide and anhydrous sodium sulfate (98%) of analytical grade were purchased from Sinopharm Chemical Reagent (Beijing, China).
An Agilent 6890N GC/5973 MS was used to analyze the monosultap. Centrifugation was performed in two different instruments: an Anke TDL-40B centrifuge equipped with a bucket rotor (4 × 100 mL) (Shanghai, China). A water bath kettle (Medical Equipment Factory of Shanghai Medical Instruments Co., Ltd) was used for derivatizing reaction. BS1100S analytical balance (Sartorius, Germany), IKA A11-basic grinder (IKA, Germany), HAZ-C shaker (Donglian Electron Technology Co., Ltd., Harbin, China) and QL-901 Vortex (Kylin-bell Lab Instruments Co., Ltd, Jiangsu, China) were used for preparing the samples.
Field experiments including the residue dynamic and final residue in supervised field trials designed according to the pesticide label (already registered by Chinese government) recommendations were conducted in Beijing and Hunan according to ‘the Guideline for Pesticide Residue Field Experiment’ issued by the Institute of the Control of Agrochemicals, Ministry of Agriculture, the People’s Republic of China. A number of field plots each with 30 m2 area were prepared; 1 m distance was used as a buffer area to separate each plot in the same field.
To investigate the dissipation of monosultap in water, soil and rice plant, rice field were sprayed with the commercial formulations once in the experiments plots each with three replicates. The dosage was 360 g ha−1. Representative samples were randomly collected at intervals of 0 (2 h post treatment), 12 h, 1, 2, 3, 4, 5 and 7 days after application. The final residue experiments were performed with two dosage levels, 180 g ha−1 (low concentration, recommended dosage from the label use) and 360 g ha−1 (high concentration, 2 times of the recommended dosage). Both low and high levels were sprayed 1 time and 2 times with an interval of 7 days between each application. A plot with the same size but on monosultap application was compared simultaneously. Three replicates were carried out for each treatment. Representative soil, rice plant and husked rice samples were randomly collected at PHI of 21 and 30 days from each plot. All samples were stored at −20°C until analyzed.
200 g samples were obtained from 1 kg samples collected from trial field by concentrating them using the quartation method. Soil samples were dried in the air under shade at 25 ± 5°C and screened through 40 mesh sieves. Water sample was collected in the plastic bottles and filtered through a Buchner funnel. Husked rice was ground to powder. Rice plant samples were cut into small pieces using a mechanical slicer.
5 g previously homogenized samples were placed in a 50 mL centrifuge tube and 30 mL 0.2 M HCl was added. The centrifuge tube was capped and agitated on a shaker for 30 min, and centrifuged for 5 min at 3,800 r min−1. Then 20 mL 0.2 M HCl was added in sample scraps for extracting the second time. The supernatants were combined.
The pH of the supernatants was adjusted to pH 8.5–9.0 with 0.2 M NaOH, and 1 mL 0.2 mol L−1 Na2S was also added as catalyzer. The mixture was then held for 2.5 h in a water bath at 70°C for derivatization. After derivatizated, the mixture was then cooled down to room temperature and 5 mL toluene was added. The mixture was mixed vigorously by vortexing for 1 min and centrifuge extracted for 5 min at 3,800 r min−1. After separated and dried by anhydrous sodium sulfate, the toluene layer was filtered through a 0.22 μm filter membrane and transferred into autosampler vial for GC–MS analysis.
An Agilent 5890NGC/5973MS (Agilent Technologies, USA), equipped with a HP-5 capillary column, 30 m × 0.32 mm, 0.25 μm, was used for the analysis. The column temperature was set at 60°C for 1 min, and then it was programmed to heat from 60 to 180°C at 20°C min−1 (hold 1 min), from 180 to 280°C at 15°C min−1 (hold 10 min). The temperature of the injection was set at 250°C. Splitless injection mode was used. The carrier gas was helium, and its flow rate was set at 1 mL min−1. The mass spectrometer was operated in electron ionization mode with impact ionization voltage 70 eV, the trap temperature of 230°C and the quadrupoles temperature of 150°C. The acquisition was performed in selected ion monitoring. The ionization and dissociation rules were shown in Fig. 1. The transition m/z70, m/z103 and m/z149 were chosen for quantification and confirmation.
Results and Discussion
The linearity of water, soil, rice plant and husked rice samples was studied in the range of 0.02–2 mg L−1 with five calibration points (0.02, 0.05, 0.5, 1 and 2 mg L−1) by matrix-matched standard calibrations method. Linear calibration graphs were constructed by least-squares regression of concentration versus peak area of calibration standards. Linearity values of water, soil, rice plant and husked rice samples, calculated as determination coefficients (R2), were 1, 0.999, 1 and 0.999, respectively. Quantification was accomplished using the standard curve constructed by plotting analyte concentrations against peak areas. The limits of detection (LOD) of monosultap in water, soil, rice plant and husked rice were all 0.003 mg kg−1, at a signal-to-noise (S/N) ratio of 3. The limits of quantification (LOQ) were 0.01 mg kg−1 at a signal-to-noise ratio of 10.
The efficiency of the method has been evaluated by spiking blank water, soil, rice plant and husked rice samples with a corresponding volume of monosultap working solution at three different levels (0.05, 0.5 and 1 mg kg−1). Five samples of each concentration were processed. The mean recoveries from all fortified samples were in the range of 75.0%–109.2%. The relative standard deviation (RSD) ranged from 1.2% to 5.1% and suggested that extraction and clean-up procedure could be considered suitable for routine analysis of monosultap in experimental matrices. The recovery and relative standard deviation are shown in Table 1.
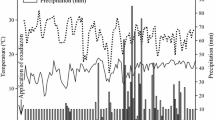
The proposed methodology was applied to a dissipation study of the monosultap after its application in an experimental field. The dissipation curve of monosultap in water, soil and rice plant under field conditions was showed in Fig. 2. Half-life and other statistical parameters of monosultap residue dissipation were calculated from the experimental data and summarized in Table 2. The initial residue level of monosultap in water, soil and rice plant collected from two different geographic zones were in the range 2.57–3.20, 0.45–0.89 and 3.06–5.46 mg kg−1, respectively. As expected, a gradual and continuous decrease of the pesticide residues in the treated plants was observed as a function of time. The results showed that monosultap dissipated faster in water, soil and rice plants, the average half-lives were 1.5, 1.8 and 1.5 days, respectively. It was seen that more than 50% of the initial residues of monosultap had dissipated in both plants and soil within 2 days. About 90% of the residues had dissipated by the 7th day after the treatment. Initial deposits of monosultap in plants and soil differed among the two experimental sites. The results in the study indicated that monosultap disappear rapidly in rice plant and field under natural conditions and exhibited a first-order kinetics dissipation. Usually, the degradation of pesticides in the plant besides the effect of some physical and chemical factors like light, heat, pH and moisture, growth dilution factor might have played a significant role (Dhananjay et al. 2005). Dissipation of monosultap was faster in Hunan than in Beijing. It might be affected by some physical and chemical factors, growth dilution factor, soil characteristics and microorganisms.
The final residue of monosultap in soil, rice plant and husked rice samples collected from the treated plots are summarized in Table 3. At PHI of 21 and 30 days, monosultap residue were 0.01–0.06 mg kg−1 in soil, 0.01–0.19 mg kg−1 in rice plants, 0.01–0.09 mg kg−1 in husked rice, respectively. In the final residue experiment, there was only a trace of monosultap residue in the soil, rice plants and husked rice. It showed that when monosultap was used under the designed experiment, there was no significant pesticide residue in rice, which indicates safe use and consumption of monosultap on rice.
There is no available MRLs value for monosultap in rice or other commodity in USA, EU and CAC. However, the MRL of monosultap in rice was set at 0.5 mg kg−1. The acceptable daily intake (ADI) for monosultap has not been established yet. In China, 20% Monosultap Abamectin ME is registered for use on rice at 180 g ha−1 and spray one time with PHI of 21 days. In twelve trials that followed the above GAP conditions conducted (three repetition every zone) in China, the concentrations of monosultap residues were below 0.09 mg kg−1 in all husked rice samples. STMR value may be assumed to be 0.01 mg kg−1. The dietary intake of rice grain is in the range 250–400 g every day reference from dietary guideline published by Health Ministry of the People’s Republic of China (Keyou et al. 2007). Based on the Chinese food consumption data and the worst case scenario, estimated daily intake of monosultap in rice is 0.0001 mg/kg bw (assuming that average body weight of Chinese adult as 60 kg).
When 20% Monosultap Abamectin ME was applied at GAP coditions, the results shows that there is no significant potential health risk induced by monosultap consume in husked rice in China. Therefore, monosultap could be considered as a good alternative to high-toxicity pesticides in China, and can be used in rice fields safely.
References
Dhananjay KT, Vipin K, Ravindranath SD, Adarsh S (2005) Dissipation behavior of bifenthrin residues in tea and its brew. Food Control 16:231–237
Fisher DH, Xie Y, Loring R (1993) Analysis of nereistoxin using HPLC and electrochemical detection. Anal Lett 26:1051–1063
Ge K, Jia JB, Liu H (2007) Food-based dietary guidelines in China practices and problems. Ann Nutr Metab 51(Suppl 2):26–31
Hernando MD, Ferrer C, Ulaszewska M, García-Reyes JF, Molina-Díaz A, Fernández-Alba AR (2007) Application of high-performance liquid chromatography-tandem mass spectrometry with a quadrupole/linear ion trap instrument for the analysis of pesticide residues in olive oil. Anal Bioanal Chem 389:1815–1831
Kmellár B, Fodor P, Pareja L, Ferrer C, Martínez-Uroz MA, Valverde A, Fernandez-Alba AR (2008) Validation and uncertainty study of a comprehensive list of 160 pesticide residues in multi-class vegetables by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1215:37–50
Mol HG, Rooseboom A, van Dam R, Roding M, Arondeus K, Sunarto S (2007) Modification and re-validation of the ethyl acetate-based multi-residue method for pesticides in produce. Anal Bioanal Chem 389:1715–1754
Namera A, Watanabe T, Yashiki M, Kojima T, Urabe T (1999) Simple and sensitive analysis of nereistoxin and its metabolites in human serum using headspace solid-phase microextraction and gas chromatography-mass spectrometry. J Chromatogr Sci 37:77–82
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC Text with EEA relevance, Brussels (2005)
Tao CJ, Hu JY, Li JZ (2007) Determination of insecticide monosultap residues in tomato and soil by capillary gas chromatography with flame photometric detection. Canadian J Anal Sci Spectrosc 52:295–304
Acknowledgments
This study was funded by Program for Science Research for the 11th Five-year Plan (No. 2009BADB7B03-6), People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, F., Wang, L., Zhou, L. et al. Dissipation and Residues of Monosultap in Rice Plant and Environment. Bull Environ Contam Toxicol 88, 362–367 (2012). https://doi.org/10.1007/s00128-011-0437-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0437-2