Abstract
The degradation of Alizarin Yellow dye is studied here using the laser-induced photo-catalysis process in the presence of a zinc doped tungsten oxide catalyst. For optimization of the photo-catalytic process, the following parameters were investigated: dye concentration, laser irradiation time and incident laser energy. The calculated value of the reaction rate was found to be of a high value (k) = 0.197 (with estimated half-life of 6.5 min). This high value of k indicates the efficiency of this method in removing Alizarin Yellow dye from wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
A large amount of organic waste is produced worldwide in different forms as the result of utilization of many organic products (dyes, phenols, benzene, etc.) in the manufacturing industry. Organic dyes used in many applications in the textile industry, leather industry, paints and in other manufacturing processes. The widespread contamination of water by these organic compounds is currently recognized as an issue of great concern. Organic dyes are carcinogenic to humans and are a considerable health concern, even at low (0.001 mg/L) concentrations (Slein and Sansone 1980). Hence a solution to the treatment of wastewater containing organic matter like dyes is highly desirable. It is estimated that 1.3 billion people have no access to clean drinking water in developing countries (WHO/UNICEF, Report. Global water supply 2000).
Numerous conventional methods are available for the removal of organic contaminants such as dyes from wastewater include microbial degradation, adsorption on activated carbon (Brandt et al. 1997), biosorption (Aksu and Yener 1998), chemical oxidation (using agents such as ozone, hydrogen peroxide, and chlorine), deep-well injection, incineration, solvent extraction and irradiation (Bertoncini et al. 2003; Khalid et al. 2004; Denizli et al. 2005; Munaf et al. 1997). These methods have advantages and disadvantages. For example, the high cost of activated carbon, solvent extraction and oxidation treatments have stimulated interest to use cheaper methods for wastewater treatment. Due to the limitations and drawbacks of the above mentioned methods, alternative techniques have to be developed. The Advanced Oxidation Process (AOP) is one of the emerging technologies (Fox and Dulay 1993) that is capable of converting organic pollutants into harmless products such as carbon dioxide, water and mineral salts (Li et al. 2006; Gondal et al. 2008). This method is therefore beneficial as it does not require any further separation of the by-products in an aqueous solution. In the AOP process, very active species, e.g. hydroxyl radicals (OH*) which react with organic contaminants and other oxidizable components are generated (Fox and Dulay 1993; Kamat 1993; Gondal et al. 2008).
Heterogeneous photo-catalysis (Fox and Dulay 1993; Gondal et al. 2009a, b) is a branch of AOP where a semiconductor catalyst under UV (ultraviolet) irradiation is highly successful in the mineralization of many organic pollutants such as dyes. In this paper, the removal of Alizarin Yellow Dye is investigated by heterogeneous laser-induced photo-catalysis which uses zinc doped tungsten oxide (WO3) as a semiconductor catalyst.
Materials and Methods
Zinc doped tungsten oxide was prepared using the impregnation method as described in Hameed et al. (2004). Prior to use for photo-catalytic reaction studies, Zn doped tungsten oxide was heated to 500°C with the temperature rising at the rate of 10°C/min and it was kept at 500°C for another 7 h to ensure the complete removal of impurities and to preserve the moisture content. A standard solution of a concentration of 100 ppm of phenol was prepared using deionized water for the investigation of activity of zinc doped catalyst for the degradation of Alizarin.
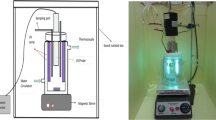
For the irradiation of 100 ppm Alizarin Yellow solution in water, A 355 nm wavelength high power laser beam generated from the third harmonic of the Spectra Physics Nd: YAG laser (Model GCR 250), with a pulse width of ~8 ns was employed. The important parameters which significantly affect the removal process are: laser energy, dye concentration, laser irradiation time, stirring rate and laser beam diameter. The parameters i.e. stirring rate and laser beam diameter were kept constant for all studies while the other parameters i.e. laser energy, dye concentration and amount of catalyst were carefully optimized and investigated in this work. The destructive effect of the focused laser beam was minimized by expanding the diameter of the laser beam to 1 cm by using lenses and mirrors.
An optimized amount (300 mg) of zinc oxide catalyst powder was suspended in 80 mL of deionized water at 100 ppm Alizarin Yellow solution. The photo-catalytic degradation of Alizarin occurred in a batch reactor and the removal of Alizarin was investigated by suspending an optimized amount (300 mg) of the zinc oxide semiconductor powder in 80 mL of de-ionized water. Argon was used as the purge gas to remove dissolved gases. A UV/VIS spectrophotometer was used to measure the UV-VIS spectra of the Alizarin solutions after laser irradiation at regular time intervals of 5 min. The pH changes in the solution during laser irradiation were measured using a pH meter calibrated using the buffers of pH 4, 7 and 10. The concentration of the phenol was also estimated by analyzing the laser exposed samples at regular interval of 10 min by using a gas chromatograph and UV spectrometer.
Results and Discussion
Most of the earlier work on the removal of organic contaminates from water using heterogeneous photo-catalysis was carried out with custom made setups using broad spectral lamps and a TiO2 semiconductor catalyst (Fox and Dulay 1993; Pichat 2003). Heterogeneous photo-catalysis is still in the developing stage and several aspects of the photo-catalytic process are yet to be investigated. The two basic inherited problems associated with the conventional setups are long term reaction time and low photonic efficiencies. Once these challenges are overcome, heterogeneous photo-catalysis can be commercialized as an economic and valuable technique for the synthesis of chemical compounds and to degrade toxic air and water pollutants.
In this work, the above mentioned problems associated with heterogeneous photo-catalysis processes are addressed by modifying the existing conventional setups being used to study the photo-catalytic processes. Instead of using a conventional lamp based photo-catalytic reactor, a simple reactor free of all logistics, such as heating, cooling and evacuating assemblies, was designed. The main problem in using the UV lamps is their inherited defect in delivering a constant output power over longer periods of time. The UV lamps warm up very quickly and their output power drops after a few hours of operation. In addition, UV lamps emit over a broad spectral range and most of their spectrum lies outside the absorption band of the photo-catalyst. In order to overcome these drawbacks, a monochromatic source like lasers have been applied in this work to achieve high photonic efficiencies. The use of coherent and monochromatic source like a laser is mainly to enhance the photo-catalytic activity of semiconductor photo-catalysts for the degradation process.
Figure 1 shows the pattern of removal of Alizarin Yellow at different laser irradiation times. The amount of Alizarin Yellow removed by the photocatalytic process was estimated by a UV absorption spectrophotometer. It is noticed that as the Alizarin concentration decreases, absorbance decreases, indicating the removal of the dye. The percentage degradation, estimated from UV analysis as presented in Fig. 1, versus the laser irradiation time is plotted in Fig. 2.
Figure 3 depicts the dependence of dye removal on incident laser energy. Increasing laser energy, more photons were generated which activated more catalyst particles. Activation of catalyst particles leads to the generation of more active species, which resulting in an increase in degradation of Alizarin dye molecules.
The relation between the percentage of the degradation of the dye and its concentration is shown in Fig. 4. It is clear from this figure that an increase in the concentration of the dye will decrease the percentage of the amount being degraded. It was also noted that the percentage of the amount of the dye degraded is affected by the concentration of the catalyst used. This means that extra catalyst excitation sites are required to excite more dye molecules. Therefore, the concentration of the catalyst was increased from the value of 300 mg in 100 mL, considered as optimum, to a value of 400 mg at which 100% mineralization of dye happened.
In order to calculate the rate of removal of Alizarin dye (or the reaction rate), the experimental data on Alizarin dye removal obtained from UV spectrometry at various laser irradiation times was fitted to pseudo-first order kinetics:
where C 0 and C t are the concentrations at the beginning of the reaction and any time t, respectively where k obs is the first order reaction rate. Solving Eq. 1, we get
By plotting the −ln (C t /C o ) versus time, one can get the value of k obs. A plot of −ln C t /C 0 versus time for our data is depicted in Fig. 5. A k obs value of 0.197 min−1 was estimated from the slope of −ln C t /C 0 versus time plot (Fig. 5). Accordingly, the photo-catalytic degradation of Alizarin Yellow follows the first order kinetics and has a large reaction rate.
In summary, laser induced photocatalytic removal of Alizarin Yellow dye was investigated using a zinc doped tungsten oxide catalyst. Parametric dependence demonstrated that dye removal strongly depends on incident laser energy, catalyst concentration and laser irradiation time. A high removal rate around 0.19 mM/min was estimated using first order kinetics.
References
Aksu Z, Yener J (1998) Investigation of the biosorption of phenol and monochlorinated phenols on the dried activated sludge. Proc Biochem 33:649–655
Bertoncini C, Raffaelli J, Fassino L, Odetti HS, Bottani EJ (2003) Phenol adsorption on porous and non-porous carbons. Carbon 41:1101–1111
Brandt S, Zeng A, Deckwer WD (1997) Adsorption and desorption of pentachlorophenol on cells of Mycobacterium chlorophenolicum PCP-1. Biotechnol Bioeng 55:480–489
Denizli A, Cihangir N, Tüzmen N, Alsancak G (2005) Removal of chlorophenols from aquatic systems using the dried and dead fungus Pleurotus sajor caju. Bioresour Technol 96:59–62
Fox MA, Dulay MT (1993) Heterogeneous photocatalysis. Chem Rev 93:341–357
Gondal MA, Sayeed MN, Seddighi Z (2008) Laser enhanced photo-catalytic removal of phenol from water using P-Type NiO semiconductor catalyst. J Hazard Mater 155:83–89
Gondal MA, Sayeed MN, Yamani ZH, Arfaj A (2009a) Efficient removal of phenol from water using fe2o3 semiconductor catalyst under uv laser irradiation. J Environ Sci Heal A44:515–521
Gondal MA, Dastageer A, Khalil A (2009b) Synthesis of Nano-WO3 and its catalytic activity for enhanced antimicrobial process for water purification using laser induced photo-catalysis. Catal Comm 11:211–219
Hameed A, Gondal MA, Yamani ZH (2004) Effect of transition metal doping on photocatalytic activity of WO3 under laser illumination: role of 3d-orbitals. Catal Comm 5:715–719
Kamat PV (1993) Photochemistry on nonreactive and reactive (semiconductor) surfaces. Chem Rev 93:267–301
Khalid M, Joly G, Renaud A, Magnoux P (2004) Removal of phenol from water by adsorption using zeolites. Ind Eng Chem Res 43:5275–5280
Li L, Zhu W, Zhang P, Zhang Q, Zhang Z (2006) TiO2/UV/O3-BAC processes for removing refractory and hazardous pollutants in raw water. J Hazard Mater 128:145–149
Munaf E, Zein R, Kurniadi R, Kurniadi I (1997) The use of rice husk for removal of phenol from waste water as studied using 4-aminoantipyrine spectrophotometeric method. Environ Technol 18:355–358
Pichat P (2003) Photocatalytic degradation of pollutants in water and air: basic concepts and applications. In: Tarr MA (ed) Chemical degradation methods for wastes and pollutants: environmental and industrial applications. Marcel Dekker, New York, pp 77–119
Slein MW, Sansone EB (1980) Degradation of chemical carcinogens. New York, Van Nostrand Reinhold
WHO/UNICEF, Report. Global water supply and sanitation assessment report. New York and Geneva, 2000
Acknowledgment
The author greatly appreciates the scientific cooperation with Prof. M. A. Gondal, Laser Research Laboratory, Physics Department, King Fahd University of Petroleum & Minerals, Dhahran, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seddigi, Z.S. Removal of Alizarin Yellow Dye from Water Using Zinc Doped WO3 Catalyst. Bull Environ Contam Toxicol 84, 564–567 (2010). https://doi.org/10.1007/s00128-010-9995-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-010-9995-y