Abstract
Total mercury levels were quantified in Tilapia mossambicus, Cirrhinus mrigela and Labio rohita, captured from East Calcutta Wetlands and Titagarh sewage fed aquaculture ponds. The bioconcentration factor of collected fish was assessed. Total mercury level ranged from 0.073 to 0.94 μg/g in both pre and post monsoon season. T. mossambicus in both season and C. mrigela at pre monsoon, cross the Indian recommended maximum limit (0.50 μg/g wet weight) for food consumption and according to World Health Organization guidelines all fish were not recommended for pregnant women and individuals under 15 years ages. A significant correlation was observed between mercury content of aquaculture pond water and fish muscle tissue. Total mercury concentration in experimental sites were higher than the control area (Wilcoxon Ranked-Sum test p > 0.05), which suggested the connection between mercury bioaccumulation and sewage fed aquaculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury is one of the major toxic elements, contaminating in the environment by natural as well as anthropogenic activities and accumulating in the biota. Mercury pollution in aquatic ecosystems has received great attention since the discovery of mercury as the cause of Minamata disease in Japan in the 1950s. Large quantity of mercury is being released in the environment and is washed into aquatic systems, where it is biologically converted into methylmercury and accumulates in aquatic organisms. Fish accumulate mercury directly from their food and the surrounding water (Rainbow 1985). Mercury speciation involves specific microbes, which subsequently concentrate mercury through the food chain and accumulate in the tissue of fish and aquatic animals (Altindag and Yigit 2005). Mercury is a well know neurotoxic compound (Chang 1977; Clarkson 1987; Louria 1992) which is listed by the International Program of Chemical Safety (IPCS) as one of the six most dangerous chemicals in the world’s environment (IRIS-Integrated Risk Information System 1993). Mercury bioaccumulation in fish was found in river contaminated by chlor-alkali industry in Cuba (De La Rosa et al. 2009). Fresh water fish found in Ghana and South Africa accumulated mercury ranging from 50 to 510 ng/g wet weight (Campbell et al. 2003). The general population is primarily exposed to mercury through consumption of contaminated fish. Fish serve as a valuable and nutritious component and rich protein source of the Bengali diet (Darnton-Hill et al. 1988). Mercury through this important food sources are major threats to human health. People who consume significant amounts of contaminated fish may be at risk of mercury toxicity. Fish are also important indicators of mercury contamination in surface water through municipal waste and industrial effluents (Agarwal et al. 2007).
Kolkata, the capital of the State West Bengal, India is one of the major commercial and industrial cities of Eastern India. The city dose not have any conventional effluent treatment plant. Wastewater recycle zone of East Calcutta wetlands (88°20′–88°35′E; 22°25′–22°40′N) acts as effluent treatment plants of Kolkata, reduce high BOD load of the sewage through the existing microorganisms and algae present in the shallow water bodies of the area. Like this system Titagarh municipality (22°44′11″N; 88°22′25″E) also culture fish in the maturation ponds of their wastewater lagoon system. Both of the sewage fed fisheries supply fresh water fish to market of Kolkata and suburb. The Titagarh area is also an old industrial area having a big thermal power station and a chlor-alkali plant.
The objective of the present paper was to investigate mercury accumulation level of some common fish consumed by the local people and also to identify the probable sources of mercury contamination and their potential human health risk.
Materials and Methods
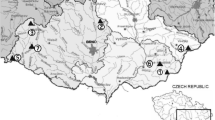
Twenty-five individuals of Tilapia mossambicus, Cirrhinus mrigela and Labio rohita were collected randomly from two sampling sites (Fig. 1) in both pre and post monsoon seasons of 2007 for determining total mercury level in fish muscle tissues. Site 1 and Site 2 were East Calcutta Wetlands and Titagarh sewage fed aquaculture ponds respectively. Candipur village of Notrh 24 Parganas (Fig. 1) was selected as control sites as because no domestic sewage and industrial effluents contaminate the aquaculture ponds of this areas. The fish were collected directly from the fisherman and dissected on a polyethylene dissection board using a clean stainless steel scalpel. The muscle tissues were removed and washed with deionized water. The tissues was dried at 50–55°C in hot air oven, homogenized to a fine powder, and sealed in a desiccator with a silica gel desiccant before total mercury analysis. Wet weight of the samples was calculated from dry weight assuming that the tissues contain 80% water (Zhang et al. 2007). About 0.5–1 g of samples was taken in a round bottom flask, in which 10–12 ml of concentrated nitric acid and 1–2 ml of sulfuric acid was added for digestion through a Bethge’s apparatus. The mercury was determined by a cold-vapour atomic absoption spectrophotometer (Mercury Analyzer-5840, ECIL, detection limit 0.1 μg/l) at 253.6 nm using 20% SnCl2 and 10% HNO3 (APHA, 1995). Relative standard solution was run with the samples simultaneously to check the precision of the instrument. A quantitative recovery of standard used during analysis was 95%. The detection limit of this method is 0.1 ppb. Accuracy and precision data were found to be ±5 μg/l. Pond water sample was collected in a sterile glass bottle with addition of HNO3. Before analysis of mercury, the water sample was centrifuged and filtered through Whatman No. 42 filter paper.
Bio-concentration factors of the tissue were calculated using the ratio of the tissue mercury concentration (μg/g wet weight) and the mercury concentration (μg/ml) where they grew. Log values of bio-concentration factors were plotted for preparing the graph. The data of total mercury level of pond water was used to estimate bio-concentration factors only; the data was not shown in the current communication.
The Wilcoxon Ranked-Sum test was used to determine the statistical significance of differences (p > 0.05) between total mercury level in the muscle at test and control sites. All data were tested for normality using the Shapiro–Wilk test prior to analysis. Minitab 13 and SPSS 11 software was used for the statistical analysis.
Results and Discussion
Mercury concentration in collected fish ranged from to 0.073 to 0.94 μg/g wet weight are presented in Table 1. Lower accumulation was observed in all tested species collected from control areas and the highest mercury accumulation was found in East Calcutta Wetlands areas. T. mossambicus was the highest accumulator of mercury followed by C. mrigela and L. rohita. All tested samples accumulated more mercury in pre-monsoon than the post monsoon season. The highest seasonal variation was observed in L. rohita (46%) followed by C. mrigela and T. mossambicus at Site-1, where as total opposite result was found in Site-2 (Fig. 2). Like present observation, Sinha et al. (2007) observed that total mercury accumulation in fish from the River Ganges at Varanasi was higher in pre-monsoon ranging from 0.205 to 4.369 μg/g. Our data resembles with the observation of Altindag and Yigit (2005), Carvalho et al. (2005) and Gammons et al. (2006) where mercury concentration of fish varied from 0.012 to 2.74 μg/g (wet weight). The total mercury level of the present study was higher than the fish collected from the River Gomoti, India (Agarwal et al. 2007). Paul (1987) also reported that total mercury level of 10 common edible fish species from the Stanley reservoir at Tamil Nadu ranging from 0.135 to 0.200 μg/g. These data is also less than the range of total mercury level observed in our study.
The highest bio-concentration factor was observed in T. mossambicus followed by C. mrigela and L. rohita. Same pattern of bioaccumulation was observed in both the seasons at all study areas (Fig. 3). Mercury biologically magnifies in aquatic ecosystem and accumulates in higher concentration in top level consumer of the food chain (Morel et al. 1998). Sinha et al. (2007) found that bio-concentration factors of fish collected from the River Ganges was 3–9 times more than that of the reference fish samples.
Mercury concentration in T. mossambicus in all season and C. mrigela in pre monsoon season had cross permissible level for human consumption (0.23–0.47 μg/g of wet weight of fish/day) recommended by World Health Organization (World Health Organization 1990), regulatory bodies of United Kingdom, and the United States (Gammons et al. 2006). The values also exceed the maximum residue limit of Prevention of Food Adulteration Act, India (PFA Act 1954, amended in 2002) (Fig. 4). Consumption of all the collected fish samples was vulnerable for pregnant women, individuals under 15 years or frequent fish consumers (World Health Organization 1990). L. rohita was supposed to be safe fish for human consumption for both the seasons in all experimental sites.
All the fish captured from the Site 1 and Site 2 accumulated more mercury in the muscle tissue than that of the control Site. After analysis of Wilcoxon Ranked-Sum test, it could be inferred that fish from industrial wastewater and domestic sewage fed fisheries produce significant difference with the aquaculture practices in the rural areas (p > 0.05). Our observations were comparable with the work of Zhang et al. (2007) where sewage fed aquaculture impoundment was the major cause for significant difference of mercury accumulation in fish tissues.
Our data however indicate that consumption of the fish collected from two sewage fed aquaculture farms, may impose the risk of mercury toxicity among the regular consumers though no incident of mercury toxicity had been reported from areas till now. This study may help in the follow up investigation and also help to prepare suitable policy for the consumers.
References
Agarwal R, Kumar R, Behari JR (2007) Mercury and lead content in fish species from the River Gomti, Lucknow, India, as biomarkers of contamination. Bull Environ Contam Toxicol 78:118–122
Altindag A, Yigit S (2005) Assessment of heavy metal concentrations in the food web of lake Beysehir, Turkey. Chemosphere 60:552–556
APHA (1995) Standard methods, 19th edn. American Public Health Association, Washington, DC
Campbell LM, Dixon DG, Hecky RE (2003) Review of mercury in Lake Victoria, East Africa: implications for human and ecosystem health. J Toxicol Environ Health Part B 6:325–356
Carvalho ML, Santiago S, Nunes ML (2005) Assessment of the essential element and heavy metal content of edible fish muscle. Anal Bioanal Chem 382:426–432
Chang LW (1977) Neurotoxic effects of mercury—a review. Environ Res 14:329
Clarkson TW (1987) Metal toxicity in the central nervous system. Environ Health Perspect 75:59–64
Darnton-Hill I, Hassan N, Karim R, Duthie MR (1988) Tables of nutrient composition of Bangladeshi foods. English version with particular emphasis on vitamin A content. Helen Keller International, Dhaka, Bangladesh
De La Rosa D, Lima L, Olivares-Rieumont S, Graham DW, Enriquez I, Diaz O, Bastýás JM, Munoz O (2009) Assessment of total mercury levels in Clarias gariepinus from the Sagua la Grande River, Cuba. Bull Environ Contam Toxicol 82:101–105
Gammons CH, Slotton DG, Gerbrandt B, Weight W, Young CG, McNearny RL, Camac E, Calderon R, Tapia H (2006) Mercury concentration of fish, river water, and sediment in the Rio Ramis-Lake Titicaca Watershed. Peru Sci Total Environ 368:637–648
IRIS- Integrated Risk Information System (1993) EPA, Office of Research and Development, Washington, DC
Louria DB (1992) Trace metal poisoning. In: Wyngaarden JB, Smith LH Jr, Bennet JC (eds) Cecil textbook of medicine, vol 2, 19th edn. Saunders Co., Philadelphia 2380 p
Morel FM, Kraepiel MAML, Amyot M (1998) The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst 29:543–566
Paul RS (1987) Incidence of methylmercury in fish from Stanley reservoir (Mettur), Tamil Nadu, India. J Environ Biol 8:151–155
PFA Act (1954) The Prevention of Food Adulteration Act, 1954, together with Prevention of Adulteration Rules, 1955, and Notification and Commodity Index, India (amended, 2002) Eastern Book Company, Lucknow. Table No. R-57:106–110
Rainbow PS (1985) The biology of heavy metals in the sea. Int J Environ Stud 25:195–211
Sinha RK, Sinha SK, Kedia DK, Kumari A, Rani N, Sharma G, Prasad K (2007) A holistic study on mercury pollution in the Ganga river system at Varanasi, India. Curr Sci 92(9):1223–1227
World Health Organization (1990) Environmental Health Criteria 101: methylmercury. World Health Organization, Geneva
Zhang L, Zang X, Xu J, Xie P, Zhu Z, Su J (2007) Mercury bioaccumulation in fishes of three Gorges reservoir after impoundment. Bull Environ Contam Toxicol 78:262–264
Acknowledgments
The authors gratefully acknowledge Department of Environmental Science, University of Kalyani for providing support and necessary facilities for research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharyya, S., Chaudhuri, P., Dutta, S. et al. Assessment of Total Mercury Level in Fish Collected from East Calcutta Wetlands and Titagarh Sewage Fed Aquaculture in West Bengal, India. Bull Environ Contam Toxicol 84, 618–622 (2010). https://doi.org/10.1007/s00128-010-9972-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-010-9972-5