Abstract
Analysis of heavy metals in top soil samples from Nzoia sugarcane farms in Western Kenya found elevated levels of heavy metals in the soils with mean concentrations (mg kg−1 dry weight) of 142.38, 59.12, 73.35, 116.27, 409.84 (dry season) and 144.22, 50.29, 72.14, 158.81, 368.83 (wet season) for Cr, Pb, Cu, Zn and Fe, respectively, compared with a control soil sample from an adjacent field where fertilizers are not applied having mean concentrations of 117.27, 61.87, 63.68, 123.49, 282.93 (dry season) 108.00, 50.68, 66.10, 114.23, 167.01 (wet season), respectively. The heavy metal loads in the sugarcane farms were above international standards. The levels of the same metals in the fertilizers used in the sugarcane farms were within acceptable international standards. A risk assessment of the continued use of phosphate fertilizer (DAP) in the farms based on a 50-year period, did not exceed international threshold. The soil pH values (6.18 dry season and 5.66 wet season) were low compared to the control (7.46 dry season and 7.10 wet season) a situation that could accelerate heavy metal solubility and mobility in the farm soil. Lowering of soil pH was attributed mainly to fertilizer application and partly to increased organic matter content as shown by the high mean total organic carbon content values of 8.63% (dry season) and 8.43 (wet season) in comparison with a control soil meant total organic carbon content value of 4.76% (dry season) and 5.02 (wet season).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mortvedt (1995) has reported that fertilizers and biosolids, mixtures of scum and sludge, contain heavy metals that accumulate in soil with repeated fertilizer application. Heavy metals in fertilizers and other soil inputs are therefore a big threat to the sustainability of farming practices and to the ecology of the local environment. The bioaccumulation and bio-concentration of toxic heavy metal residues in the food chain can put terrestrial consumers including humans and birds at risk (Wang 1987; Gough and Herring 1993; Driscoll et al. 1994; Ongley 1996; Sekhar et al. 2003). The risks of heavy metal accumulation through agricultural activities and problems posed by heavy metals in fertilizers and other soil inputs have therefore increasingly drawn the attention of farmers, environmental organizations, consumers, and public policymakers worldwide (USEPA 1999; Lawrence and Brian 2002; Oliver 2004; Pekey et al. 2004).
The physicochemical processes taking place within the soil environment, including soil erosion, leaching, plant uptake and volatilization, are the primary determinants of the transport and fate of heavy metals emanating from fertilizers and related products (Alloway 1995a). Although heavy metals are not susceptible to chemical degradation like organic contaminants, chemical conditions in the soil are important secondary determinants of transport and fate of both anthropogenic and naturally-occurring heavy metals (Duinker et al. 1982; Alloway 1995b; Ford et al. 2001). The four various physico-chemical interactions between metals and the solid phase of the soil matrix, soil water, and air within and above the soil which include specific adsorption, co-precipitation, cation exchange, and organic-ligand complexation, respectively, depend on a variety of chemical reactions. These chemical reactions include covalent bonding with soil matrix and metal-anion exchange on the soil surface as well as formation of water insoluble precipitates from metal cations and anions such as carbonates, sulfides and/or phosphates (Stevenson 1979; Duinker et al. 1982; Alloway 1995b; Ford et al. 2001).
The organic carbon fraction has a significant influence on heavy metal transport and, anthropogenic heavy metals such as Pb, Cu, Zn and Cr are strongly correlated with organic carbon (Chen and Stevenson 1979; Xue 2008). The soil pH is also an important secondary determinant of heavy metal transport and fate at the application sites, with their ionization, water solubility and mobility increasing at low soil pH (Alloway 1995b; Moody and Aitken 1997). Ionization of metals increases at low pH thereby increasing their water solubility and mobility. Hydroxonium ions displace most other cations on negative surface charges which results in the reduction of metal adsorption through cation exchange and organic complexation (White 1987; Ford et al. 2001). In addition Duinker et al. (1982) found that the use of commercial fertilizers affects soil chemistry, specifically by lowering the soil pH hence increasing heavy metal solubility and mobility.
This study was aimed at determining heavy metal concentrations, total organic carbon (TOC) and pH in soil in Nzoia Nucleus estate sugarcane farms in Kenya where inorganic fertilizers are used extensively and the impact of fertilizers on soil with respect to heavy metal concentration levels, soil and water pH as well as seasonal variations on these parameters. The study area was the Nucleus Estate sugarcane farms of Nzoia Sugar Company Limited (lying between 340 50′49′′E–350 35′41′′E longitudes and 00 4′55′′N–00 20′11′′S latitudes) which is located in Bungoma District, Western Province. The company premises occupy a total surface area of 4,629 Ha (hectares) and the factory serves over 30,000 out-grower farmers within Bungoma, Kakamega and Lugari Districts in Kenya. The Nucleus Estate farms which are owned by the sugar company and cover a total area of 4,000 Ha, supplies 20% of sugarcane requirement for the refined sugar processing company. The rest of the cane requirement is met by private out-grower farmers. The Nucleus Estate farms have many water canals which run across the farms and discharge waste water into River Kuywa which flows through the farms before joining River Nzoia and the waste water coming from the farms is of significant concern to the local communities which depend on river water. Currently, the area under cane within the Nucleus Estate farms is 3,500 Ha with an average plant crop yield of 124 TCH (Tones of Cane per Hectare) and a Ratoon crop yield of 93 TCH. The Nucleus Estate farms have black nitrosol soils that are deep and well drained (DDP 1994) and intensive sugarcane farming is done with fertilizers including Di-ammonium Phosphates (DAP) and urea being applied during the onset of the long rains at the rate of 3.4 50-kg bags/Ha DAP and 3.6 50-kg bags/Ha Urea per year, respectively. Most of the rainfall is received during the long rain season which is from March to May while the short rains season is from September to November and the average annual temperature ranges from 15°C minimum to 30°C maximum (DDP 1994; DDP 2002). The control site was at Kanduyi District Education Board Primary School football pitch which lies within the same geographical region as Nzoia Sugar Company and also has black nitrosol soils that are deep and well drained (DDP 1994).
Materials and Methods
A complete randomized block design was used in sampling of the fertilizers whereby three 100 g replicates from each of the 50 bags sampled at random in one store of the Department of Agronomy, Nzoia Sugar Factory were collected in plastic bags and transported to the laboratory in Maseno University for analysis. A similar complete randomized design was also used to sample three 100 g replicates of top soil samples from each sampling point (Fig. 1) using a PVC pipe of 1.5 cm diameter, inserted up to 20 cm into the soil. The soil samples were transported in black plastic bags to the laboratory for analysis. The same procedure was replicated at the control site in a football pitch of Kanduyi District Education Board Primary School. All the samples were air dried and crushed with a pestle and mortar, sieved through a 45 μm mesh and kept in clean plastic containers before acid digestion. The moisture contents of the soil samples were determined.
Aqua ragia digestion method was used to extract total heavy metals present in samples and the extract analyzed for heavy metals (Pb, Cr, Cu, and Zn) using a Shimadzu AA-6200 Atomic Absorption Spectrophotometer. Strontium chloride (1.5 mg mL−1) was used to remove interference in absorbance of the specific metal by other metals at the same wavelength and to improve efficiency by preventing unnecessary ionization of the ions. For the soil pH, a method adopted from Rhodes (1982) was used: 50 mL of de-ionized water was added to 20 g of crushed soil sample. The mixture was stirred well for 10 min and allowed to stand for 30 min before stirring again for 2 min. The pH was then measured using a pH meter (3071 Jenway). A method, as described by Okalebo et al. (2002), was used to determine total organic carbon of soil samples. In this method 10 g of well mixed air dried soil sample was heated in an oven for 3 h at 105°C in a crucible and the difference in weights noted as the moisture content. The sample, in a cricuble, was then placed in a Vulcan A-550 muffle furnace and the temperature raised slowly from 100, 200, to 550°C, respectively, and then maintained at 550°C for 8 h. The grayish white ash sample was removed and cooled in a desiccators and then weighed. The difference in weight between the moisture free sample and the ash represented the total organic carbon of the sample.
Recovery studies were done for each metal to ascertain the accuracy of the extraction procedures by taking analytical grade potassium dichromate, lead nitrate, copper sulphate, zinc nitrate and iron nitrate, respectively, to make a 10,000 ppm stock solution for spiking samples. Twenty 1 g acid-washed soil and fertilizer samples were each spiked with 10 μL of the stock solution and mixed well. The spiked samples were digested together with some blanks of the respective samples and solvents used in the extraction process and the digests were analysed by AAS. Various concentrations of the analytical reagent of each metal were analyzed by the AAS and a calibration curve for each standard metal drawn. Each of the digested samples was then analyzed for each metal by the AAS and extrapolation using the respective calibration curve.
The means and ranges of the data collected were determined in this study. Confidence limits of 5% were applied to test the significance of the analytical results. Analysis of variance (ANOVA) (p ≤ 0.05) (two factor experiment for soil and one factor for fertilizers) and students T-test (p ≤ 0.05) were used to check the variations. Statistical analysis was performed using MSTATC.
Results and Discussion
The analytical method found mean % recovery values for Cr, Pb, Cu, Zn and Fe of 93, 89, 94, 88 and 84, respectively, with detection limits of 0.001, 0.001, 0.003, 0.008 and 0.01 ng mL−1, respectively. The wet weight:dry weight ratios were 1.015 ± 0.014 and 1.029 ± 0.009, for the farm soil and control soil samples, respectively. The results of the heavy metal concentrations, soil pH and TOC are shown in Tables 1, 2, 3, 4, 5, 6 and 7. The soils of Nzoia Sugar belt in Kenya are not “clean” with reference to standard background values of countries such as Netherlands, Taiwan and Dutch (Chen et al. 1996, 1999; Zueng-Sang 2000) (Table 5). However, no background values for soils in Kenya have been developed hence the necessity to do so in order to have compatible background values with regional characteristics in Kenya. Hence, by adopting those standards, the soils in our studied area were ‘not clean’. However, soils in the Nucleus Estate farms had a higher concentration of heavy metals than soils from the control site (Tables 1–4). This was an indication that fertilizer use in the farms had a negative impact on the environment due to elevated concentration levels of heavy metals in the soil.
Analysis of fertilizers used in the farms revealed that they are contaminated with the studied heavy metals though not above international standards (Tables 3, 4). Risk assessment analysis (Table 7) of DAP fertilizer used in the farms within the Nzoia Nucleus Estate for 50 years (in Kg/ha) in comparison with international standards (USEPA 1999) revealed that fertilizer application did not pose a threat to the farms. However, of great concern, was the pH difference in the two farms because during the wet season Nzoia Nucleus Estate farms soil pH dropped to approximately 5.66 from 6.18 (Tables 1, 2) while the control site soil maintained an almost constant soil pH value i.e. 7.10 (wet season) and 7.46 (dry season) (Tables 3, 4). The low soil pH values aid in solubility and mobility of heavy metals in soils (Alloway 1995b) even the naturally occurring ones. A part from fertilizers playing a great role in pH reduction (Duinker et al. 1982), the soil organic matter represented by the total organic carbon content in soil could also contribute to lowering of the soil pH, although to a lesser extent, in the present study (Tables 1–4). A similar observation has been made in other studies (Chen and Lee 1995; Xue 2008). The total organic content level was high in the farms as compared to the control soils due to agricultural activities, especially agricultural residues such as bio-solids. Alloway (1995a) has also reported similar high TOC values in farms as result of agricultural activities. In this study, a formula was used to calculate the risk of DAP fertilizer application in the Nucleus Estate farms. The USEPA formula for risk assessment used is: Kg/Hectare = (ppm metal/1,000,000) × %Ph × Application Rate of the Fertilizer × 0.454 kg/Ib × 2.47 Ac/hectare × 50 years (USEPA 1999). The results obtained by using this formula are given in Table 7 where they are compared with other international standards.
In conclusion, we found heavy metal loads in soil samples taken from Nzoia Sugar Company Nucleus Estate farms to be above international background levels hence there is need to determine, in more detail, levels of toxic heavy metals in such farms in Kenya where there is heavy usage of fertilizers and to determine the impact of fertilizer use on the farm soil and on water bodies downstream. In this study, the heavy metal concentration levels in the farm soil samples were higher than in control showing the impact of fertilizer use on the elevated levels of the heavy metals analysed. Similar findings have been reported in other countries in farms including sugarcane farms where contamination with heavy metals as a result of fertilizer use and biosolids have impacted negatively on streams downstream (Schroeder et al. 1996; Lawrence and Brian 2002; Sekhar et al. 2003; Oliver 2004; Pekey et al. 2004). In this study, it was also found that the use of fertilizers affects the soil chemistry by lowering of the soil pH which in turn can aid in heavy metal solubility and subsequent mobility from the soil matrix. Our results indicate the need for continuous environmental monitoring in similar agricultural systems where intensive fertilizer application is practiced for mitigation purposes.
References
Alloway BJ (1995a) Impact of fertilizers on soil. In: Alloway BJ (ed) Heavy metals in soils, 2nd edn. Blackie, New York
Alloway BJ (1995b) Soil processes and the behavior of heavy metals in soils. In: Alloway BJ (ed) Heavy metals in soils, 2nd edn. Blackie, New York, pp 11–37
Anonymous (2006) The soil profile. In: Public health concerns with hazardous materials in fertilizers. A newsletter providing information on issues relating to soils and plant nutrition in New Jersey. vol. 16 ROTGERS, the State University of New Jersey
Chen ZS, Lee DY (1995) Heavy metal contents of representative agricultural soils in Taiwan. J Chinese Inst Environ Engineer 5:205–211
Chen ZS, Lee DY, Lin CF, Lo SL, Wang YP (1996) Contamination of rural and urban soils in Taiwan. In: Naidu R, Kookana RS, Oliver DP, Rogers S, McLaughlin MJ (eds) Contaminants and the soil environment in the Australasia-Pacific region. Kluwer Academic Publishers, London, pp 691–709
Chen Y, Stevenson FJ (1979) In: Chen Y, Avinmelech Y Martinus Nijhoff (eds) The role of organic matter in modern agriculture. Dordrecht, pp 73–112
Chen ZS, Tsai CC, Tsui CC (1999) Proposed regulation of soil pollutants in Taiwan soils. In: Chen ZS (ed) Proceedings of 6th Workshop on soil pollution and prevention: soil remediation techniques on soils contaminated by organic pollutants. Taipei, Taiwan ROC. pp. 169–207. (In Chinese, with English abstract and Tables)
DDP (1994) District development plan of Kenya (Bungoma District) for the period 1994–1996. Government Printers, Nairobi
DDP (2002) District development plan of Kenya (Bungoma District) for the period 2002–2008. Government printers, Nairobi
Driscoll CT, Otton JK, Iverfield CK (1994) Trace metal speciation and cycling. In: Molden B, Eemy J (eds) Biogeochemistry of small catchments: a tool for environmental research. John Wiley, New York, pp 299–322
Duinker JC, Nolting RF, Michel D (1982) Effects of salinity, pH and redox conditions on behaviour of Cd, Zn, Ni, and Mn in the Scheldtestuary. Thalassia Jugosl 18:191–201
Ford RG, Scheinost AC, Sparks DL (2001) Frontiers in metal sorption/precipitation mechanisms on soil mineral surfaces. Adv Agronomy 74:41–62
Gough LP, Herring JR (1993) Geologic research in support of sustainable agriculture. Agric Ecosystem and Environ 46:55–68
Lawrence RC, Brian WS (2002) Heavy metal in fertilizers: considering for setting regulations in oregon, oregon department of agriculture salem Oregon. Department of environment and molecular toxicology Oregon State University Corvallis Oregon
Moody PW, Aitken RL (1997) Soil acidification under some tropical agricultural systems: I. Rates of acidification and contributing factors. Australian J of Soil Res 35:163–173
Mortvedt JJ (1995) Heavy metal contaminants in inorganic and organic fertilizers. In: Nutrient cycling in agroecosystems, Springer, Netherlands, 43: 55–61
Okalebo RJ, Kenneth WG, Poul LW (2002) Laboratory methods of soil and plant analysis: a working manual, 2nd edn. Sacred Africa Publishers, Nairobi, Kenya
Oliver DC (2004) Environmental impacts of sugar production. CABI Publishers, United Kingdom
Ongley ED (1996) Control of water pollution from agriculture: FAO irrigation and drainage paper 55. FAO Food and Agriculture Organization of the United Nations, Rome
Pekey H, Karakas D, Bakacoglu M (2004) Source apportionment of trace metals in surface waters of polluted stream using multivariate statistical analysis. Mar Pollut Bull 49:809–818
Rhodes JD (1982) Methods of soil analysis, part 2. 2nd edn (Page AL, R.H.M and Keeney DR, (eds). American Society of Agronomy, Madison, USA
Schroeder BL, Turner PET, Meyer JH, Robinson JB (1996) Advances in quantifying soil acidity and acidification rates in the South African sugar industry. In: Wilson JR, Hogarth DM, Campbell JA, Garside AL (eds) Sugarcane: research towards efficient and sustainable production. CSIRO Division of Tropical Crops and Pastures, Brisbane, pp 256–258
Sekhar CK, Chary NS, Kamala CT, Raj DS, Rao SA (2003) Fractionation studies and bioaccumulation of sediment bound heavy metals in Kolleru Lake by edible fish. Environ Int 29:1001–1008
Stevenson FJ (1979) Encyclopeadia of Soil Sciences, Fairbridge RW and Finkl CW. Dowden (eds), Hutchinson and Ross, Stroudsburg, pp 195–205
USEPA (1999) Estimating risk from contaminants contained in agricultural fertilizers, EPA 68-W-98-0085
Wang W (1987) Factors affecting metal toxicity and accumulation by aquatic organisms- an overview. Environ Internat 13:437–457
White RE (1987) Introduction to the principles and practice of soil science, 2nd edn. Blackwell, Oxford
Xue SW (2008) Correlations between heavy metals and organic carbon extracted by dry oxidation procedure in urban roadside soils. Environ Geol 54(2):261–273
Zueng-Sang C (2000) Relationship between heavy metal concentrations in soils of Taiwan and uptake by crops. Department of Agricultural chemistry, National Taiwan University Taipei 106, Taiwan
Acknowledgment
We wish to thank all the technical staff of Maseno University Chemistry Department for their unquantifiable help. This work was partly supported by the IAEA CRP Project 13695/RO and we are grateful to the IAEA for financial and technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Omwoma, S., Lalah, J.O., Ongeri, D.M.K. et al. Impact of Fertilizers on Heavy Metal Loads in Surface Soils in Nzoia Nucleus Estate Sugarcane Farms in Western Kenya. Bull Environ Contam Toxicol 85, 602–608 (2010). https://doi.org/10.1007/s00128-010-0133-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-010-0133-7


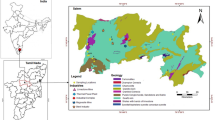


 , Kuywa River
, Kuywa River 