Abstract
A simple and sensitive high pressure liquid chromatographic method has been developed for the simultaneous determination of Thiram and its transformation product using isocratic mixture of methanol–water (65:35) at flow rates of 0.75 mL min−1, PDA detector using UV absorbance (λmax) at 217 nm for Thiram and 265.5 nm for the transformation product. The transformation product was isolated from the commercial DS formulation and has been tentatively assigned the structure by 1H NMR and ESI-MS spectral data. The separation is dependent on the nature of the mobile phase, its flow rate and the nature of the HPLC column. The detection limit (signal/noise; S/N = 3) for both Thiram and its transformation product was 0.2 ppm. The method has been successfully applied to analysis of soil and soybean samples spiked with Thiram, its transformation product and a commercial sample containing these products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Thiram [Bis (dimethylthiocarbamoyl)-disulfide (Synonym: tetramethyl thiuram disulfide TMTD] is a well-known dithiocarbamate fungicide used to prevent crop damage in field and during storage or transport. It is widely used as a foliar treatment on fruits, vegetables, ornamentals and turf crops and a seed treatment to control a number of fungal diseases (Kunkur et al. 2007). Thiram is available as dust, wettable powder and dry and wet flowable formulations as well as admixture with other fungicides. It is also an environmental degradation product of Ferbam and Ziram (Anonymous 1991). Several analytical methods are available for quantification of dithiocarbamate fungicides in different crop matrices and environmental samples. Gas chromatographic methods have been employed by determination of CS2 formed on acid hydrolysis of dithiocarbamates in food using a headspace technique (Jianren et al. 1989; Hill 1992; Friedrichs et al. 1995) or after partitioning of the CS2 into an organic phase (Harrington et al. 1998). Although gas chromatographic methods are sensitive, they suffer from lack of selectivity since all dithiocarbamate pesticides evolve CS2 on acid hydrolysis. Reverse phase HPLC methods have also been proposed for the analysis of Thiram and other dithiocarbamate fungicides using UV or electrochemical detectors (Aulakh et al. 2004; van Lishaut and Schwack 2000; del Silva et al. 1999). It has been observed that during long storage, Thiram is transformed into products with reduced antifungal activity. Analysis of such transformation products is therefore vital for understanding usefulness and fate of Thiram during storage. Since no report is available on the analysis of Thiram and its transformation product, the present paper describes the isolation, identification and simultaneous LC analysis of Thiram and its transformation product in Thiram 75% DS formulation.
Materials and Methods
HPLC grade water was prepared in laboratory by passing the city supply through a reverse osmosis (RO) unit and a water purification system (US Filter, Purelab classic). HPLC grade methanol (Qualigens India, a unit of GlaxoSmith-Kline) and Thiram DS formulation (Devidayal Agrochemicals) were procured from the market. The solvents were degassed and filtered through a filtration system before use. Thin layer chromatography was performed on silica gel-coated plastic or aluminium TLC plates supplied by Merck Schruchardt, Germany. The plates were developed in (hexane:ethyl acetate, 40:60) and visualized under UV-light or iodine vapours. Reverse phase LC system consisted of a Waters 600 quaternary pump with a manual injector (20 μL fixed loop), and a 2,996 photodiode array detector (Waters, USA). A computer using an “Empower” software programme integrated the peak areas automatically.
A shelf life expired sample (25 g) of Thiram formulation (Thiram 75% DS) was extracted with acetone (150 ml × 3) and dichloromethane (100 ml × 3). The combined acetone extract was concentrated in vacuo and crystallized from methanol to afford a transformation product (6.5 g). The dichloromethane extract was similarly processed to obtain Thiram (4 g). On TLC plate, these gave respective R f values of 0.6 and 0.7. The chromatographic analysis was performed at ambient temperature (25–30°C) on a RP-18e Purosphere STAR and RP-Select B LiChrospher (250 mm × 4 mm i.d., Merck, Darmstadt, Germany) columns. The physico-chemical properties of LC columns used in the study are listed in Table 1. The mobile phase comprised of isocratic mixture of methanol–water (65:35) at flow rates of 0.75 mL min−1. For quantitation, the photodiode array detector was set at 217 nm for Thiram and 265.5 nm for transformation product. A 20 μL sample was injected each time.
Thiram and the transformation product were subjected to MS-analysis (Thermo Finnigan). Mass spectroscopy was carried out using Thermo Electron LC-MS-MS spectrometer equipped with P-2000 binary pumps, MAX-detector (Thermo Electron Corporation USA) and electrospray ionization (ESI) source. Detection of mass was done by a Finnigan LCQ tune plus programme using Xcalibur software for the study of fragment ions and identification of masses. The MS was recorded in direct infusion mode with following parameters: Sheath gas flow rate 15, spray voltage 4.5 KV, spray current 0.5 μA, capillary temperature 190°C, capillary voltage 22 V and mass ranges 50–1,000 amu. Standard solutions of Thiram and its transformation product (1,000 μg mL−1) were prepared by dissolving 25 mg of each in 25 mL of methanol. Working standard solutions for LC were prepared in the range of 1–250 μg mL−1 through a serial dilution of the standard solution with the mobile phase. Recovery experiments on Thiram and its transformation product were carried out on soybean seed and soil samples at 50 and 100 ppm levels. Ten gram sample each was fortified at 50 and 100 μg mL−1 levels and kept for 4 h at room temperature. Samples were extracted with dichloromethane (10 × 3 mL) and filtered through Whatman no. 4 filter paper. The pooled extract was evaporated on rotary evaporator under reduced pressure and the residue dissolved in 5 mL methanol (HPLC grade) for HPLC analysis.
Thiram and its transformation product were evaluated for their antifungal activity against the phytopathogenic fungi Macrophomina phaseolina by Poisoned Food Technique (Agarwal et al. 2001).
Results and Discussion
Dichloromethane extract of the Thiram formulation furnished a compound which on the basis of its mass (MS) and 1H NMR was identified as Thiram. In the ESI-mass spectrum it exhibited three molecular adduct ion peaks at m/z 241.4 (M + H)+, 263.5 (M + Na)+ and 286 (M + 2Na)+ which were characteristic of Thiram molecule. The MS-MS of the di-sodium and mono sodium adduct ion peaks at m/z 286 and 263 further gave peaks at m/z 240.9 and 143.0 (Fig. 1). These peaks further proved their ultimate origin from thiram molecule. In its 1H NMR spectrum the proton peak at δ 3.43–3.64 integrating for 12 protons were attributed to four methyl groups of the thiram molecule.
Acetone extract of the Thiram formulation furnished another compound which on the basis of MS and 1H NMR analysis was identified as the dimeric transformation product of Thiram. In its ESI-MS, it exhibited molecular adduct ion peak at m/z 614.8. ESI-MS-MS of this molecular adduct ion peak gave characteristic fragment ion peaks at m/z 319 and 176.2. On the basis of its fragmentation pattern, the compound was tentatively identified as dimer of Thiram with two additional sulfur atoms. The transformation product probably originated as a result of the dimerization of the Thiram molecule during long storage. The 1H NMR spectrum of the Thiram transformation product exhibited two singlet peaks at δ 3.42 and 3.52 which corresponded to form methyl groups of the Thiram transformation product.
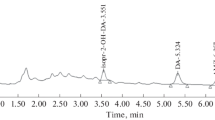
Thiram and its transformation product exhibited UV absorption at 217 and 265.5 nm, respectively. High performance liquid chromatographic method with UV/PDA detector was considered more suitable for their analysis. Reversed-phase RP-C18 bonded phase columns (RP-Select B LiChrosphere and RP-18e Purosphere STAR) were chosen for the analysis. The analytical columns were optimized for solvent mixture composition, flow rate and limits of detection and quantification (Tables 2, 3). Methanol–water (65:35) at flow rate of 0.75 mL min−1 was the best. RP-Select B LiChrosphere column gave good separation in a reasonable time. LC chromatograms of Thiram and its transformation product extracted and isolated from Thiram DS formulation is given in Figs. 2 and 3. The retention times of Thiram and its transformation product were 5.2 and 6.4 min, respectively under the above described conditions.
Fivefold injections of 20, 40, 100 μg mL−1 of Thiram and its transformation product were used to determine the accuracy and standard deviation. The accuracy of this method was adequate for determination of both the products (Table 4). The injection precision—a measure of the method variability, and relative standard deviation (RSD) were determined for six replicate injections of a representative batch of both the compounds separately at the target concentration of 100 μg mL−1. R.S.D.s of the response factor of Thiram and its transformation product peaks were, respectively, 1.55% and 1.86% for the six injections, indicating a good precision of the method.
Three calibration curves with five standard solutions each were prepared. Concentration range of 2–200 μg mL−1 was used with the target concentration of 100 μg mL−1 for both the compounds. The UV detector response for both the compounds was linear over this range with correlation coefficients of 0.999 and 0.996 for Thiram and its transformation product, respectively. Peak area (y) and concentration (x) of each injection was subjected to regression analysis to calculate the calibration equation and correlation coefficients. Linearity was confirmed as the R.S.D. values of the slope (1.14 and 2.80) and the intercept (2.80 and 2.56) were less than 3%. Least-square regression calibration curves were constructed by plotting peak areas of thiram and its transformation product as a function of concentration in the standard working solutions. The calibration curves could be represented by the following regression equations:
The optimized LC conditions were applied to the determination of thiram and its transformation product in fortified soil and soybean seed samples. Soybean samples spiked with 50 and 100 μg mL−1 were used in triplicate to assess accuracy. The method required negligible clean up. The seed blank did not show any peak interfering with either thiram or the transformation/degradation products. In any clean up experiment the recovery of an analyte from the collection medium must be 75% or greater. The present recovery of Thiram (Table 5) ranged from 86.9 to 93.4 in soil and 94.5–99.0 in soybean seeds with R.S.D. values less than 1.0%. The present recovery of the transformation product on the other hand ranged from 88.6 to 93.3 in soil and 95.7–99.4 in soybean seeds with R.S.D. <1.0%. The amount of thiram and the transformation product were calculated from related regression equations. Good recoveries achieved from both soil and soybean seed samples indicated that the method has sufficient accuracy for the determination of Thiram and its transformation product either alone or in combination. Specificity of the HPLC method is illustrated in Fig. 4. A complete separation of thiram and its transformation product from the extracted seed and soil sample was noticed. No interfering peaks were observed at the retention times of Thiram and its transformation product.
Detection limit (LOD), the lowest amount of an analyte in a sample that can be detected but not necessarily quantitated, is usually evaluated as the signal to noise ratio that is equivalent to three times the standard deviation of the noise (S/N = 3). The limit of quantification (LOQ), the lowest concentration that can be determined with acceptable accuracy and precision, is evaluated at signal to noise ratio equivalent to ten times the standard deviation (S/N = 10) of the noise. The lower limit of detection for thiram and the transformation product was established as 0.20 μg mL−1 as their 20 μL injection gave a measurable peak. The limit of quantification of both the compounds was experimentally verified by six injections and found to be 0.40 and 0.50 μg mL−1, respectively (Table 6). Results on the effect of stationary phase particle size, carbon load, surface area and mobile phase using Purosphere RP 18e and Lichrosphere RP-Select B columns in RP-HPLC separations are reported in Tables 1 and 3. Purosphere RP 18e column was distinctly more favourable with significant difference in detection and quantification.
Thiram exhibited significant antifungal activity against the phytopathogenic fungus Macrophomina phaseolina. At 50 and 25 ppm test concentrations, it exhibited 100% and 80% inhibition of the fungal growth. The transformation product on the other hand was inactive and did not show any activity at these concentrations. The decreased activity of the expired sample of Thiram was attributed to the presence of the increased amount of the transformation product in the sample.
Thiram, a dithiocarbamate fungicide is available for use as dust, wettable powder, and dry or wet flowable. During long storage there is a likelihood of the active ingredient being transformed/degraded to less active transformation products. An isocratic reverse phase LC method has been developed for the simultaneous analysis of Thiram and its transformation product of close polarity and chromatographic behaviour. The proposed method has been verified for accuracy, precision, and selectivity. It requires short time (<45 min) for sample clean up and analysis. The two analytes can be separated within 20 min. The use of RP-Select B LiChrosphere column and an isocratic mobile phase offers improved resolution and increased peak height and eliminates the need for gradient separation. The method can be recommended for routine assay of thiram or its transformation products alone and/or in combination in the technical materials and formulations.
References
Agarwal M, Walia S, Dhingra S, Khambay BPS (2001) Insect growth inhibition, antifeedant and antifungal activity of compounds isolated/derived from Zingiber officinale Roscoe (ginger) rhizomes. Pest Manag Sci 57:289–300
Anonymous (1991) International Agency for Research on Cancer (IARC)—Summaries & Evaluations. 53:403
Aulakh JS, Sharma VK, Mahajan RK, Malik AK (2004) HPLC-UV determination of tetramethylthiuram disulphide (Thiram) and thiourea in wheat grains and in a commercial sample. EJEAF Che 3(5):751–757
del Silva MP, Procopio JR, Hernandez L (1999) Electrochemical detection in the determination of several dithiocarbamates by reverse-phase liquid chromatography. J Liq Chromatogr Relat Technol 22:463–475
Friedrichs K, Winkeler HD, Gerhards P (1995) Determination of dithiocarbamate residues in food stuff by headstuff gas chromatographiy and flame photometric detection. Z Lebensm Unters Forsch 201:69–73
Harrington P, Horner J, Hird S, Griffiths T, Reynolds SL (1998) Modification of the method for measurement of dithiocarbamate residues as carbon disulfide in fruit and vegetables. Central Science Laboratory, York UK, p 1
Hill ARC (1992) Headspace methods for dithiocarbamates. In: Cairns T, Sherma J (eds) Modern methods for pesticide analysis. Emerging strategies for pesticide analysis. CRC Press, London, p 213
Jianren Y, Yongquan Z, Shuzshen J, Zhengguo W, Fuzhen Z (1989) Gas chromatographic headspace determination of dithiocarbamate fungicide residues on vegetables. Sci Agric Sin 22:76–80
Kunkur VK, Hunje R, Biradar Patil NK, Vyakarnhal BS (2007) Effect of seed coating with polymer, fungicide and insecticide on seed quality in cotton during storage. Karnataka J Agric Sci 20(1):137–139
van Lishaut H, Schwack W (2000) Selective trace determination of dithiocarbamate fungicides in fruits and vegetables by reversed-phase ion-pair liquid chromatography with ultraviolet and electrochemical detection. J AOAC Int 83:720–727
Acknowledgments
Authors are thankful to Indian Council of Agricultural Research (ICAR) for financial assistance under Emeritus Scientist Scheme, and to the Head, Division of Agricultural Chemicals, IARI, New Delhi for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walia, S., Sharma, R.K. & Parmar, B.S. Isolation and Simultaneous LC Analysis of Thiram and Its Less Toxic Transformation Product in DS Formulation. Bull Environ Contam Toxicol 83, 363–368 (2009). https://doi.org/10.1007/s00128-009-9754-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-009-9754-0