Abstract
Ten soil samples collected in Linfen were analyzed for 21 organochlorine pesticides. The concentration of total organochlorine pesticides ranged from 4.3 to 23.2 ng g−1 in soil from urban areas and from 26.3 to 247.4 ng g−1 in soil from industrial plants. The highest levels of contamination were observed in northwest and central Linfen, reflecting the distribution of industrial plants. The HCH and DDT profiles revealed that the sources were associated mainly with lindane and technical DDT, respectively, while HCHs in the soil of industrial plants might originate from a new source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Soil is an important reservoir for many persistent organic pollutants (POPs) including organochlorine pesticides (OCPs) (Zhang et al. 2005). OCPs have common physical–chemical properties, such as semivolatility, high levels of stability, and hydrophobicity, which are favorable for long-range transport and widespread distribution in the environment (Zhang et al. 2005). Hence, as POPs are released or escape into the environment, the burden in soils globally is a complex function of the balance between inputs and losses. A full understanding of the fate of OCPs requires knowledge of their concentrations and distributions in the soil of all major ecological zones. At present, there is a shortage of data from China (Qiu et al. 2005); however, the information is needed for reliable calculation of the global stocks of OCPs.
The city of Linfen is located in the southwest of Shanxi Province, China, an area of 20,275 km2. The urban area is located mainly in the central part of Linfen, with 339,800 residents. The climate is dominated by temperate semi-wet monsoon, with a mean annual temperature of 12.2°C. China clay is the main representative soil type in Linfen, which is the heavy chemical industry base of China. With the development of industry in recent years, Linfen has provided increasing amounts of chemical products and secondary energy, such as coke and electric power. At the same time, environmental contamination has become more and more serious, but the levels of OCP residues in the soil of Linfen have not been investigated in detail. There is a paucity of data for the levels of environmental OCPs in China, so we undertook this study to determine both the concentration and the profile of OCPs in soil samples obtained from urban areas of Linfen. The main objectives of this study were to determine the spatial distribution of OCPs in the environment, to identify possible sources of pollution, and to explore the factors affecting contamination in order to prevent further environmental deterioration in Linfen.
Materials and Methods
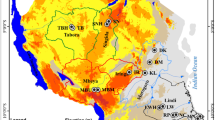
Surface soil samples (0–10 cm depth) were obtained from five urban sites and five industrial plants in Linfen, Shanxi Province (Fig. 1), in January 2006. Any overlying vegetation was removed before samples of the surface soil were collected in triplicate using a hand-held coring device. Each composite urban soil sample consisted of nine sub-samples in the same grid pattern at each sampling site. Each composite industrial plant soil sample consisted of three sub-samples. The subsamples were freeze-dried, mixed thoroughly, sieved to 60 mesh (International standard size, 250 μm), transferred to amber glass and stored at 4°C. The remaining water content in the soil was determined gravimetrically after drying individual composite soil samples in air at 105°C for 12 h. All results are reported on a dry weight basis.
The mixed OCPs standard solution (1,000 μg mL−1; purchased from Chem service (West Chester, PA, USA) included α-HCH, β-HCH, γ-HCH, δ-HCH, heptachlor, heptachlor epoxide, γ-chlordane, α-chlordane, α-endosulfan, β-endosulfan, endosulfan sulfate, diedrin, endrin, endrin aldehyde, endrin ketone, aldrin, pp′-DDT, pp′-DDD, and pp′-DDE. The op′-DDT standard solution (1,000 mg mL−1) was purchased from the National Research Center for Certified Reference Materials of China (Beijing, China). Hexachlorobenzene (HCB) and 2,4,5,6-tetrachloro-m-xylene (TCMX, as surrogate) were purchased from Supelco (Bellefonte, PA, USA). The working standards were prepared by dilution with isooctane. Florisil (60–100 mesh) was purchased from Supelco (Bellefonte, PA, USA) and activated in air at 130°C for 16 h. Anhydrous sodium sulfate (Beijing Chemical Factory, Beijing, China) was heated at 600°C for 12 h to destroy any organic material. All solvents used were of pesticide grade (J.T. Baker, NJ, USA).
A 5 g portion of each soil sample was weighed and then ground with anhydrous sodium sulfate into a free-flowing powder. The samples were extracted with 30 mL of hexane/acetone (1:1, v/v) by ultrasonication for 4 min then separated by centrifugation. This process was repeated three times. Before extraction, TCMX was added as a surrogate standard. The concentrated extracts were reduced to a volume of 1 mL under a gentle stream of N2.
OCPs were cleaned using a chromatography column (30 cm × 10 mm i.d.) containing 4 g of activated Florisil® and 2 g of anhydrous sodium sulfate. The column was washed through with 40 mL of hexane/diethyl ether (4:1, v/v) before loading the sample. The fraction containing 21 OCPs was eluted using 60 mL of hexane/diethyl ether (4:1, v/v). The solvent was evaporated under a gentle stream of N2 to reduce the volume to 100 μL.
Analysis of 21 OCPs was done with an Agilent 6890 gas chromatograph (GC) equipped with a micro-cell 63Ni electron capture detector (μ-ECD). Separation was done in a 30 m DB-5MS (30 m × 0.25 mm i.d., 0.25 μm film thickness) capillary column with an injector temperature of 230°C and a detector temperature of 305°C. The GC column was maintained at 100°C for 2 min, then the temperature was raised to 160°C at 10°C min−1, then raised to 230°C at 4°C min−1, and finally raised to 280°C at 10°C min−1 and kept at this temperature for 10 min. The total run time was 40.5 min. Quantification of the samples was done by an external standard method.
To confirm the OCP results, selected samples were checked using an Agilent 6890 series gas chromatograph coupled to an Agilent 5973 mass spectrometer (MS) using an electron impact ionization source (EI) in the selected ion monitoring (SIM) mode. In EI mode, the MS source temperature was 230°C, the transfer line temperature was 300°C and the electron energy was 70 eV. GC separation was performed as described above.
We used a laboratory method control group to demonstrate the lack of interference and cross-contamination. We also ran a procedural blank in parallel with every set of six samples to further check for interference and cross-contamination. We analyzed duplicate samples in the laboratory along with the regular samples for additional quality-control assessment to ensure valid results. We determined the instrument stability and relative response factor variance by analyzing the calibration standards in each sample batch.
The identification of 21 OCPs was confirmed, and concentrations were measured using an external quantification standard consisting of known amounts of all the target compounds. For accuracy and precision of the analysis, method blanks were run first using the same solvents as those used for real samples. No contaminant of OCPs was found in the method blanks (n = 3). The average recovery experiments were carried out in triplicate by spiking known concentrations of standards in a matrix blank. The limits of detection were calculated as three times the response of the signal-to-noise ratio, and the limits of quantification were calculated as five times the signal-to-noise ratio. These parameters are shown in Table 1. Before extraction, each soil sample was spiked with a known amount of TCMX to monitor any loss of components. The recoveries of TCMX were in the range 70%–90%, which was considered satisfactory and no correction of analytical data was used for the samples.
Results and Discussion
A total of 21 OCPs were identified in all samples (Table 2). The detection rates of OCPs in the soils were up to 100%, which indicates widespread occurrence of these compounds in Linfen. The concentration of total OCPs (defined as the sum of 21 OCPs) ranged from 4.3 to 23.2 ng g−1 (median 6.1 ng g−1, dry weight) in urban soil. The median concentration in the industrial plant soil was much higher, ranging from 26.3 to 247.4 ng g−1 (median 46.2 ng g−1, dry weight).
In comparison with other areas of China, the concentrations of OCPs in the soil of urban Linfen were lower than those in the agricultural soils of Jiangsu Province (173.3 ng g−1, dry weight) (Wang et al. 2005), the soils of the Pear River delta (17–155 ng g−1, dry weight) (An et al. 2005), and agricultural surface soils from greenhouses in suburan areas of Beijing (80.2 ng g−1, dry weight) (Ma et al. 2003). The levels were lower than those reported for Romania (29.2 ± 27.1 ng g−1) (Covaci et al. 2001) and Poland (11 ± 29 ng g−1) (Falandysz et al. 2001) but higher than those in urban soil in some developing countries in tropical/subtropical areas where pollutants evaporate readily from the soil, e.g. India (N.D.−3.6 ng g−1) (Kawano et al. 1992) and Egypt (N.D.−16.2 ng g−1) (Kabbany et al. 2000).
The distribution of OCPs in the soil samples shown in Fig. 2 reveals an increasing trend in northwest and central Linfen, reflecting the distribution of industrial plants that were built mainly in the north and the central areas of Linfen.
When considering HCH composition patterns, the ratio of α-HCH/γ-HCH is relatively stable with a value of 4.64–5.83 for the technical HCHs and nearly zero for lindane (Zhang et al. 2004), which can be used to monitor whether the source was from technical HCHs or lindane. In the present study, the ratio of α-HCH/γ-HCH varied from 0.19 to 0.36 in the urban areas and from 0 to 5.10 in the industrial plants. Compared with the fraction of β-HCHs in technical HCHs (5–14%) (Qiu et al. 2004), the relatively high percentage of β-HCHs means that there was a lack of new HCH sources in the areas studied. In the present study, β-HCHs accounted for 43%–85% in the urban areas but were absent from the industrial plant soil. It could be concluded that HCHs in the soil of urban areas in Linfen might originate from a relatively old source of lindane, while HCHs in the industrial plant soils might originate from a new source of lindane.
The ratio of op′-DDT/pp′-DDT was used to distinguish DDT pollution caused by technical DDTs from that caused by dicofol (Qiu et al. 2005). Since op′-DDT in the environment is more unstable than pp′-DDT, it would be impossible for the ratio of op′-DDT/pp′-DDT to be higher than technical DDTs, whereas the characteristics of pollution from dicofol would have a higher ratio of op′-DDT/pp′-DDT than that of technical DDTs. It is well known that dicofol contains approximately 3%–7% DDTs as impurities. Generally, the ratio of op′-DDT/pp′-DDT ranges from 0.2 to 0.3 in technical DDTs, and from 1.3 to 9.3 or higher in dicofol (Qiu et al. 2005). In the present study, the ratio varied from 0.2 to 1.1, with 0.5 as the median, which outlined the important contribution of technical DDTs. When considering the DDT profiles, the ratio of (pp′-DDE + pp′-DDD)/pp′-DDT can be used as an indicator of the residence time of pp′-DDT in the environment, because the levels of the parent compounds (pp′-DDT) in the natural environment would decrease with time, and the major metabolites are expected to be pp′-DDE and pp′-DDD (Qiu et al. 2004). A ratio >1 is generally expected for old sources in the environment and a ratio <1 indicates relatively recent exposure to the parent DDT (Jaga and Dharmani 2003). In the present study, the ratios of (pp′-DDE + pp′-DDD)/pp′-DDT were >1 in all samples, indicating that the contamination by technical DDT occurred in the past. It can be concluded that DDTs in the soils in urban areas and in industrial plants in Linfen might originate from a relatively old source of technical DDTs.
According to the guidelines of the Chinese environmental quality standard for soil (GB15618-1995), a soil is classified as having (I) no pollution, (II) low pollution, (III) middle pollution, or (IV) high pollution. A pollution level below grade I, is defined as no pollution; if the pollution level is between grade I and grade II, it is defined as low pollution, and so on. For HCHs and DDTs, the median value for all soil samples in this study was below the maximum allowable concentration of class I soil in China. This might be attributed to the prohibited use of selected OCPs in China for nearly 30 years. However, the HCHs contamination of industrial plant sample sites all originated from a new source of lindane. The half-life of OCPs in soil is longer, and OCPs together with its metabolites can act as endocrine disruptors; therefore, it is necessary to monitor the status of OCPs continuously.
References
An TC, Chen JX, Fu JM, Sheng GY, Li GY, Hu ZY, Kuang YQ (2005) The pollution situation and control strategy of persistent organic pollutants in the Pearl River delta, China. Ecol Environ 14:981–986 (in Chinese)
Covaci A, Hura C, Schepens P (2001) Selected persistent organochlorine pollutants in Romania. Sci Total Environ 280:143–152. doi:10.1016/S0048-9697(01)00820-8
Falandysz J, Brudnowska B, Kawano M, Wakimoto T (2001) Polychlorinated Biphenyls and organochlorine pesticides in soils from the southern part of Poland. Arch Environ Contam Toxicol 40:173–178. doi:10.1007/s002440010160
Jaga K, Dharmani C (2003) Global surveillance of DDT and DDE levels in human tissues. Int J Occup Med Environ Health 16:7–20
Kabbany SE, Rashed MM, Zayed MA (2000) Monitoring of the pesticide levels in some water supplies and agricultural land in ElHaram, Giza. J Hazard Mater 72:11–21. doi:10.1016/S0304-3894(99)00174-0
Kawano M, Ramesh A, Thao VD, Tatsukawa R (1992) Persistent organochlorine insecticide residues in some paddy, upland and urban soils of India. Int J Anal Chem 48:163–174. doi:10.1080/03067319208027397
Ma LL, Chu SG, Xu XB (2003) Organic contamination in the greenhouse soils from Beijing suburbs, China. J Environ Monit 5:786–790. doi:10.1039/b305901d
Qiu X, Zhu T, Li J, Pan H, Li Q, Miao G, Gong J (2004) Organochlorine pesticides in the air around the Taihu Lake, China. Environ Sci Technol 38:1368–1374. doi:10.1021/es035052d
Qiu X, Zhu T, Yao B, Hu J, Hu S (2005) Contribution of dicofol to the current DDT pollution in China. Environ Sci Technol 39:4385–4390. doi:10.1021/es050342a
Wang X, Dong YH, An Q, Wang H (2005) Difference in analyzing organochlorines between US EPA 8080 and GB/T 14550–93. Soils 37:105–108 (in Chinese)
Zhang ZL, Huang J, Yu G, Hong HS (2004) Occurrence of PAHs, PCBs and organochlorine pesticides in Tonghui River of Beijing, China. Environ Pollut 130:249–261. doi:10.1016/j.envpol.2003.12.002
Zhang H, Lu Y, Dawson RW, Shi Y, Wang T (2005) Classification and ordination of DDT and HCH in soil samples from the Guanting Reservoir, China. Chemosphere 60:762–769. doi:10.1016/j.chemosphere.2005.04.023
Acknowledgments
This study was supported by the National Natural Scientific Foundation of China (No. 20707031) and the evaluation item of city eco-system and geographic chemistry of economic region in the Loess Plateau basin of Shanxin Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, H.X., Fu, S., Liu, Y.H. et al. Organochlorine Pesticides in the Soil in Linfen, China. Bull Environ Contam Toxicol 81, 599–603 (2008). https://doi.org/10.1007/s00128-008-9544-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-008-9544-0