Abstract
This study presents an evaluation of Norwegian kyanite quartzites from Gullsteinberget, Knøsberget, Kjeksberget, Sormbrua, Tverrådalen, Juovvačorrú and Nasafjellet as potential deposits of high-purity quartz (HPQ) for use as raw material for special applications in high-technology industries. Fine-grained quartz, which forms 70 to 85 vol.% of these rocks, generally contains less than 50 μg g−1 (total sum) of the structurally incorporated trace elements B, Li, Al, Ge, Ti, Fe, Mn, K and P. The concentrations are in the same range as those found in HPQ products, which are being mined and produced in Norway and elsewhere. Quartz analyses were performed using laser ablation–inductively coupled plasma mass spectrometry. Complimentary whole-rock analyses and cathodoluminescence studies of quartz were carried out to reveal processes, which have led to the low trace-element concentrations in quartz. This discovery, together with a better knowledge of the processes leading to the formation of HPQ in kyanite quartzites, could lead to the recognition of a new global type of HPQ resource applicable for industrial use. However, the processing technology necessary to separate HPQ from kyanite quartzite economically has not been developed so far.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The demand for the raw material quartz is increasing worldwide, in particular, the demand for high-purity quartz (HPQ; e.g. Moore 2005; Haus 2005). Therefore, quartz has been recently considered as a strategic mineral because it represents the raw material for special applications in high-tech industry. The trace-element contents of quartz are its most important quality criteria. Quartz is designated high purity when it contains not only less than 50 μg g−1 of impurities, which mainly comprise structurally bound trace elements (B, Li, Al, Ge, Ti, Fe, Mn, Ca, K, Na and P) in the quartz lattice, but also micro-inclusions of minerals and trapped fluids. HPQ is rare in nature and larger deposits even more so. The few HPQ deposits found around the world include certain types of quartz-rich granitic pegmatite (e.g. IOTA® 2005; Norwegian Crystallites AS 2006) and hydrothermal quartz veins.
Norway is one of the most important producers of silicon in the world. To ensure the availability of raw materials for future production of raw material quartz, the Geological Survey of Norway (NGU) has investigated quartz resources in Norway in recent decades. During these investigations, Wanvik (2004) discovered that the kyanite quartzite from Nasafjellet (Nordland, Norway) contains quartz grains with very low contents of lattice-bound trace elements. Kyanite quartzites are rare, exotic rocks that do not contain feldspar and biotite like ordinary sedimentary quartzites, and their origin is still under discussion (e.g. Larsson 2001 and references therein). Traditionally, such quartzites have been mined for kyanite, e.g. at Willis Mountain and East Ridge in the Appalachian belt of Virginia, USA (Kyanite Mining Corporation 2005).
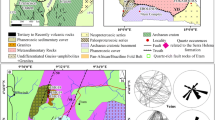
Further studies were carried out to verify, on a broader scale, if the low trace-element concentrations found in the quartz in the Nasafjellet kyanite quartzite are a common feature of such rocks. All known kyanite quartzites in Norway, located in Solør, Surnadal, Skjomen and Nasafjellet were sampled (Fig. 1). In addition, two non-Norwegian kyanite quartzites, from Hålsjöberg in Sweden and from Rio Levele in Niassa Province, Mozambique, were included. Concentrations of B, Be, Mn, Li, Al, Ge, Ti, Fe, Ca, K, Na and P in quartz from these rocks were analysed in situ by laser ablation–inductively coupled plasma mass spectrometry (LA–ICP-MS). The concentrations of most of these elements are <10 μg g−1 and, thus, the in situ analysis of such low concentrations is a challenge even with a state-of-art equipment (Flem et al. 2002; Müller et al. 2003b). Flem et al. (2002) developed an analytical procedure for routine trace-element determinations in quartz at the LA–ICP-MS laboratory of NGU. For this study, the procedure of Flem et al. (2002) has been improved by adding a new standard blank to the calibration.
Kyanite quartzite occurrences in Norway according to Ihlen (2000)
Scanning electron microscope cathodoluminescence (SEM-CL) of quartz has been applied to reveal possible growth textures, alteration structures and sequences of quartz precipitation reflecting the crystallisation processes involved in HPQ formation in the kyanite quartzites.
In this study, Al and Ti are used as indicators of the quality of the quartz. Besides these elements, Li, P and B are important quality-determining trace elements depending on the industrial application being considered for the raw material. High Ti concentrations, for example, change the absorption behaviour of optical silica glass. Metallic silicon and polysilicon are bred in quartz crucibles, and high P and B concentration in the quartz glass of the crucibles contaminates the growing silicon crystals, which are used for solar cells or computer chips. Moreover, Al, Ti and Li are difficult to remove during refinement of quartz. Therefore, the industry is highly interested in quartz resources with low initial trace-element concentrations to lower production costs and to improve the quality of the quartz products.
Geology and petrography of kyanite quartzites
Geological setting
Kyanite quartzite-form, strata-bound lens-shaped bodies, which may extend for several kilometres as trains of lenses, are commonly associated with metamorphosed acid and intermediate volcanic and subvolcanic rocks of island arc setting (e.g. McCauley 1961; Nystuen 1969; Spence et al. 1980; Hora 1998; Ihlen 2000; Bibikova et al. 2001; Larsson 2001). The largest deposits occurring in the Fennoscandian shield and the Appalachian belt are of Archaean (Russia), Proterozoic (Scandinavia) and Palaeozoic (USA) ages. In Norway and Sweden, they occur in Palaeoproterozoic volcano-sedimentary sequences, which are intruded by genetically related granitic plutons of the Transcandinavian Igneous Belt (Ihlen 2000). The rocks typically underwent upper greenschist-facies to amphibolite-facies metamorphism (>380°C and >280 MPa). The presence of kyanite depends on the peak metamorphic grade, and examples of quartzites with sillimanite or andalusite as the dominant Al silicate are also known (Ihlen 2000).
Kyanite quartzites occur in four areas in Norway: Solør, Surnadalen, Nasafjellet and Skjomen (Ihlen 2000; Fig. 1). Four large kyanite quartzite bodies occur in Solør at Gullsteinberget, Knøsberget, Kjeksberget and Sormbrua. The largest of these is the Gullsteinberget body with a length of 2 km and a thickness of up to 200 m (Fig. 2), which makes it the largest of deposit known in Norway. About 16 small bodies (20 to 140 m in length) are exposed in Skjomen, belonging to the Sørdalen supracrustal belt (Fig. 3a), which is part of the Palaeoproterozoic Rombak basement window (Korneliussen and Sawyer 1989). A nearly 70-km-long folded zone of muscovite-rich rocks with lenses of kyanite quartzites is known from Nasafjellet (Dahl 1980). The folded zone forms a part of the Caledonian Gargatis nappe, which consists of Svecokarelian (1.6 to 1.9 Ga) granitic gneisses, metarhyolites, metagabbros, muscovite and biotite schists (Gjelle 1988). The four known kyanite quartzite deposits are 150, 400, 500 and 600 m long and up to 30 m wide (Fig. 3b). They are embedded in muscovite schist. A small kyanite quartzite body (5 × 10 m) occurs at Tverrådalen in the Surnadalen area (Wanvik 1998). The deposit is situated in the border zone between basal, autochthonous Proterozoic gneiss units and allochthonous Caledonian nappe rocks that extend from the Trondheim region along Surnadalen and further westwards to the Molde area. The kyanite quartzite lens is hosted by autochthonous basement granitic gneiss of Mesoproterozoic age (1600–1000 Ma; Tveten et al. 1998).
a Weathering-resistant lumps of kyanite quartzite at Skjomen. b Previous sampling locality of kyanite quartzite at Nasafjellet for dimension stone. Iron oxides (brown) on fractures result from weathering of accessory pyrite. The lower edge of the photograph corresponds to 2 m. c Centimeter-scale layering of kyanite-rich quartzite (turquoise), kyanite-poor quartzite (white) and rutile-bearing kyanite quartzite (brownish) at Skjomen. d Hand specimen scan of rutile-rich kyanite quartzite from Sormbrua. e Fine-grained greenish kyanite quartzite from Gullsteinberget with fine-disperse pyrite (black dots). f Kyanite quartzite from Sormbrua with blue agglomerations consisting of wavellite, kyanite and lazulite
Petrography
The kyanite quartzites listed above are fine-grained, laminated metamorphic rocks containing 70 to 85 vol.% quartz and >15 vol.% kyanite. Their composition changes gradually into kyanite-poor or kyanite-free pyrophyllite/muscovite-bearing quartzites between the layers and towards the contact with the host rocks. Alternating quartz-rich, kyanite-rich and pyrophyllite/muscovite-rich layers are from several centimetres up to several metres in thickness (Fig. 3c). Kyanite-rich layers contain up to 45 wt% kyanite. The average grain size of the quartz varies between 100 and 1,000 μm. The quartz texture consists either of elongated, sutured and recrystallised grains or forms a granoblastic–polygonal texture depending on the local deformation history. Kyanite plates are commonly 100–2,000 μm long and occasionally contain inclusion of quartz and/or rutile. Up to 2 cm long crystals occur in centimeter-thick layers at Nasafjellet. Kyanite-rich layers can be altered to muscovite-rich layers due to retrograde metamorphism. The kyanite quartzites do not contain feldspar or biotite, which are common minor minerals in quartzites of sedimentary origin. Common accessories are rutile, zircon and pyrite. Rutile can be a minor mineral in centimeter-thick layers with concentrations of up to 2 wt% (Fig. 3d). Fine-grained pyrite (1 mm) is dispersed in the rock, and it forms up to 3 wt% (Fig. 3e). Sporadic accessories are pyrophyllite, muscovite, topaz, fluorite, lazulite, wavellite, barite, apatite, Al–Sr phosphates and Cr mica (Fig. 3f; Müller et al. 2005).
Analytical methods
X-ray fluorescence spectrometry
Major and trace-element concentrations of kyanite quartzites were determined by X-ray fluorescence spectrometry (XRF) using a Phillips PW1480 spectrometer equipped with an Sc/W X-ray tube at the Geological Survey of Norway (NGU). Approximately 3 g sample material milled to ∼40 μm were fused at 1,030°C for 10 min. Loss on ignition (LOI) was determined gravimetrically and was used as an approximate measure of volatiles such as H2O and CO2. An amount of 0.6 g of the sample material was then fused in a Pt crucible with lithium tetraborate (Li2B4O7) at 1,120°C, yielding a homogeneous, optically flat glass disc. Major element analyses were carried out for Na, Mg, Al, Si, P, K, Ca, Ti, Mn and Fe on the glass discs. The total Fe concentration was calculated as Fe2O3. Accuracy and limits of detection (LODs) of major elements are listed in Table 1. For trace-element determination, pressed powder pellets bound with the agent wax Hoechst C were used.
Laser ablation–inductively coupled mass spectrometry
LA–ICP-MS was used for the in situ determination of Li, Be, B, Ge, Na, Al, P, K, Ca, Ti, Mn and Fe in quartz grains in the kyanite quartzites. The ICP-MS used in this study is a double-focusing sector field instrument (model-ELEMENT-1, Finnigan MAT, Bremen, Germany), which has been described in detail by Gießmann and Greb (1994) and Feldmann et al. (1994). The configuration used in this work includes the CD-2 Guard Electrode. A Finnigan MAT, UV laser probe operating at 266 nm with a Gaussian beam profile, as described by Schroeder et al. (1998), was used for ablation. A repetition rate of 20 Hz and pulse energy of 0.7–0.8 mJ with continuous ablation on an area of approximately 180 × 250 μm were applied. The laser was not equipped with an aperture, but the laser beam was adjusted to give a spot size of approximately 30 μm. Helium was used as a carrier gas to enhance transport efficiency of ablated material (Günther and Heinrich 1999). The helium carrier was mixed with argon as a makeup gas before entering the ICP-MS to maintain stable and optimum excitation conditions. Key operating parameters of the laser probe and the ICP-MS are summarised in Table 2. With this laser, there could be difficulties in controlling ablation diameter and ablation depth. The ablation parameters therefore had to be carefully selected. Ideally, a shorter wavelength UV laser should be used, e.g. an eximer laser or Nd:YAG laser operating at 193 nm and with an aperture for the precise control of spot size and with a flat beam profile yielding flat craters.
The existence of spectroscopic interferences required the use of variable mass resolutions. Li, Be, B, Mn, and Ge were analysed at low mass resolution (m/Δm ≈ 300), whereas Al, Na, Ca, P, Ti and Fe required medium mass resolution (m/Δm = 3,500) and K high mass resolution (m/Δm > 8,000). The isotope 29Si was used as an internal standard at low mass resolution and the 30Si at medium and high mass resolution. In addition, the argide 40Ar40Ar+ was measured in medium and high mass resolution between each analytical measurement and used as lock masses to compensate for time-dependant instrumental mass drift.
External calibration was done using four silicate glass reference materials produced by the National Institute of Standards and Technology (NIST SRM 610, NIST SRM 612, NIST SRM 614, and NIST SRM 616). In addition, the standard reference material NIST 1830, the soda-lime float glass (0.1 wt% Al2O3) from NIST, the high-purity silica BCS 313/1 reference sample from the Bureau of Analysed Samples, UK, the certified reference material “pure substance No. 1” silicon dioxide SiO2 from the Federal Institute for Material Research and Testing, Berlin, Germany and the Qz–Tu synthetic pure quartz monocrystal provided by Andreas Kronz from the Geowissenschaftliches Zentrum Göttingen (GZG), Germany, were used. Certified, recommended and proposed values for the reference materials were taken from their respective certificates of analysis when available, and if not, from Govindaraju (1994), Pearce et al. (1997), Horn et al. (1997), Gao et al. (2002) and Flem and Bedard (2002). Each measurement consists of 15 scans of each isotope, with a measurement time varying from 0.15 s per scan of K in high resolution to 0.024 s per scan of, e.g. Mn in low resolution. An Ar blank was run before each standard and sample measurement. The background signal was subtracted from the instrumental response of the standard before normalisation against the internal standard. This was done to avoid memory effects between samples. A weighted linear regression model including several measurements of the different standard was used for calculation of the calibration curve for each element.
Ten successive measurements on the Qz–Tu were used to estimate the LODs. LODs are based on three times standard deviation (3σ) of the ten measurements divided by the sensitivity S. Quartz analyses were performed at several days resulting in slightly different LODs for each measurement day and sequence. Examples of typical detection limits are given in Table 3. Flem et al. (2002) gave a more detailed description of the measurement procedure.
Scanning electron microscope cathodoluminescence
SEM-CL images were obtained from polished thin sections coated with carbon using the LEO 1450VP analytical SEM with an attached CENTAURUS BS BIALKALI-type cathodoluminescence (CL) detector. The applied acceleration voltage and current at the sample surface were 20 kV and ∼3 nA, respectively. Due to the generally low CL intensity of quartz, thin sections were coated with a thin layer of carbon (10 to 15 Å) using the POLARON RANGE CC7650 Carbon Coater. The coating time was chosen as 2 s instead of the 5 s usually applied for SEM investigations. The BIALKALI tube has a CL response range from 300 nm (violet) to 650 nm (red). It peaks in the violet spectrum range around 400 nm. The CL images were collected from one scan of 43 s photospeed and a processing resolution of 1,024 × 768 pixels and 256 grey levels. The brightness and contrast of the collected CL images were improved with the PhotoShop software.
Results
Whole-rock chemistry
Whole-rock analyses of major and some trace elements of the kyanite quartzites are listed in Table 4. The analysed samples contain 13–30 wt% Al2O3. Sample R1021b with 0.19 wt% Al2O3 represents a pure quartzitic layer within the kyanite quartzite unit of Juovvačorrú. The most characteristic chemical features of the kyanite quartzites are the low concentrations of CaO, Na2O and K2O less than 1 wt% (Fig. 4a). The low alkali metal contents and >10 wt% Al2O3 are the main criteria for distinguishing kyanite quartzites from ordinary quartzites of sedimentary origin.
a Major element plot of Norwegian kyanite quartzites. Compositions of Norwegian quartzites of sedimentary origin are plotted for comparison. The data of the Norwegian quartzites are from the NGU industrial mineral database. b Zr versus Cr/Zr plot of Norwegian kyanite quartzites and quartzites of sedimentary origin
Kyanite quartzites have elevated concentrations of Ba, W, Zr, Sr, Cr and V. Ba and Sr are highly variable depending on the assemblage of accessory minerals. Zr varies between 73 and 312 μg g−1. Kyanite quartzites from Juovvačorrú are depleted in Ba, Zr and Sr compared to the other Norwegian kyanite quartzites. Norwegian kyanite quartzites plot relatively closely in the Zr vs Zr/Cr diagram (Fig. 4b). The Swedish kyanite quartzite from Halsjöberget, which is considered to be genetically comparable to the deposits in the Solør area (Müller et al. 2005), has a lower Zr content than the Norwegian ones. The Zr/Cr ratio of kyanite quartzites resembles that of quartzites of sedimentary origin.
Quartz texture and SEM-CL
The quartz intergrowth texture in the fine-grained kyanite quartzites is commonly equigranular. The quartz grains in kyanite-rich layers are smaller (100–300 μm) than in kyanite-poor layers (300–1,000 μm). Some of the larger grains (>500 μm) have undulatory extinction with sub-grains forming a stripe-like pattern. Some quartzite varieties, e.g. from Juovvačorrú and Gullsteinberget, contain quartz with straight grain boundaries and 120° triple-junctions between grains forming a granoblastic–polygonal crystal texture (‘foam texture’). The texture indicates the stable arrangement of the crystal framework due to grain boundary area reduction during lattice recovery (e.g. Passchier and Trouw 1998). Quartz from the Tverrådalen and Nasafjellet deposits has sutured and irregular grain boundaries (Fig. 5a), generally interpreted as non-equilibrium ‘frozen’ mobile boundaries formed by late-stage retrograde deformation. The texture is attributed to strain-induced grain boundary migration (e.g. Passchier and Trouw 1998). Impurities and tiny accessory minerals are preferentially enriched along grain boundaries and trans-granular micro cracks (Fig. 5b).
a Quartz texture in the kyanite quartzite from Tverrådalen. Crossed nicols. mus Muscovite. b Enrichment of impurities at the grain boundary (black arrows). Crossed nicols. c SEM-CL image of quartz from the Sormbrua deposit. Quartz shows concentric shadows of brighter CL (white arrows). d SEM-CL image of quartz from the Tverrådalen deposit. Tongues of dull luminescent quartz replaced quartz with brighter CL (white arrows). The tongues show weak contrasted oscillatory banding. rt Rutile
Quartz has a more or less homogeneous, weak, dull red-brown luminescence. However, some quartz grains show slight variations of the CL intensity visible as different grey shades in the SEM-CL images. Concentric shadows of brighter CL have been observed in crystals from the Sormbrua deposit, which may reflect former grain boundaries or diffusion zoning (Fig. 5c). In the samples from Tverrådalen and Nasafjellet, tongues of dull luminescent quartz seem to replace quartz with brighter CL (Fig. 5d). The tongues show weak contrasted oscillatory banding.
Trace-element abundances in quartz from kyanite quartzites
Trace-element concentrations of quartz grains in kyanite quartzites determined by LA–ICP-MS are listed in Table 5. Generally, quartz contains very low concentrations, around 1 μg g−1 or less, of Li, Be, B, Mn, Ge, K and Fe. Al in quartz from Norwegian kyanite quartzites varies between 5 and 33 μg g−1 and Ti between <0.1 and 31 μg g−1. The outstanding high Ti of analysis 2907406-A (31 μg g−1) is probably caused by a rutile micro-inclusion, which was hit during laser ablation. The relatively large ablation volume of the laser (180 × 250 × 40 μm) during sampling involves the risk that fluid and mineral inclusions in quartz can contribute to the measured concentrations of trace elements in the quartz lattice. Rutile inclusions in quartz are very common in the kyanite quartzites (Figs. 5a,d). The LODs for Na, Ca and P are too high to draw any conclusions. However, LA–ICP-MS analyses done by Wanvik (2004) on quartz from the Nasafjellet deposit gave concentrations between <1 and 6 μg g−1 for Na and <1 μg g−1 for K (Table 5).
Al and Ti concentrations in quartz from the kyanite quartzites and of refined high-quality quartz products fabricated by Norwegian Crystallites in Norway (e.g. Drag NCA and Drag NC1) and Unimin in USA (Iota STD and Iota 8) are plotted in Fig. 6. Al and Ti are used as indicator elements of the quartz quality because they are the most common trace elements. Both elements can hardly be removed during processing of the raw material quartz, and the chemical cleaning results in high production costs. The distinction of HPQ from quartz with lower purity in Fig. 6 is based on published concentrations of trace elements in industrial HPQ products, which are sold worldwide (Table 5).
Al vs Ti plot of quartz in kyanite quartzites from Norway, Sweden and Mozambique and of refined HPQ products. Quartz with Al <25 μg g−1 and Ti <10 μg g−1 is considered as ‘high-purity quartz’. Concentrations of the quartz products Drag NC1, Drag NCA, Iota STD and Iota 8 were analysed by solution ICP-MS (Norwegian Crystallites AS 2006; Moore 2005). The company IOTA® is the world-leading producer of HPQ
Eighty-four percent of the analyses of quartz from Norwegian kyanite quartzites plot in the HPQ field. Quartz from the Knøsberget, Juovvačorrú and Nasafjellet deposits is the purest among the Norwegian kyanite quartzites when comparing the average concentrations. The Tverrådalen and Gullsteinberget deposits contain quartz with slightly elevated Ti and Al concentrations, which plot at the boundary of the HPQ field. However, low Al and Ti observed in the quartz from Norwegian kyanite quartzites do not seem to be a universal feature of kyanite quartzites occurring worldwide. Quartz in kyanite quartzites from Halsjöberget (Sweden) and Rio Levele (Mozambique) has significantly higher Al, Ti, K and Fe and plots in the medium and low-quality fields.
Discussion
Causes of the low trace-element concentrations in quartz
Compared with published data, the trace-element concentrations of quartz in the upper greenschist- to amphibolite-facies (380–550°C and 280–500 MPa) kyanite quartzites are low. However, reliable datasets comprising the trace-element concentrations in quartz from metamorphic rocks determined in situ by micro beam techniques are rare. Van den Kerkhof et al. (2004a) detected highly variable trace-element concentrations in the epiclastic Patsoana quartzites, South Africa, which range from <8 to 1,000 μg g−1 for Ti and 20 to 2,000 μg g−1 for Al. The early Archean Patsoana quartzites were affected by amphibolite-facies and contact metamorphism (>600°C). Korneliussen et al. (2006) reported 33 to 69 μg g−1 Ti and 5 to 186 μg g−1 Al in the Eidfjord quartzites and 2 to 3 μg g−1 Ti and 9 to 15 μg g−1 Al from the Horgaberg quartzite. These Norwegian Proterozoic quartzites underwent upper amphibolite- and greenschist-facies metamorphism, respectively. The few data show a strong variation of trace elements in quartz from quartzites of sedimentary origin.
Müller et al. (2003a), Larsen et al. (2004a,b), Spear and Wark (2004), Wark et al. (2004) and Wark and Watson (2006) suggested that Ti in quartz increases with temperature. Formation temperatures of quartz in kyanite quartzites were calculated applying the Ti-in-quartz geothermometer by Wark and Watson (2006; Table 6). According to the geothermometer, the general formation temperature of HPQ with <10 μg g−1 Ti should be <530°C. The geothermometer can be applied without restrictions on quartz in kyanite quartzites because it crystallised in the presence of rutile. The calculations give adequate formation temperatures for the quartz in the Gullsteinberget and Halsjöberget deposits (considering the non-contaminated analysis 3107401-A; Table 5) and for samples with relative constant Ti concentrations (Tverrådalen, Nasafjellet and Rio Levele kyanite quartzites). The Ti values of quartz from the Knøsberget, Kjeksberget, Sormbrua and Juovvačorrú deposits fall within two ranges, i.e. 2.5–6.1 and 0.1–0.3 μg g−1 (Table 5). The upper concentration range corresponds to temperatures between 438 and 495°C and the lower range to 290 to 333°C. However, Sawyer (1986) suggests a formation temperature of about 575°C for the Sørdalen supracrustal belt including the Juovvačorrú kyanite quartzite. The calculated temperatures in Table 6 may not record the peak conditions of kyanite quartzite formation if quartz was formed on the prograde path and if it did not recrystallise at the highest temperature. Thus, the lower temperature range may represent quartz grains formed during retrograde recrystallisation (e.g. Fig. 5d). Widespread grain boundary area reduction together with the associated lattice recovery with grain boundary migration is a common feature related to the retrograde overprint. This process may purify and re-equilibrate the quartz lattice by healing crystal defects and expelling trace elements to the grain boundaries (Fig. 5b) and/or concentrate them in micro-inclusions (Van den Kerkhof and Müller 1999; Van den Kerkhof et al. 2004b). It is generally accepted that incipient recrystallisation leads to strain reduction by slight boundary movements into neighbouring grains that are more strained. Tongues of newly formed, low-stress quartz on the grain boundary migrate into neighbouring grains of higher strain and defect concentration. The process is frozen in Fig. 5d, where dull luminescent (defect-poor) quartz grows at the cost of the brighter luminescent (relative defect-rich) crystal. Bright luminescent high-temperature quartz is commonly richer in Ti than weakly luminescent quartz (e.g. Müller et al. 2000, 2002, 2003a; Van den Kerkhof et al. 2004a). However, the generally weak CL of quartz in the kyanite quartzites indicates relatively low concentrations of lattice defects and luminescence active trace elements (CL activators) in the quartz lattice (e.g. Götze et al. 2001).
Origin of kyanite quartzites
Kyanite quartzites exhibit a number of features, which distinguish them from ordinary quartzites originating from sandstone and arkose. Kyanite quartzites (1) form rare lens-shaped bodies from a meter-scale up to a length of several kilometres, (2) are commonly associated with metamorphosed acidic and intermediate volcanic and sub-volcanic rocks or with mafic volcanic and intrusive rocks of island arc settings (e.g. McCauley 1961; Nystuen 1969; Spence et al. 1980; Bibikova et al. 2001), (3) do not contain feldspar and biotite and (4) contain rare mineral assemblages of Fe–Ti oxides and sulfides, pyrophyllite, Al phosphates, lazulite, apatite, topaz, fluorite, Cr mica and gold (e.g. Spence et al. 1980).
A number of petrogenetic models for the genesis of kyanite quartzite have been presented over the last 50 years but no consensus has been reached. Ihlen (2000) gave a review of discussions and distinguished three general models for the development of kyanite quartzites:
-
(1)
Metamorphosed advanced argillic alteration zones in felsic and intermediate volcanites and subvolcanic intrusions of island arc settings are suggested to be the pre-metamorphic protoliths of kyanite quartzites by Larsson (2001), Bibikova et al. (2001) and others. Fluid convection above hot granitic magma bodies giving off acid volatiles during degassing causes the acid leaching of igneous rocks, leading to the development of zones with strong argillic alteration composed dominantly of quartz, Al silicates (clays) and subordinate Al hydroxides (gibbsite). During metamorphism, clay minerals transform to quartz and kyanite, andalusite or sillimanite, depending on the metamorphic grade.
-
(2)
Metamorphism of high-alumina sediments. Willner et al. (1990) and others suggest that kyanite quartzites originate from metamorphosed aluminous clays and quartz sand, which are weathering products of altered subvolcanic and volcanic rocks. The Al-rich siliceous sediments were redistributed and deposited in basins where further Al enrichment occurred due to hydrothermal activity associated with adjacent volcanic centres.
-
(3)
Structurally controlled syn-metamorphic metasomatism. The close proximity of some kyanite quartzites to faults and shears in metamorphic terrains, e.g. kyanite-rich metasomatic rocks along the Protogine Zone in southern Sweden, has led to models advocating shear-induced syn-metamorphic metasomatism of quartzo-feldspathic rocks (Andréasson and Dallmeyer 1995; Ihlen and Marker 1998).
The three models of kyanite quartzite formation explain, in different ways, the strong enrichment of alumina and silica in the protoliths and the depletion of alkalis, which make the source rocks and their metamorphic products unique in a chemical sense. Model 1 is the most suitable to explain the field relationships and analytical data obtained. However, the limited dataset presented in this study does not permit a final conclusion.
Economic aspects
The Norwegian kyanite quartzite deposits show the following advantages for utilisation as HPQ material:
-
1)
Low concentrations of trace elements in the quartz grains
-
2)
Absence of fluid inclusions in quartz
-
3)
Straight and planar grain boundaries of quartz in the Gullsteinberget, Knøsberget, Kjeksberget, Sormbrua and Juovvačorrú deposits, which reduce energy consumption during rock crushing and grain liberation during milling
-
4)
The size and exposure of the kyanite quartzite bodies allow open pit mining. The largest deposit is the Gullsteinberget kyanite quartzite in Solør where more than 30,000,000 t of kyanite quartzite is available assuming an open pit depth of 40 m.
-
5)
Quartz and kyanite can presumably be easily separated after milling by gravity due to their density contrast. Quartz has a density of 2.62 g cm−1 and kyanite of 3.61 g cm−1. Kyanite concentrates can be used for refractory production.
The disadvantages for their utilisation are:
-
1)
Intimate intergrowths of quartz with kyanite, pyrophyllite and muscovite, generating contaminated quartz grains
-
2)
The heterogeneous distribution of kyanite and mica in the rock (centimeter- to meter-scale layering)
-
3)
The small grain size of quartz (100–1,000 μm)
-
4)
Enrichment of impurities along grain boundaries
-
5)
Common occurrence of micro-inclusions of zircon, apatite, pyrite and prismatic rutile in quartz.
Taking all the advantages and disadvantages of the investigated Norwegian kyanite quartzites into account, the Norwegian kyanite quartzites may be considered as potential future resources of HPQ. However, their grain size and the intimate intergrowth of HPQ, kyanite and other mineral constituents will be a challenge for the mineral-processing engineers.
Summary
High-performance trace-element analyses of trace elements in quartz grains of Norwegian kyanite quartzites by LA–ICP-MS reveal low concentrations of Li, Be, B, Ge, Al, P, K, Ti, Mn and Fe, which define the quartz grains as HPQ. The rarity of kyanite quartzites, the almost absent alkalis and their unique mineral assemblage suggest that they are not of sedimentary origin and were affected by leaching during evolution. Although the mechanisms for the incorporation of trace elements into the atomic structure of quartz are rather well known, knowledge of the processes and conditions favouring formation of HPQ and its deposits is fragmentary. The protoliths probably contained quartz with initial low trace-element concentrations, which were formed in a hydrothermal alteration zone. The early formation conditions seem to be an important prerequisite for the development of HPQ in kyanite quartzites. Widespread grain boundary migration during retrograde overprint and re-equilibration further purified the quartz lattice, whereby impurities were expelled from the lattice and enriched at the grain boundaries and in micro-inclusions. Kyanite and rutile may have acted as sinks for Al and Ti, respectively.
References
Andréasson PG, Dallmeyer RD (1995) Tectonothermal evolution of high-alumina rocks within the Protogine Zone, southern Sweden. J Metamorph Geol 13:461–474
Bibikova EV, Ihlen PM, Marker M (2001) Age of the hydrothermal alteration leading to garnetite and kyanite pseudo-quartzite formation in the Khizovaara segment of the late Archean Keret Greenstone Belt, Russian Karelia. EUG XI Strasbourg, 8.–12.4.2001. J Conf Abstr 6:277
Dahl Ø (1980) Nasa og Stødi kyanittfelter-resultater fra diamantboring og geologiske undersøkelser sommeren 1980. Aspro rapport 1115. Bergvesenrapport BV 3506, p 21
Ek R, Nysten P (1990) Phosphate mineralogy of the Hålsjöberg and Hökensås kyanite deposits. Geol Foren Stockh Forh 112:9–18
Feldmann I, Tittes W, Jakubowski N, Stuewer D, Giessmann U (1994) Performance characteristics of inductively-coupled plasma-mass spectrometry with high-mass resolution. J Anal At Spectrom 9:1007–1014
Flem B, Bedard LP (2002) Determination of trace elements in BCS CRM 313/1 (BAS) and NIST SRM 1830 by inductively coupled plasma-mass spectrometry and instrumental neutron activation analysis. Geostand Newsl 26:287–300
Flem B, Larsen RB, Grimstvedt A, Mansfeld J (2002) In situ analysis of trace elements in quartz by using laser ablation inductively coupled plasma mass spectrometry. Chem Geol 182:237–247
Gao S, Liu X, Yuan H, Hattendorf B, Günther D, Chen L, Hu S (2002) Determination of forty-two major and trace elements in USGS and NIST SRM glasses by laser ablation-inductively coupled plasma-mass spectrometry. Geostand Newsl 26:181–196
Gießmann U, Greb U (1994) High resolution ICP-MS—a new concept for elemental mass spectrometry. Fresenius J Anal Chem 350:186–193
Gjelle S (1988) Geologisk kart over Norge, berggrunnskart Saltdal, M 1: 250.000. Norges Geologiske Undersøkelse, Trondheim, Norway
Götze J, Plötze M, Habermann D (2001) Origin, spectral characteristics and practical applications of the cathodoluminescence (CL) of quartz—a review. Mineral Petrol 71:225–250
Govindaraju K (1994) Compilation of working values and sample description for 383 geostandards. Geostand Newsl 18(Special Issue):1–158
Günther D, Heinrich CA (1999) Enhanced sensitivity in LA–ICP-MS using helium–argon mixtures as aerosol carrier. J Anal At Spectrom 14:1369–1374
Haus R (2005) High demands on high purity. Ind Miner 10:62–67
Hora ZD (1998) Industrial minerals in island arcs. In: Metallogeny of volcanic arcs. B.C. Geological Survey, Short Course Notes, Open File 1998-8, Section L. Accessed 20th May 2005. http://www.em.gov.bc.ca/Mining/Geolsurv/MetallicMinerals/metallogeny/L98_ Abstract_Hora.HTM
Horn I, Hinton RW, Jackson SE, Longerich HP (1997) Ultra-trace element analysis of NIST SRM 616 and 614 using laser ablation microprobe inductively coupled plasma mass spectrometry (LAM–ICP-MS): a comparison with secondary ion mass spectrometry (SIMS). Geostand Newsl 21:191–203
Ihlen PM (2000) Utilisation of sillimanite minerals, their geology, and potential occurrences in Norway—an overview. Norg Geol Unders Bull 436:113–128
Ihlen PM, Marker M (1998) Kyanite-rich metasomatic rocks along crustal-scale shear zones in the Baltic Shield: evidence of shear induced fluid migration during tectonic dissection of Palaeoproterozoic supracrustal sequences? In: Phillippov N (ed) Abstract volume for Svekalapko Europrobe Project Workshop 1998, Repino, Russia. The Ministry of Natural Resources of Russian Federation/State Company ‘Mineral’, pp 25–26
IOTA® (2005) IOTA® high-purity quartz. Accessed 20th May 2005. http://www.iotaquartz.com/welcome.html
Korneliussen A, Sawyer EW (1989) The geochemistry of Lower Proterozoic mafic to felsic igneous rocks, Rombak Window, North Norway. Norg Geol Unders Bull 415:7–21
Korneliussen A, Wanvik JE, Müller A, Ihlen PM (2006) Kystnære kvartsressurser i Hordaland. Geological Survey of Norway, Report 2006.045
Kyanite Mining Corporation (2005) Kyanite Mining Corporation—our facilities. Accessed 10th May 2005. http://www.kyanite.com/index.html
Larsen RB, Henderson I, Ihlen PM, Jacamon F (2004a) Distribution and petrogenetic behaviour of trace elements in granitic pegmatite quartz from South Norway. Contrib Mineral Petrol 147:615–628
Larsen RB, Jacamon F, Ihlen PM, Henderson I (2004b) Natural refinement of quartz raw materials for the production of polycrystalline silicon. In: Pecchio M et al (eds) Applied Mineralogy—developments in science and technology, vol. 2. ICAM-BR, Sao Paulo, pp 693–696
Larsson D (2001) Transition of granite to quartz–kyanite rock at Hålsjöberg, southern Sweden: consequence of acid leaching and later metamorphism. GFF 123:237–246
McCauley JF (1961) Carolina Geological Society Guidebook. Division of Geology, State Development Board, Columbia, S.C., Geological Notes 5/5, p 13
Moore P (2005) High-purity quartz. Ind Miner 455:53–57 (August)
Müller A, Seltmann R, Behr H-J (2000) Application of cathodoluminescence to magmatic quartz in a tin granite—case study from the Schellerhau Granite Complex, Eastern Erzgebirge, Germany. Miner Depos 35:169–189
Müller A, Lennox P, Trzebski R (2002) Cathodoluminescence and micro structural evidence for crystallisation and deformation processes in quartz of S- and I-type granites in the Eastern Lachlan Fold Belt (SE Australia). Contrib Mineral Petrol 143:510–524
Müller A, Rene M, Behr H-J, Kronz A (2003a) Trace elements and cathodoluminescence of igneous quartz in topaz granites from the Hub Stock (Slavkovský Les Mts., Czech Republic). Mineral Petrol 79:167–191
Müller A, Wiedenbeck M, van den Kerkhof AM, Kronz A, Simon K (2003b) Trace elements in quartz—a combined electron microprobe, secondary ion mass spectrometry, laser-ablation ICP-MS, and cathodoluminescence study. Eur J Mineral 15:747–763
Müller A, Wanvik JE, Kronz A (2005) Norwegian kyanite quartzites—potential resources of high-purity quartz? Norg geologiske undersøkelse, Rapport 2005.039
Norwegian Crystallites AS (2006) Norwegian Crystallites AS-products-crystal quartz analyses. Accessed 20th September 2006. http://www.norcryst.no
Nystuen JP (1969) Precambrian ash-flow tuff and associated volcanic rocks at Elverum, southern Norway. Norg Geol Unders Bull 258:240–241
Passchier CW, Trouw RAJ (1998) Microtectonics. Springer, Berlin, pp 289
Pearce NJG, Perkins WT, Westgate JW, Gorton MP, Jackson SE, Neal CR, Chenery SP (1997) A compilation of new and published major and trace element data for NIST SRM 610 and NIST SRM 612 glass reference materials. Geostand Newsl 21:115–144
Sawyer E (1986) Metamorphic assemblages and conditions in the Rombak basement window. Geological Survey of Norway, Report 88.116
Schroeder E, Hamester M, Kaiser M (1998) Properties and characteristics of a laser ablation ICP-MS system for the quantitative elemental analysis of glasses. Appl Surf Sci 127–129:292–298
Spear FS, Wark D (2004) TITANiQ: potential applications of the Ti-in-quartz (+rutile) thermometer in metamorphic rocks. Eos Trans AGU, Joint Assembly Suppl, Abstract 85:JA491
Spence WH, Worthington JP, Jones EM, Kiff IT (1980) Origin of the gold mineralization at the Haile Mine, Lancaster County, South Carolina. Min Eng 32:70–73
Tveten E, Lutro O, Thorsnes T (1998) Geologisk kart over Norge, berggrunnskart Ålesund, 1: 250.000. Norges geologiske undersøkelse, Trondheim, Norway
Van den Kerkhof AM, Müller A (1999) Fluid inclusion re-equilibration and trace element redistribution in quartz: observations by cathodoluminescence microscopy. ECROFI XV Abstracts, Terra Nostra 99/6:161–162
Van den Kerkhof AM, Kronz A, Simon K, Riganti A, Scherer T (2004a) Origin and evolution of Archean quartzites from the Nondweni greenstone belt (South Africa): inferences from a multidisciplinary study. S Afr J Geol 107:559–576
Van den Kerkhof AM, Kronz A, Simon K, Scherer T (2004b) Fluid-controlled quartz recovery in granulite as revealed by cathodoluminescence and trace element analysis (Bamble sector, Norway). Contrib Mineral Petrol 146:637–652
Wanvik JE (1998) Kyanite investigations in Tverrådalen, Surnadal. Geological Survey of Norway, Report 1998.080
Wanvik JE (2004) Supplerende kvartsundersøkelser på Saltfjellet. Geological Survey of Norway, Report 2003.106
Wark DA, Watson EB (2006) TitaniQ: a titanium-in-quartz geothermometer. Contrib Mineral Petrol 152:743–754
Wark DA, Anderson AT, Watson EB (2004) Probing Ti in quartz: application of the TITANiQ thermometer to the Bishop Tuff. Eos Trans AGU, Joint Assembly Suppl, Abstract 85:JA494
Willner A, Schreyer W, Moore JM (1990) Peraluminous metamorphic rocks from the Namaqualand metamorphic complex (South Africa): geochemical evidence of an exhalation-related, sedimentary origin on a Mid-Proterozoic rift system. Chem Geol 81:221–240
Acknowledgements
This study was supported by the Geological Survey of Norway Project 286100. The authors are grateful to R.B. Larsen and S. Swanson for their helpful reviews. The data from the Rio Levele deposit are included with the permission of the National Directorate of Geology, Mozambique. We greatly appreciate the critical comments and language improvement of R. Boyd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: B. Lehmann
Rights and permissions
About this article
Cite this article
Müller, A., Ihlen, P.M., Wanvik, J.E. et al. High-purity quartz mineralisation in kyanite quartzites, Norway. Miner Deposita 42, 523–535 (2007). https://doi.org/10.1007/s00126-007-0124-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00126-007-0124-8