Abstract
Aims/hypothesis
There is controversy regarding the performance of HbA1c in old age. We evaluated the prognostic value of HbA1c and other glycaemic markers (fructosamine, glycated albumin, fasting glucose) with mortality risk in older adults (66–90 years).
Methods
This was a prospective analysis of 5636 participants (31% with diagnosed diabetes, mean age 76, 58% female, 21% black) in the Atherosclerosis Risk in Communities (ARIC) study, baseline 2011–2013. We used Cox regression to examine associations of glycaemic markers (modelled in categories) with mortality risk, stratified by diagnosed diabetes status.
Results
During a median of 6 years of follow-up, 983 deaths occurred. Among older adults with diabetes, 30% had low HbA1c (<42 mmol/mol [<6.0%]) and 10% had high HbA1c (≥64 mmol/mol [≥8.0%]); low (HR 1.32 [95% CI 1.04, 1.68]) and high (HR 1.86 [95% CI 1.32, 2.62]) HbA1c were associated with mortality risk vs HbA1c 42–52 mmol/mol (6.0–6.9%) after demographic adjustment. Low fructosamine and glycated albumin were not associated with mortality risk. Both low and high fasting glucose were associated with mortality risk. After further adjustment for lifestyle and clinical risk factors, high HbA1c (HR 1.81 [95% CI 1.28, 2.56]), fructosamine (HR 1.96 [95% CI 1.43–2.69]), glycated albumin (HR 1.81 [95% CI 1.33–2.47]) and fasting glucose (HR 1.81 [95% CI 1.24, 2.66]) were associated with mortality risk. Low HbA1c and fasting glucose were no longer significantly associated with mortality risk. Among participants without diabetes, associations of glycaemic markers with mortality risk were less robust.
Conclusions/interpretation
Elevated HbA1c, fructosamine, glycated albumin and fasting glucose were associated with risk of mortality in older adults with diabetes. Low HbA1c and fasting glucose may be markers of poor prognosis but are possibly confounded by health status. Our findings support the clinical use of HbA1c in older adults with diabetes.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
HbA1c is central to the diagnosis and management of diabetes. However, our understanding of the association of HbA1c with clinical outcomes is based primarily on studies of middle-aged adults. There has been recent debate regarding the interpretation of age-related increases in HbA1c and whether age-related increases reflect true hyperglycaemia [1,2,3,4]. This has implications for the use and interpretation of HbA1c in older age.
Among middle-aged adults, J- or U-shaped curves between HbA1c and risk of mortality have been reported in both adults with and without diabetes in some studies [5,6,7,8,9] but not others [10, 11]. There is controversy regarding why low HbA1c might be associated with mortality risk [12, 13] and whether there is a J-shaped association in older age [14,15,16,17,18,19]. This issue is particularly relevant to clinical care for frail older adults with diabetes or those with a presumed limited life expectancy due to concerns of potential overtreatment and risks of hypoglycaemia in the setting of strict glycaemic control [20].
Fructosamine and glycated albumin are serum markers of hyperglycaemia that are highly correlated with HbA1c [21]. Both tests are Food and Drug Administration (FDA)-cleared for clinical use in the USA but are not routinely used. Fructosamine and glycated albumin track with HbA1c and are similarly associated with microvascular [22] and macrovascular outcomes in middle-aged adults [23], however there are scant data on fructosamine and glycated albumin in older adults. Because these biomarkers are haemoglobin-independent and, thus, not subject to the same factors that can affect the interpretation of HbA1c [21], they can help us understand whether associations with outcomes are glycaemic or non-glycaemic in nature.
We sought to test whether HbA1c was associated with all-cause mortality risk in older adults aged 66–90 years with and without diabetes, and compared these associations to those for fructosamine, glycated albumin and fasting glucose. Based on the prior literature in middle-aged adults, we hypothesised there would be J-shaped associations between glycaemic markers and mortality risk among older adults with and without diabetes. We also explored determinants of low HbA1c.
Methods
Study design
The ARIC study is a prospective multi-centre community-based cohort, which began in 1987–1989, when participants were aged 45–64 years [24]. Multiple clinic visits have since taken place. At each clinic visit, participants underwent an interview, physical examination and blood tests. All participants provided written informed consent and ARIC protocols were approved at Institutional Review Boards for each study centre.
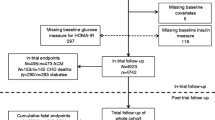
We conducted a prospective cohort analysis with visit 5 (2011–2013, attended by 65% of the participants still alive) as baseline when participants were 66 to 90 years old. Of the 6538 visit 5 participants, we excluded 439 who were missing any of the glycaemic measurements, 247 with non-fasting blood tests and 181 where information was missing on other model covariates. Additionally, participants who were neither black nor white as well as black participants at the Maryland and Minnesota centres were excluded due to small numbers (n = 35). This resulted in an analytic sample of 5636 participants (31% with diagnosed diabetes) (see electronic supplementary material [ESM] Fig. 1 for details).
Diabetes and glycaemic measurements
We conducted all analyses stratified by diagnosed diabetes status, which was defined as history of self-reported physician diagnosis or current medication use for diabetes (self-reported at visit 5). We did not have information on diabetes type. Duration of diagnosed diabetes at visit 5 was estimated based on the date of the annual (or semi-annual) phone calls where diagnosed diabetes was first reported, and was dichotomised at the median (≥10 vs <10 years). Glycaemic markers were measured at visit 5 in all participants (with and without diagnosed diabetes). HbA1c was measured in whole blood using a Tosoh G7 Automated HPLC Analyzer (Tosoh Bioscience, USA), which was standardised to the DCCT assay. Fructosamine and glycated albumin were both measured in serum on the Roche Modular P800 Chemistry Analyzer (Roche Diagnostics, USA). Fructosamine was measured using a colorimetric assay by Roche Diagnostics. Glycated albumin was measured using a complex method by Asahi Kasei Pharma (Japan). Fasting glucose was measured in serum using the hexokinase method. The laboratory intra-assay CV based on blind duplicate samples for HbA1c was 1.3% and for fasting glucose was 5.7%. For fructosamine, CVs were 3.2% at a concentration of 212.6 μmol/l and 2.5% at a concentration of 856.7 μmol/l. For glycated albumin, CVs were 2.3% at a concentration of 1.579 g/dl and 2.8% at a concentration of 0.426 g/dl.
Other measurements
Participants self-reported their demographic characteristics (including race), smoking and drinking status. BMI was calculated from measured height and weight. BP was measured three times, and we used the mean of the second and third measurements. eGFR was calculated using the CKD-EPI equation based on cystatin C and creatinine [25]. Total cholesterol and HDL-cholesterol were measured in plasma using colorimetric methods. Haemoglobin was measured using an Automated Haematology Analyser (ABX Horiba Diagnostics MICROS 60-CS, USA). Anaemia was defined as haemoglobin <135 g/l for men and <120 g/l for women; moderate/severe anaemia was defined as haemoglobin for men <120 g/l and <100 g/l for women [26]. Liver enzymes (alanine and aspartate aminotransferase [ALT, AST] and γ-glutamyl transferase [GGT]) were measured in serum. Elevated liver enzymes were defined by sex-specific 95th percentile thresholds [27]. Participants were asked to bring their medication containers to each visit, and these were transcribed and coded. Prevalent CVD included CHD (defined as either definite or probable myocardial infarction), stroke (definite or probable) and heart failure. Frailty status was based on a standard definition used in prior studies, defined by the presence of ≥3 of the following: low energy, low physical activity, low strength, slowed motor performance and unintentional weight loss [28].
Outcome ascertainment
All-cause mortality was identified through annual (semi-annual since 2012) follow-up telephone calls to participants or their proxies, state records and linkage to the National Death Index up to 31 December 2018.
Data analysis
We present baseline (visit 5) participant characteristics across HbA1c categories, stratified by diagnosed diabetes status. We categorised HbA1c among participants without diabetes as <31 mmol/mol, 31–<39 mmol/mol, 39–<48 mmol/mol and ≥48 mmol/mol (undiagnosed diabetes) (<5.0%, 5.0–<5.7%, 5.7–<6.5%, ≥6.5%), and among those with diagnosed diabetes as <42 mmol/mol, 42–<53 mmol/mol, 53–<64 mmol/mol, and ≥64 mmol/mol (<6.0%, 6.0–<7.0%, 7.0–<8.0%, ≥8.0%). Diabetes-specific percentile equivalent cut-points to HbA1c were used for fructosamine and glycated albumin [23]. Fasting glucose was modelled using clinically relevant cut-points in those individuals without diabetes as <5.5, 5.5–<7.0, and ≥7.0 mmol/l. In those with diagnosed diabetes we categorised fasting glucose as <5.5, 5.5–<8.3, 8.3–<11.1, and ≥11.1 mmol/l.
We used Cox proportional hazards regression models to examine the associations between categories of glycaemic markers and risk of mortality among participants with and without diabetes. We also modelled the glycaemic biomarkers more flexibly using restricted cubic splines. We compared two models: in Model 1, we adjusted for age, sex and race-centre (Forsyth County, North Carolina-white; Forsyth County, North Carolina-black; Washington County, Maryland-white; Minneapolis, Minnesota-white; Jackson, Mississippi-black). In Model 2, we additionally adjusted for current smoking status (yes/no), current drinking status (yes/no), BMI (continuous), systolic BP (continuous), antihypertension medication use, eGFR (continuous, spline with knot at eGFR <60 ml min−1 [1.73 m]−2), HDL (continuous), total cholesterol (continuous), cholesterol-lowering medication use (yes/no) and haemoglobin (continuous). We verified the proportional hazards assumption for each model. We used seemingly unrelated regression to compare the strength of the HRs for the associations of HbA1c, fructosamine, glycated albumin and fasting glucose with mortality risk [29]. In a sensitivity analysis, we further adjusted for markers of comorbid health status: prevalent CVD, elevated liver enzymes (AST, ALT, GGT) and frailty.
To evaluate cross-sectional determinants of low levels of HbA1c and other glycaemic markers, we used logistic regression models adjusted for age, sex and race-centre. In these models, we selected variables (BMI, eGFR, CVD, liver disease, frailty, anaemia, diabetes duration and medication use) that have been previously linked to low HbA1c in prior literature and that could confound the association between low glycaemic levels and risk of mortality [30].
We examined results stratified by race, given the ongoing controversy regarding the interpretation of racial differences in HbA1c [31]. We also conducted analyses stratified by anaemia, stage 3+ chronic kidney disease (CKD), and frailty status and formally tested for multiplicative interaction by these variables. A p value <0.05 was considered statistically significant.
Results
The mean age of the 5636 participants was 76 years, 58% were female and 21% were black. Overall, 31% of the study population had diagnosed diabetes. Over a median of 6 years of follow-up, 983 deaths occurred (400 occurring in those with diagnosed diabetes).
Older adults without diabetes
In people without diagnosed diabetes, compared with those with normal HbA1c 31–<39 mmol/mol (5.0–<5.7%), individuals with low HbA1c <31 mmol/mol (<5.0%) were more likely to be male, black, have prevalent CVD and have moderate or severe anaemia (Table 1). Individuals with HbA1c ≥48 mmol/l (≥6.5%, undiagnosed diabetes) were more likely to be female, black, obese, and to have CVD than those with normal HbA1c 31–<39 mmol/mol (5.0–5.7%).
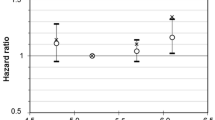
Among older people without diabetes, HbA1c was weakly associated with fructosamine (Pearson’s r = 0.19), glycated albumin (r = 0.28) and fasting glucose (r = 0.36) (ESM Table 1). Low HbA1c <30 mmol/mol (<5.0%) was associated with higher mortality risk after adjustment for demographic variables compared with those with HbA1c 31–<39 mmol/mol (5.0–<5.7%) (Model 1; Fig. 1a and Table 2), but not after additional adjustment for lifestyle and clinical risk factors (Model 2). Undiagnosed diabetes (HbA1c ≥48 mmol/mol or ≥6.5%) was not significantly associated with mortality risk. Individuals with fructosamine and glycated albumin in the ≥98th percentile (vs 6th–55th percentile) had elevated mortality risk (Model 1): HR 1.99 (95% CI 1.39, 2.84) for fructosamine and HR 1.88 (95% CI 1.28, 2.75) for glycated albumin. After adjustment for lifestyle and clinical risk factors, the associations remained significant for fructosamine (HR 1.63 [95% CI 1.12, 2.35]) but was attenuated for glycated albumin (HR 1.38 [95% CI 0.93, 2.06]) (Fig. 1b-c and Table 2). Fasting glucose was not significantly associated with risk of mortality in older adults without diabetes (Fig. 1d and Table 3). Associations between all four glycaemic markers and mortality were not appreciably altered when we adjusted for all variables in Model 2 plus markers of comorbid health status: prevalent CVD, elevated AST, ALT or GGT, and frailty.
Associations of glycaemic markers with all-cause mortality, stratified by diabetes diagnosis status (a–d, no diagnosed diabetes; e–h, diagnosed diabetes), for the ARIC study 2011–2018, showing HR (95% CI) and proportion of the population (%). Restricted cubic splines adjusted for age, sex and race. Knots at 5th, 35th, 65th, and 95th percentiles, and centred at the median; >99th and <1st percentile removed to minimise influence of extreme values and for ease of display
There were no statistically significant differences for the association of any of the glycaemic biomarkers with mortality risk according to race (Model 1: all p values for interaction >0.17; ESM Fig. 2a-b), anaemia (p-interactions >0.21; ESM Fig. 3a-b), CKD (p-interactions >0.60; ESM Fig. 4a-b), or frailty (p-interactions >0.26; ESM Fig. 5a-b). While not statistically significant, there were, however, some qualitative differences for race and anaemia. High fructosamine was significantly associated with increased risk of mortality in white people but not black people. High HbA1c was associated with increased risk of mortality in individuals without anaemia but not in those with anaemia. In the subset with frailty and no diabetes, no glycaemic markers were significantly associated with mortality risk (ESM Fig. 5a-b).
Individuals with low HbA1c <31 mmol/mol (<5.0%) were more likely to have stage 3+ CKD (OR 1.55 [95% CI 1.03, 2.34]), prevalent CVD (OR 1.92 [95% CI 1.25, 2.94]) or moderate/severe anaemia (OR 3.34 [95% CI 1.76, 6.35]) (ESM Table 2), compared with those in the normal HbA1c range 31–<39 mmol/mol (5.0–<5.7%). Individuals with low (vs normal) fructosamine (OR 2.52 [95% CI 1.14, 5.56]) were more likely to have moderate/severe anaemia.
Older adults with diabetes
In people with diagnosed diabetes, compared with those with low HbA1c <42 mmol/mol (<6.0%), individuals with high HbA1c ≥64 mmol/l (≥8.0%) were more likely to be black, obese, have a longer duration of diabetes or to be currently taking diabetes medications (Table 1).
In individuals with diabetes, biomarkers of hyperglycaemia were highly correlated: HbA1c and fructosamine r = 0.75; HbA1c and glycated albumin r = 0.81; HbA1c and fasting glucose r = 0.64 (ESM Table 1). HbA1c, fructosamine and glycated albumin all had similarly shaped associations with mortality risk (Fig. 1e–g). With adjustment for demographic factors, low (HR 1.32 [95%CI 1.04, 1.68]) and high HbA1c (HR 1.86 [95% CI 1.32, 2.62]) were both associated with an increased risk of mortality vs HbA1c 42–52 mmol/mol (6.0–6.9%). Low fructosamine and glycated albumin were not significantly associated with mortality risk. After additional adjustment for lifestyle and clinical risk factors, high levels (≥91st percentile) of HbA1c, fructosamine and glycated albumin were associated with mortality risk, compared with those with markers between the 32nd and 73rd percentile, e.g. HbA1c HR 1.81 (95% CI 1.28, 2.56). Similar to the association observed for HbA1c, fasting glucose followed a J-shaped association in the demographic-adjusted model (Fig. 1h and Table 3); after adjustment for lifestyle and clinical risk factors, elevated glucose carried higher mortality risk, and low fasting glucose was no longer statistically significantly associated with mortality risk (Table 3). When we further adjusted Model 2 for prevalent CVD, elevated liver enzymes and frailty, the associations between HbA1c, fructosamine, glycated albumin and fasting glucose and mortality risk were unchanged.
Associations of the biomarkers of hyperglycaemia and mortality risk were not statistically significantly different according to race (p-interactions >0.08, ESM Fig. 2c-d), anaemia (p-interactions >0.07, ESM Fig. 3c-d), CKD (p-interactions >0.13, ESM Fig. 4c-d), or frailty (p-interactions >0.12, ESM Fig. 5c-d). In the subset of participants with diabetes and who were frail, low (<42 mmol/mol, <6.0%) and high (≥8.0%) HbA1c were associated with elevated risk of mortality; fructosamine and glycated albumin were not associated with risk of mortality in this subgroup (Models 1–2, ESM Fig. 5c-d).
In participants with diabetes, those with low HbA1c (<42 mmol/mol, <6.0%) were less likely to be overweight or obese, had a shorter diabetes duration, and were less likely to use diabetes medications than those between 42 and <53 mmol/mol (6.0–<7.0%) (ESM Table 2).
Discussion
Among older adults with diabetes, elevated HbA1c, fructosamine, glycated albumin and fasting glucose were robustly associated with risk of mortality; low HbA1c and fasting glucose were associated with mortality (J-shaped) but these associations were not statistically significant after further adjusting for confounding factors. In older adults without diabetes, associations between glycaemic markers and mortality risk were less consistent. Elevated fructosamine was associated with an increased risk of mortality. Low HbA1c was associated with elevated risk of mortality after adjusting for demographic characteristics, but this association was no longer significant with further adjustment. Our findings support the clinical use of HbA1c among older adults with diabetes and suggest that the performance of fructosamine, glycated albumin and fasting glucose as markers of risk is similar compared with HbA1c.
The strength of risk factor associations differ across the life course; typically, risk factors measured in mid-life are more strongly associated with cardiovascular outcomes [32, 33] and mortality risk [32,33,34] than those same risk factors measured in late-life. Understanding the HbA1c-mortality relationship in late-life has implications for diabetes care in older adults. HbA1c is an important risk factor for microvascular outcomes (CKD, end-stage renal disease, retinopathy) [35, 36], CVD [37,38,39] and all-cause mortality [37, 38, 40, 41] in middle-aged adults. In our study population with diabetes (aged 75+), we found that HbA1c was positively associated with risk of mortality. Consistent with prior observations, the association between HbA1c and mortality risk was less robust than that observed in middle-age (aged 48–67) [23, 38]. Indeed, in individuals without diabetes, we did not observe significant associations of HbA1c with mortality risk after adjustment for confounding factors.
Previous research in older adults has not examined alternative glycaemic markers with risk of mortality; this can help us discern if associations are glycaemic or non-glycaemic in nature. We found that glycated albumin and fructosamine were correlated with HbA1c—especially in individuals with diabetes—and their associations with mortality were generally similar in magnitude and shape as compared with HbA1c. This suggests that the associations that we observed between HbA1c and mortality risk primarily reflect hyperglycaemia-related processes.
Some [5,6,7,8,9, 15] but not all [10, 11, 14] prior studies in individuals without diabetes have reported J-shaped curves between HbA1c and mortality risk. In a prior ARIC analysis, no one specific cause of death (cancer, cardiovascular, respiratory, digestive/liver, genitourinary/kidney) accounted for the elevated risk among individuals with low HbA1c who were middle-aged at the time [41]. After accounting for potential confounding factors, we did not find robust associations between low HbA1c and mortality risk in this older population. Liver disease has been postulated as a mechanism by which low HbA1c leads to mortality [42]. In analyses of the National Health and Nutrition Examination Survey (NHANES), adults without diabetes who had very low HbA1c (<20 mmol/mol; <4.0%) had much higher prevalence of elevated liver enzymes, hepatitis C seropositivity [5, 42] and ultrasound-detected steatosis as compared with those with HbA1c between 31 and <37 mmol/mol (5.0–<5.5%) [42]. In middle-aged ARIC participants without diabetes, a J-shaped curve between HbA1c and risk of liver hospitalisations has also been reported [41]. By contrast, in our study among the now older ARIC participants, liver enzymes were not particularly elevated among individuals with low HbA1c (or other glycaemic biomarkers). Anaemia and conditions relating to red-cell turnover are also plausible explanations linking low HbA1c and mortality [43]. We found that moderate or severe anaemia was a strong determinant (threefold higher) of having low HbA1c (vs normal) in older adults without diabetes. Moderate or severe anaemia was also a predictor of having low fructosamine (and glycated albumin, albeit non-statistically significantly), which are not haemoglobin-dependent glycaemic markers. The effect estimates between the individual glycaemic markers and mortality risk were slightly larger among individuals without anaemia than among those with anaemia. We did not find, however, any statistical evidence to suggest that the association between HbA1c and other glycaemic markers differed according to anaemia status (nor did adjustment for haemoglobin affect our results) in older adults with or without diabetes.
In older individuals with diabetes, some experts have raised concerns of potential overtreatment and whether goals for glycaemic control should be less stringent in frail individuals or those with complex health status. Low HbA1c may be a confounded marker of poor prognosis in older adults with diabetes, particularly those who are frail. In our study population, low HbA1c, in older adults with diabetes, was not a robust predictor of mortality risk after adjustment; similar patterns were observed for fructosamine, glycated albumin and fasting glucose. These results suggest that, in older adults, well-controlled diabetes (i.e. HbA1c <53 mmol/mol or <7.0%) is not a robust marker for mortality. Indeed, individuals with diabetes and low HbA1c were more likely to have a shorter duration of diabetes and were less likely to be taking glucose-lowering medications.
There are limitations of this study. First, we had limited power for testing for interaction and estimating moderate effects in subgroups. Second, elevated HbA1c without a diagnosis of diabetes—i.e. undiagnosed diabetes—was uncommon in this study population, limiting our power to detect associations of undiagnosed diabetes with mortality risk. Lastly, while we adjusted for major confounders, residual confounding remains a possibility [30]. Strengths of this study include the standardised assessment and rigorous measurement of covariates in a research setting and active surveillance for mortality. To our knowledge, our study is the first examination of glycated albumin and fructosamine with mortality risk in a community-based population of older adults with and without diabetes.
In conclusion, elevated HbA1c, fructosamine, glycated albumin and fasting glucose were associated with risk of mortality in older adults with diabetes. We also found evidence that low levels of HbA1c and fasting glucose were associated with mortality risk (J-shaped associations) in those with diabetes, and that low HbA1c was associated with mortality risk in those without diabetes, but these associations were attenuated after accounting for confounding characteristics. Among participants without diabetes, associations of glycaemic markers with risk of mortality were less robust. Our results support the notion that low HbA1c is a confounded marker of poor outcomes, rather than a direct risk factor for mortality. Collectively, our findings support the complementary nature of glycaemic biomarkers and the clinical utility of HbA1c among older adults.
Data availability
The datasets analysed within this study are not publicly available due to the possibility that some information in these data might compromise research participants’ privacy or consent. However, data may be available from the corresponding author on request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- ARIC:
-
Atherosclerosis Risk in Communities
- AST:
-
Aspartate aminotransferase
- CKD:
-
Chronic kidney disease
- GGT:
-
γ-Glutamyl transferase
References
American Diabetes Association (2020) Standards of Medical Care in Diabetes—2020. Diabetes Care 43(Suppl 1):S1–S212
Selvin E, Parrinello CM (2013) Age-related differences in glycaemic control in diabetes. Diabetologia 56(12):2549–2551. https://doi.org/10.1007/s00125-013-3078-7
Warren B, Rawlings AM, Lee AK, Grams M, Coresh J, Selvin E (2017) Increases in biomarkers of hyperglycemia with age in the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 40(8):e96–e97. https://doi.org/10.2337/dc17-0075
Pani LN, Korenda L, Meigs JB et al (2008) Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care 31(10):1991–1996. https://doi.org/10.2337/dc08-0577
Carson AP, Fox CS, McGuire DK et al (2010) Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes 3(6):661–667. https://doi.org/10.1161/circoutcomes.110.957936
Mongraw-Chaffin M, Bertoni AG, Golden SH et al (2019) Association of low fasting glucose and HbA1c with cardiovascular disease and mortality: the MESA study. J Endocr Soc 3(5):892–901. https://doi.org/10.1210/js.2019-00033
Brewer N, Wright CS, Travier N et al (2008) A New Zealand linkage study examining the associations between A1C concentration and mortality. Diabetes Care 31(6):1144–1149. https://doi.org/10.2337/dc07-2374
Saydah S, Tao M, Imperatore G, Gregg E (2009) GHb level and subsequent mortality among adults in the U.S. Diabetes Care 32(8):1440–1446. https://doi.org/10.2337/dc09-0117
Levitan EB, Liu S, Stampfer MJ et al (2008) HbA1c measured in stored erythrocytes and mortality rate among middle-aged and older women. Diabetologia 51(2):267–275. https://doi.org/10.1007/s00125-007-0882-y
Pfister R, Sharp SJ, Luben R, Khaw KT, Wareham NJ (2011) No evidence of an increased mortality risk associated with low levels of glycated haemoglobin in a non-diabetic UK population. Diabetologia 54(8):2025–2032. https://doi.org/10.1007/s00125-011-2162-0
Chonchol M, Katz R, Fried LF et al (2010) Glycosylated hemoglobin and the risk of death and cardiovascular mortality in the elderly. Nutr Metab Cardiovasc Dis 20(1):15–21. https://doi.org/10.1016/j.numecd.2009.02.007
American Diabetes Association (2020) 12. Older adults: Standards of Medical Care in Diabetes—2020. Diabetes Care 43(Suppl 1):S152. https://doi.org/10.2337/dc20-S012
Schottker B, Rathmann W, Herder C et al (2016) HbA1c levels in non-diabetic older adults - No J-shaped associations with primary cardiovascular events, cardiovascular and all-cause mortality after adjustment for confounders in a meta-analysis of individual participant data from six cohort studies. BMC Med 14:26. https://doi.org/10.1186/s12916-016-0570-1
Palta P, Huang ES, Kalyani RR, Golden SH, Yeh HC (2017) Hemoglobin A1c and mortality in older adults with and without diabetes: results from the National Health and Nutrition Examination Surveys (1988-2011). Diabetes Care 40(4):453–460. https://doi.org/10.2337/dci16-0042
Huang ES, Liu JY, Moffet HH, John PM, Karter AJ (2011) Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 34(6):1329–1336. https://doi.org/10.2337/dc10-2377
Forbes A, Murrells T, Mulnier H, Sinclair AJ (2018) Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol 6(6):476–486. https://doi.org/10.1016/S2213-8587(18)30048-2
Hamada S, Gulliford MC (2016) Mortality in individuals aged 80 and older with type 2 diabetes mellitus in relation to glycosylated hemoglobin, blood pressure, and total cholesterol. J Am Geriatr Soc 64(7):1425–1431. https://doi.org/10.1111/jgs.14215
Doucet J, Verny C, Balkau B, Scheen AJ, Bauduceau B (2018) Haemoglobin A1c and 5-year all-cause mortality in French type 2 diabetic patients aged 70 years and older: the GERODIAB observational cohort. Diabetes Metab 44(6):465–472. https://doi.org/10.1016/j.diabet.2018.05.003
Monami M, Vitale V, Lamanna C et al (2013) HbA1c levels and all-cause mortality in type 2 diabetic patients: epidemiological evidence of the need for personalised therapeutic targets. Nutr Metab Cardiovasc Dis 23(4):300–306. https://doi.org/10.1016/j.numecd.2012.01.003
Kirkman MS, Briscoe VJ, Clark N et al (2012) Diabetes in older adults. Diabetes Care 35(12):2650–2664. https://doi.org/10.2337/dc12-1801
Parrinello CM, Selvin E (2014) Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep 14(11):548. https://doi.org/10.1007/s11892-014-0548-3
Selvin E, Rawlings AM, Grams M et al (2014) Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2(4):279–288. https://doi.org/10.1016/s2213-8587(13)70199-2
Selvin E, Rawlings AM, Lutsey PL et al (2015) Fructosamine and glycated albumin and the risk of cardiovascular outcomes and death. Circulation 132(4):269–277. https://doi.org/10.1161/circulationaha.115.015415
The ARIC Investigators (1989) The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 129(4):687–702. https://doi.org/10.1093/oxfordjournals.aje.a115184
Inker LA, Schmid CH, Tighiouart H et al (2012) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367(1):20–29. https://doi.org/10.1056/NEJMoa1114248
World Health Organization (1968) Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser 405:5–37
Lazo M, Rubin J, Clark JM et al (2015) The association of liver enzymes with biomarkers of subclinical myocardial damage and structural heart disease. J Hepatol 62(4):841–847. https://doi.org/10.1016/j.jhep.2014.11.024
Kucharska-Newton AM, Palta P, Burgard S et al (2017) Operationalizing frailty in the Atherosclerosis Risk in Communities study cohort. J Gerontol A Biol Sci Med Sci 72(3):382–388. https://doi.org/10.1093/gerona/glw144
Zellner A (1962) An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. J Am Stat Assoc 57(298):348–368. https://doi.org/10.1080/01621459.1962.10480664
Rutter MK (2012) Low HbA1c and mortality: causation and confounding. Diabetologia 55(9):2307–2311. https://doi.org/10.1007/s00125-012-2620-3
Selvin E (2016) Are there clinical implications of racial differences in HbA1c? A difference, to be a difference, must make a difference. Diabetes Care 39(8):1462–1467. https://doi.org/10.2337/dc16-0042
Lind L, Sundstrom J, Arnlov J, Lampa E (2018) Impact of aging on the strength of cardiovascular risk factors: a longitudinal study over 40 years. J Am Heart Assoc 7(1):e007061. https://doi.org/10.1161/jaha.117.007061
Sattar N, Rawshani A, Franzen S et al (2019) Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 139(19):2228–2237. https://doi.org/10.1161/circulationaha.118.037885
Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju Sh N et al (2016) Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388(10046):776–786. https://doi.org/10.1016/s0140-6736(16)30175-1
Selvin E, Ning Y, Steffes MW et al (2011) Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes 60(1):298–305. https://doi.org/10.2337/db10-1198
Lee MY, Huang JC, Chen SC, Chiou HC, Wu PY (2018) Association of HbA1C variability and renal progression in patients with type 2 diabetes with chronic kidney disease stages 3–4. Int J Mol Sci 19(12):4116. https://doi.org/10.3390/ijms19124116
Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N (2004) Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 141(6):413–420. https://doi.org/10.7326/0003-4819-141-6-200409210-00006
Selvin E, Steffes MW, Zhu H et al (2010) Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 362(9):800–811. https://doi.org/10.1056/NEJMoa0908359
Colayco DC, Niu F, McCombs JS, Cheetham TC (2011) A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 34(1):77–83. https://doi.org/10.2337/dc10-1318
Currie CJ, Peters JR, Tynan A et al (2010) Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 375(9713):481–489. https://doi.org/10.1016/s0140-6736(09)61969-3
Aggarwal V, Schneider AL, Selvin E (2012) Low hemoglobin A(1c) in nondiabetic adults: an elevated risk state? Diabetes Care 35(10):2055–2060. https://doi.org/10.2337/dc11-2531
Christman AL, Lazo M, Clark JM, Selvin E (2011) Low glycated hemoglobin and liver disease in the U.S. population. Diabetes Care 34(12):2548–2550. https://doi.org/10.2337/dc11-0944
Zoppini G, Targher G, Chonchol M et al (2010) Anaemia, independent of chronic kidney disease, predicts all-cause and cardiovascular mortality in type 2 diabetic patients. Atherosclerosis 210(2):575–580. https://doi.org/10.1016/j.atherosclerosis.2009.12.008
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions. Parts of this study were presented as an abstract at the American Heart Association Epi|Lifestyle Scientific Sessions in Phoenix, AZ, USA, from 3–6 March 2020.
Author’s relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract numbers (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Research reported in this publication was supported by the NIH/NHLBI grant T32HL007024 (MRR), NIH/NIDDK grants F30DK120160 (OT), K24DK106414 (ES) and R01DK089174 (ES). Reagents for the fructosamine assays were donated by Roche Diagnostics, and reagents for the glycated albumin (albumin) assays were donated by the Asahi Kasei Corporation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study sponsor/funder was not involved in the design of the study; the collection, analysis and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Author information
Authors and Affiliations
Contributions
MRR and ES designed the study, conducted the analyses and drafted the manuscript. All authors provided data interpretation and meaningful contributions to the revision of the manuscript. All authors gave final approval of the version to be published. MRR and ES are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM
(PDF 422 kb)
Rights and permissions
About this article
Cite this article
Rooney, M.R., Tang, O., Pankow, J.S. et al. Glycaemic markers and all-cause mortality in older adults with and without diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 64, 339–348 (2021). https://doi.org/10.1007/s00125-020-05285-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-020-05285-3