Abstract
Aims/hypothesis
We tested the hypothesis that dietary acid load may increase the risk of type 2 diabetes, and studied the association between acid load and insulin sensitivity as a possible mechanism involved.
Methods
An observational survey with prospective follow-up including 911 non-diabetic Swedish men aged 70–71 years was carried out. The gold standard euglycaemic–hyperinsulinaemic clamp technique and the OGTT were used to determine insulin sensitivity and beta cell function, respectively. Diabetes incidence was assessed during 18 years of follow-up. Renal function was estimated from serum cystatin C concentrations. Dietary acid load was calculated as potential renal acid load (PRAL) and net endogenous acid production (NEAP) algorithms from 7 day food records. Adequate dietary reporters were identified by Goldberg cut-offs.
Results
PRAL and NEAP were not associated with insulin sensitivity or beta cell function. Underlying kidney function or consideration of dietary adequate reporters did not modify these null findings. During follow-up, 115 new cases of diabetes were validated. Neither PRAL nor NEAP was associated with diabetes incidence.
Conclusions/interpretation
Our results do not support the hypothesis that dietary acid load influences insulin sensitivity, beta cell function or diabetes risk. Interventional studies modifying acid–base dietary intake are needed to further elucidate a possible role of acid load in the development of type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A healthy diet and lifestyle are effective preventive measures for reducing the risk of diabetes [1, 2]. A recent population-based study [3] of women from the French centres of the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study reported that excess dietary acid load was associated with increased diabetes risk. The rationale for that study was that dietary factors can affect acid–base balance [4] and insulin sensitivity (IS) [5]. Specifically, a Western diet rich in animal products and other acidogenic foods may lead to endogenous production of acid, which is not compensated for because of the shortage of fruit and vegetables, leading to increased metabolic acidosis [6].
Validation of this finding in men as well as in other societies with different dietary habits is important to substantiate preventive dietary recommendations. A better understanding of the mechanisms involved should also contribute to the identification of therapeutic targets. Diet is likely to influence the risk of diabetes by affecting IS or its reciprocal, insulin resistance [7]. The latter plays an important role in the pathogenesis of multiple metabolic diseases, and is considered the strongest predictor of diabetes [8]. However, the consequences of excess dietary acid depend on the ability of the kidney to excrete that acid load [9, 10], and underlying kidney dysfunction may have confounded previous observations.
Two methods are commonly used to estimate dietary acid load from dietary recalls in epidemiology studies: the potential renal acid load (PRAL) method and the net endogenous acid production (NEAP) method [11–13]. A negative PRAL value reflects a base-forming potential, whereas a positive value reflects an acid-forming potential [14]. Therefore, the present study aimed to evaluate the association between dietary acid load, IS determined using both the gold standard euglycaemic clamp method and beta cell function assessed by the OGTT, and the risk of diabetes in older community-dwelling Swedish men of similar age. Within this aim, we also assessed possible effect modification by underlying kidney function and addressed the potential bias of over-/under-reporting inherent to dietary-recall methods.
Methods
Study population
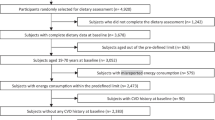
This investigation was performed in the Uppsala Longitudinal Study of Adult Men (ULSAM) (www2.pubcare.uu.se/ULSAM/). The study, initiated in the 1970s, invited all 50-year-old men living in the Uppsala region to participate, and subsequent re-examinations were planned. The present analyses are based on the third examination cycle of the ULSAM cohort. During this examination, participants were 70–71 years of age (examinations performed during 1991–1995; n = 1,221), and a detailed comorbid history, risk factor assessment and dietary records were collected simultaneously. For this specific analysis, we set some a priori exclusions (n = 310): (1) presence of diabetes at the time of examination (defined as fasting plasma glucose ≥7.0 mmol/l, 2 h post-load glucose level ≥11.1 mmol/l, or the use of oral hypoglycaemic agents or insulin; n = 179); (2) lack of data on the main variables for this study (i.e. euglycaemic clamps [n = 52], serum cystatin C [n = 24], or 7 day dietary records [n = 50]); (3) extreme values of reported energy intake (<3,200 or >18,000 kJ/day; n = 5). The present study therefore comprises 911 diabetes-free individuals with complete data. All participants gave written consent, and the ethics committee of Uppsala University approved the study.
Demographics and comorbidities
Investigations were performed under standardised conditions as described elsewhere [15]. Smoking status was defined as current smoking vs non-smoking. Regular physical activity was defined as the reporting of regular or athletic leisure time exercise habits according to four physical activity categories (sedentary, moderate, regular and athletic) [16]. Educational level was recorded as low (elementary school), medium (secondary school) or high (university). Previous cardiovascular disease (CVD) was established as history of any CVD as recorded in the Swedish Hospital Discharge Registry (ICD-8 codes 390–458 [www.wolfbane.com/icd/icd8.htm] or ICD-9 codes 390–459 [www.icd9data.com/2007/Volume1/240-279/250-259/250/default.htm]). BP was measured in duplicate using the right arm with the participant supine after resting for 10 min. Hypertension was defined as BP ≥140/90 mmHg or the use of antihypertensive medication. Hyperlipidaemia was defined as serum cholesterol >6.5 mmol/l, serum triacylglycerols >2.3 mmol/l, or treatment with lipid-lowering medication.
Laboratory measurements
Venous blood samples were drawn after an overnight fast and stored at −70°C until analysis. The assays were performed at the Department of Clinical Chemistry, University Hospital, Uppsala, which is accredited according to the Swedish Board for Accreditation and Conformity Assessment (Swedac) standard ISO/IEC 17025. Serum cystatin C was measured with latex-enhanced reagent (N Latex Cystatin C; Dade Behring, Deerfield, IL, USA) using a Behring BN ProSpec analyser (Dade Behring). The total analytical imprecision of the method was 4.8% at 0.56 mg/l and 3.7% at 2.85 mg/l. Estimated GFR (eGFR) was calculated from serum cystatin C concentrations (mg/l) by the following formula: eGFR = 77.24 × cystatin C− 1.2623; this has been shown to correlate closely with iohexol clearance [17]. Fasting plasma glucose was measured by the glucose dehydrogenase method (Gluc-DH; Merck, Darmstadt, Germany). Urinary albumin excretion rate (UAER) was measured in one overnight urine collection and expressed in μg/min. The assay used a commercially available radioimmunoassay kit (Albumin RIA 100; Pharmacia, Uppsala, Sweden).
Insulin sensitivity
The euglycaemic–hyperinsulinaemic clamp technique of DeFronzo et al [18] was used, with a slight modification to suppress hepatic glucose production for estimation of in vivo sensitivity to insulin [19]. Insulin (Actrapid Human; Novo, Copenhagen, Denmark) was infused in a primary dose for the first 10 min and then as a continuous infusion (389 pmol/min per m2 body surface area) for 2 h to maintain steady-state hyperinsulinaemia, achieving a level of about 660 pmol/l. The target plasma glucose level was 5.1 mmol/l, maintained by measuring plasma glucose every 5 min. The glucose infusion rate during the last hour was used as a measure of the glucose disposal rate (M value, mg kg−1 min−1; to convert values to SI units [mmol min−1 kg−1] multiply by 0.00555). The coefficient of variation of the M value was 12% on repeated clamp investigations within 30 days in the same individual. The Insulin Sensitivity Index (M/I ratio) is a measure of the tissue sensitivity to insulin expressed per pmol/l of insulin and was calculated by dividing M by the mean insulin concentration during the same period of the clamp. M/I thus represents the amount of glucose metabolised per unit of plasma insulin and is given in mg kg−1 min−1 (pmol/l)−1 of insulin multiplied by 100. In addition, an OGTT was performed in which the participants ingested 75 g glucose dissolved in 300 ml water. Blood samples for determination of plasma glucose and insulin were drawn immediately before and 30, 60, 90 and 120 min after ingestion of glucose. The Insulinogenic Index (IGI) for beta cell function was calculated using the following formula: IGI = (insulin30 min − insulin0 min)/(glucose30 min − glucose0 min) [20]. The OGTT and the clamp procedure took place on separate days within the same week.
Dietary acid load
Dietary habits were evaluated from a 7 day dietary record based on a validated pre-coded menu book, which was prepared and previously used by the Swedish National Food Administration (NFA) [21]. The participants were given oral instructions by a dietitian on how to perform the dietary recording, and the amounts consumed were reported in household measurements or specified as portion sizes. The daily intake of energy and macro- and micro-nutrients was calculated using a database from the NFA. In order to reduce extraneous variation and predict the effect of dietary interventions, the daily intake of macro- and micro-nutrients was corrected for total energy intake by regression analysis of the residual method [22]. PRAL and NEAP scores were derived from estimations of the intake of several nutrients [11–13]:
-
1.
\( \begin{array}{l}\mathrm{PRAL}\kern0.5em \left(\mathrm{mEq}/\mathrm{day}\right)=0.49\times \mathrm{protein}\kern0.5em \mathrm{intake}\kern0.3em \left(\mathrm{g}/\mathrm{day}\right)+0.037\times \mathrm{phosphorus}\kern0.5em \left(\mathrm{mg}/\mathrm{day}\right)-\hfill \\ {}0.021\times \mathrm{potassium}\left(\mathrm{mg}/\mathrm{day}\right)-0.013\times \mathrm{calcium}\kern0.5em \left(\mathrm{mg}/\mathrm{day}\right)-0.026\times \mathrm{magnesium}\hfill \\ {}\left(\mathrm{mg}/\mathrm{day}\right)\hfill \end{array} \)
-
2.
NEAP1(mEq/day) = [54.4 × protein intake (g/day)/potassium (mEq/day)] − 10.2
-
3.
\( \begin{array}{l}{\mathrm{NEAP}}_2\left(\mathrm{mEq}/\mathrm{day}\right)={\displaystyle \sum \left\{\left[\mathrm{phosphate}\ \mathrm{intake}\kern0.05em \left(\mathrm{mmol}/\mathrm{day}\right)\times 0.642-0.355\right]\times 1.8+\right.}\hfill \\ {}\ \mathrm{chloride}\kern0.5em \mathrm{intake}\kern0.5em \left(\mathrm{mmol}/\mathrm{day}\right)\times 0.95+0.325\times \mathrm{protein}\kern0.5em \mathrm{intake}\left(\mathrm{g}/\mathrm{day}\right)\times 0.75\times 2+41\hfill \\ {}\kern0.15em \left(\mathrm{mEq}/\mathrm{day}\right)\times \mathrm{individual}\kern0.2em \mathrm{body}\kern0.2em \mathrm{surface}\kern0.2em \mathrm{area}/1.73{\mathrm{m}}^2-\mathrm{sodium}\kern0.5em \mathrm{intake}\kern0.5em \left(\mathrm{mmol}/\mathrm{day}\right)\times 0.95\hfill \\ {}-\mathrm{potassium}\kern0.1em \mathrm{intake}\left(\mathrm{mmol}/\mathrm{day}\right)\times 0.8-\left[\mathrm{calciumintake}\left(\mathrm{mmol}/\mathrm{day}\right)\times 0.17+1.496\right]\times 2-\hfill \\ {}\ \left.\left[\mathrm{magnesium}\kern0.2em \mathrm{intake}\kern0.2em \left(\mathrm{mmol}/\mathrm{day}\right)\times 0.39-0.946\right]\times 2\right\}\hfill \end{array} \)
For the score from energy-adjusted nutrients, all nutrients were corrected for energy intake by the residual method before being introduced into equations.
Endpoint definitions
Diabetes incidence was defined according to ADA diagnostic criteria using fasting concentration of glucose (fasting plasma glucose ≥ 7.0 mmol/l) or the use of glucose-lowering medication [23]. These criteria were applied in each of the subsequent ULSAM examinations, which occurred 6 years after (where 839 participants attended), 10 years after (530 participants attended) and 18 years after (354 attended) reinvestigation. In addition, for those who did not attend the reinvestigations, the Swedish National Hospital Discharge Register recording was used to identify additional participants who developed diabetes (identified by ICD-9 codes 250 or ICD-10 codes E10–E14 [www.who.int/classifications/icd/en/]) during the 18 year follow-up (end-of follow-up 31 December 2011), and validated by inspection of medical records [23]. Death was censored, and no participant was lost to follow-up.
Statistical analysis
Values are expressed as mean ± SD for normally distributed continuous variables, median (interquartile range [IQR]) for skewed variables, or percentage of total for categorical variables. Study participants were divided into three groups according to tertiles of PRAL. The Jonckheere–Terpstra test was used to assess linear trends across these groups, and p value for trend was reported.
Multivariate linear regressions were calculated to evaluate the cross-sectional association of 1 SD increment of PRAL and NEAP with IS (M value) or with estimated beta cell function (IGI from OGTT). Three sets of models in a hierarchical fashion were investigated: (1) unadjusted model; (2) model 1 considered adjustment for age, lifestyle factors (BMI, smoking status, physical activity, education) and presence of comorbidities (CVD, hypertension, hyperlipidaemia); (3) model 2 further considered dietary factors, namely energy-adjusted fibre, monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA), saturated fatty acid (SFA) and carbohydrate intake [7]; (3) model 3 further adjusted for the renal function surrogates, UAER and eGFR. Data are expressed as regression coefficients (β) and 95% CI. As a sensitivity analysis, we performed the same analysis in the subpopulation of adequate dietary reporters (n = 504) as identified by Goldberg cut-offs [24, 25] and observed similar results.
The association between dietary acid load and diabetes risk was investigated with multivariate logistic regression models, both per SD increment as well as per prespecified multicategory (tertiles) of PRAL and NEAP. Proportional hazards assumptions were confirmed using the Schoenfeld test. The relations of PRAL and NEAP with diabetes risk were investigated in four sets of models in a hierarchical fashion: (1) model 1 adjusted for age, lifestyle (BMI, smoking status, physical activity, education) and IS (M); (2) model 2 further adjusted for comorbidities (CVD, hypertension, hyperlipidaemia); (3) model 3 further adjusted for dietary factors (energy-adjusted fibre, MUFA, PUFA, SFA and carbohydrate intake); (4) model 4 further adjusted for UAER and eGFR. Data are presented as OR and 95% CI. Confirmation of results in adequate reporters was not performed because of the low number of events.
p < 0.05 was regarded as significant. Because multiple comparisons were not taken into account, results have to be considered as descriptive. All statistical analyses were performed using statistical software Stata version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Baseline characteristics
The median PRAL and NEAP values for the study population were: PRAL 10.0 (IQR 1.2–39.1; range −40.1 to 322.9) mEq/day; NEAP1 41.0 (IQR 36.3–45.7; range 15.4–90.6) mEq/day; NEAP2 25.7 (IQR 13.2–52.3; range −71 to 425) mEq/day. PRAL and NEAP correlated positively with each other (NEAP1, ρ = 0.42, p < 0.001; NEAP2, ρ = 0.89, p < 0.001). Baseline characteristics of included participants are shown in Table 1 as stratified by tertiles of PRAL. No differences were observed across tertiles regarding demographics, lifestyle factors, comorbidities or UAER, eGFR, glucose, insulin, M or M/I. The energy-adjusted intake of protein, fat, MUFAs, SFAs, sodium, calcium and phosphorus increased, while carbohydrate, potassium and fibre intake decreased across increasing PRAL tertiles. The energy-adjusted intake of milk products, cheese and bread increased, while fruit and vegetable intake decreased across increasing PRAL tertiles.
Dietary acid load and IS
IS (M) tended to decrease with PRAL or NEAP increments (negative β coefficients), but this never reached statistical significance in any model tested (Table 2). PRAL and NEAP were not associated with estimates of beta cell function as derived from OGTT. Null associations were also observed when M/I was included as the exposure (data not shown) or in the subpopulation of adequate dietary reporters (data not shown).
Dietary acid load and risk of diabetes
During 18 years of follow-up, 115 new cases of diabetes were validated. In multivariate logistic regression models, neither PRAL nor NEAP was associated with diabetes incidence. The multicategory models largely confirmed these findings (Table 3).
Discussion
In this study of community-dwelling older men of similar age, dietary acid load was not associated with insulin sensitivity. Further, dietary acid load was not associated with diabetes incidence. Consideration of underlying kidney function or careful analysis in the subgroup of adequate dietary reporters did not modify these negative findings.
Our study cannot confirm the recent report by Fagherazzi et al [3] showing a positive association of PRAL and NEAP with type 2 diabetes incidence (1,372 events) among 66,485 women with a wide age range. The disagreement between that study and our study could possibly be attributed to the inclusion of 71-year-old men in our study. Thus, it is possible that sex differences may further explain this discrepancy, although this is currently not known. Another factor to consider in this imbroglio is the homogeneity of the individuals investigated, which renders low PRAL and NEAP variability. Certainly, the two studies also differ in sample size and number of events, and therefore we cannot exclude the possibility that a larger sample size would have allowed us to observe an association. Nevertheless, our study design has certain advantages in terms of case validation and analytical approach. First, we used 7 day food records, which is considered to be a preferable dietary-recall method to food frequency questionnaires [3]. Second, we used two different approaches to data analysis: estimation of acid load from raw nutrients and, in addition, from standardised energy-adjusted nutrients by regression analysis of the residual method. Third, compared with the use of self-reports of new-onset diabetes in the previous report [3], our study offers a more solid validation of incident cases. In addition, we took into consideration important confounders such as baseline IS and underlying kidney function. Finally, and in support of our null finding, we failed to observe a relationship between dietary acid load and IS as assessed by the gold standard euglycaemic–hyperinsulinaemic clamp technique or beta cell function as assessed by OGTT. This was a solid finding which was further confirmed in adequate dietary reporters.
Previous studies have reported a higher urinary pH in diabetic patients than in matched controls [26]. The 24 h urine pH [27], serum bicarbonate levels and anion gap [28] have also been found to be associated with IS, as estimated by surrogate indices. Although PRAL and NEAP estimations from dietary recalls are considered valid reflections of true renal net acid excretion in healthy humans and have been associated with markers of metabolic acidosis, both in the community and in patients with manifest chronic kidney disease [29, 30], it is currently unknown if the acid–base balance disturbances that are linked to insulin resistance are solely derived from dietary sources. Surrogate IS indices based on fasting insulin and glucose levels can be influenced by the effects of renal retention of these metabolites in the setting of kidney failure [31], and therefore the use of clamp-derived IS in our study is a strength. An additional explanation is that it is difficult to extrapolate the effects of a single dietary component from that of the whole dietary approach [7]. Moreover, a healthy diet is plausibly linked to healthier lifestyle-related habits and factors such as obesity, smoking and physical activity and education exposures, which can also influence IS and diabetes incidence [5].
Additional study characteristics must be considered in the interpretation of our results. Strengths are the consideration of reporting bias in dietary recalls [24, 25] and the confounding by underlying kidney function [9, 10]. Although the homogeneity of participants in our survey (same age, sex, ethnicity and geographical distribution) is also a strength producing unbiased associations, it renders a selective population that may not be representative of the general population. An additional limitation is the relatively healthy condition of the individuals in our survey, attributed in part to the lower prevalence of metabolic risk factors in Nordic countries and to the nature of our screening programme, whereby individuals more concerned about their health and lifestyle may have been more likely to participate. The influence of time is not taken into account in our multivariate logistic regression analysis. We therefore present odds rather than hazards, which is another difference from the previous report [3]. We are assuming, but cannot ensure, that dietary intake was kept constant over time. Finally, although we adjusted for many important confounders in the association of interest, residual confounding cannot be ruled out in this or in any other observational study.
Conclusions
Dietary acid load was not associated with insulin sensitivity, beta cell function or incidence of type 2 diabetes during 18 years of follow-up in older community-dwelling Swedish men. Our results therefore do not support the hypothesis that dietary acid load influences the risk of diabetes. Although solid, this evidence is observational in nature, and interventional studies modifying acid–base dietary intake are needed to elucidate the role of acid load in the initiation and progression of type 2 diabetes.
Abbreviations
- CVD:
-
Cardiovascular disease
- eGFR:
-
Estimated GFR
- IGI:
-
Insulinogenic Index
- IS:
-
Insulin sensitivity
- MUFA:
-
Monounsaturated fatty acid
- NEAP:
-
Net endogenous acid production
- PRAL:
-
Potential renal acid load
- PUFA:
-
Polyunsaturated fatty acid
- SFA:
-
Saturated fatty acids
- UAER:
-
Urinary albumin excretion rate
- ULSAM:
-
Uppsala Longitudinal Study of Adult Men
References
Tuomilehto J, Lindstrom J, Eriksson J et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350
Knowler W, Barrett-Connor E, Fowler S et al (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403
Fagherazzi G, Vilier A, Bonnet F et al (2014) Dietary acid load and risk of type 2 diabetes: the E3N-EPIC cohort study. Diabetologia 57:313–320
Pizzorno J, Frassetto LA, Katzinger J (2010) Diet-induced acidosis: is it real and clinically relevant? Br J Nutr 103:1185–1194
McAuley K, Mann J (2006) Thematic review series: patient-oriented research. Nutritional determinants of insulin resistance. J Lipid Res 47:1668–1676
Adeva MM, Souto G (2011) Diet-induced metabolic acidosis. Clin Nutr 30:416–421
Weickert MO (2012) What dietary modification best improves insulin sensitivity and why? Clin Endocrinol 77:508–512
Sung KC, Jeong WS, Wild SH, Byrne CD (2012) Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care 35:717–722
Remer T (2000) Influence of diet on acid-base balance. Semin Dial 13:221–226
Scialla JJ, Anderson CA (2013) Dietary acid load: a novel nutritional target in chronic kidney disease? Adv Chron Kidney Dis 20:141–149
Remer T, Dimitriou T, Manz F (2003) Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr 77:1255–1260
Frassetto LA, Todd KM, Morris RC Jr, Sebastian A (1998) Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68:576–583
Remer T, Manz F (1994) Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr 59:1356–1361
Engberink MF, Bakker SJ, Brink EJ et al (2012) Dietary acid load and risk of hypertension: the Rotterdam Study. Am J Clin Nutr 95:1438–1444
Vessby B, Tengblad S, Lithell H (1994) Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 37:1044–1050
Byberg L, Zethelius B, McKeigue PM, Lithell HO (2001) Changes in physical activity are associated with changes in metabolic cardiovascular risk factors. Diabetologia 44:2134–2139
Larsson A, Malm J, Grubb A, Hansson LO (2004) Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest 64:25–30
DeFronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223
Pollare T, Vessby B, Lithell H (1991) Lipoprotein lipase activity in skeletal muscle is related to insulin sensitivity. Arterioscler Thromb J Vasc Biol Am Heart Assoc 11:1192–1203
Seltzer HS, Allen EW, Herron AL, Brennan MT (1967) Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 46:323–335
Becker W (1994) Food habits and intake in Sweden 1989. The Swedish National Food Administration, Uppsala [in Swedish]
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65:1220S–1228S, discussion 1229S-1231S
Resnick HE, Harris MI, Brock DB, Harris TB (2000) American Diabetes Association diabetes diagnostic criteria, advancing age, and cardiovascular disease risk profiles: results from the Third National Health and Nutrition Examination Survey. Diabetes Care 23:176–180
Goldberg GR, Black AE, Jebb SA et al (1991) Critical-evaluation of energy-intake data using fundamental principles of energy physiology: 1. Derivation of cutoff limits to identify under-recording. Eur J Clin Nutr 45:569–581
Lichtman SW, Pisarska K, Berman ER et al (1992) Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 327:1893–1898
Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K (2006) Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol 17:1422–1428
Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K (2007) Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol 2:883–888
Farwell WR, Taylor EN (2008) Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabet Med 25:798–804
van den Berg E, Engberink MF, Brink EJ et al (2012) Dietary acid load and metabolic acidosis in renal transplant recipients. Clin J Am Soc Nephrol 7:1811–1818
Scialla JJ, Appel LJ, Astor BC et al (2011) Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin J Am Soc Nephrol 6:1526–1532
Jia T, Huang X, Qureshi AR et al (2014) Validation of insulin sensitivity surrogate indices and prediction of clinical outcomes in individuals with and without impaired renal function. Kidney Int. doi:10.1038/ki.2014.1
Acknowledgements
We are indebted to all participants in the study and are grateful to the ULSAM group for their assiduous and thorough data collection.
Funding
This work was supported by the grants from the Swedish Research Council and the Westman Foundation. Baxter Novum is the result of a grant from the Baxter Healthcare Corporation to Karolinska Institutet.
Duality of interest
BL is affiliated with Baxter Healthcare Corporation. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Contribution statement
HX carried out the studies and data analyses and drafted the manuscript. JJC conceived the study, participated in its design and coordination, and helped to draft the manuscript. The other authors participated in its design and contributed to developing the manuscript to its final form. All authors approved the final version. JJC is guarantor of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H., Jia, T., Huang, X. et al. Dietary acid load, insulin sensitivity and risk of type 2 diabetes in community-dwelling older men. Diabetologia 57, 1561–1568 (2014). https://doi.org/10.1007/s00125-014-3275-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3275-z