Abstract
Aims/hypothesis
The present study investigated the effect of agmatine, an endogenous ligand of imidazoline receptors, on plasma glucose in streptozotocin-induced diabetic rats (STZ-diabetic rats).
Methods
Plasma glucose was assessed by the glucose oxidase method. Plasma insulin and β-endorphin-like immunoreactivity in plasma or adrenal medulla were measured by enzyme-linked immunosorbent assay. Systolic blood pressure was determined by the tail-cuff method. The mRNA levels of glucose transporter subtype 4 (GLUT4) in soleus muscle and phosphoenolpyruvate carboxykinase (PEPCK) in liver were detected by northern blotting. Protein levels of GLUT4 in soleus muscle and hepatic PEPCK were estimated using western blotting analysis.
Results
After intravenous injection into fasting STZ-diabetic rats for 30 min, agmatine decreased plasma glucose in a dose-dependent manner without changing systolic blood pressure. At the same time, plasma β-endorphin-like immunoreactivity also increased in STZ-diabetic rats receiving the same treatment. Plasma glucose was significantly elevated in STZ-diabetic rats by an intravenous injection of clonidine at a dose sufficient to decrease systolic blood pressure. Involvement of I1-imidazoline receptors and/or α2-adrenoceptors in this effect of agmatine was thus unlikely. The lowering of plasma glucose and increase of plasma β-endorphin-like immunoreactivity by agmatine were abolished by pretreating the rats with BU-224 at a dose sufficient to block I2-imidazoline receptors. Both effects of agmatine were also abolished in adrenalectomised STZ-diabetic rats. Moreover, agmatine enhanced β-endorphin-like immunoreactivity release from the isolated adrenal medulla of STZ-diabetic rats, an effect also blocked by BU-224. Release of β-endorphin from the adrenal glands by I2-imidazoline receptor activation seems responsible for the plasma glucose-lowering action of agmatine. This was supported by the fact that intravenous injection of naloxone or naloxonazine at doses sufficient to block opioid μ-receptors inhibited the action of agmatine. In addition to lowering plasma glucose, repeated intravenous injection of agmatine into STZ-diabetic rats for 4 days also increased mRNA and protein levels of GLUT4 in soleus muscle. The same treatment also reversed the higher mRNA and protein levels of PEPCK in liver of STZ-diabetic rats.
Conclusions/interpretation
Our results suggest that agmatine may activate I2-imidazoline receptors in the adrenal gland. This enhances secretion of β-endorphin, which can activate opioid μ-receptors to increase GLUT4 gene expression and/or suppress hepatic PEPCK gene expression, resulting in a lowering of plasma glucose in diabetic rats lacking insulin. The results provide a potential new target for intervention in type 1 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Imidazoline receptor is thought to mediate the central antihypertensive action of clonidine and analogues [1]. Imidazoline receptor has been subclassed into two sites: I1 sites show a high affinity for clonidine and I2 sites show greater affinity for idazoxan used as α2-adrenergic antagonist and I2-imidazoline agonist [2, 3]. Functionally, peripheral imidazoline receptors mediate movement of smooth muscle, stimulate insulin release and regulate the renal excretion of sodium, potassium and water [4].
Some α2-adrenergic antagonists with a moeity of imidazoline enhanced insulin secretion in vivo and in vitro [5, 6]. These imidazoline insulin secretagogues work by blocking ATP-regulated potassium channels in pancreatic beta cells, resulting in membrane depolarisation [7]. For this reason imidazoline compounds have been used as adjuncts in the treatment of type 2 diabetes. However, little is known about the role of imidazoline receptors in regulating glucose homeostasis. Agmatine, which present in various regions of the brain from the decarboxylation of arginine, is the endogenous ligand of imidazoline receptors [4, 8]. Activation of I2-imidazoline receptors in the brain by central administration of agmatine may lower plasma glucose levels in streptozotocin-induced diabetic (STZ-diabetic) rats, a rat model of type 1 diabetes [9]. This suggests a possible beneficial effect of central I2-imidazoline receptor activation on glucose homeostasis in diabetic rats lacking insulin.
Exogenous β-endorphin induced an increase of circulating insulin in healthy and diabetic humans [10]. Also, we found that β-endorphin may increase glucose utilisation via opioid μ-receptor activation, lowering plasma glucose in STZ-diabetic rats [11]. Thus, β-endorphin seems to be involved in the regulation of glucose homeostasis. It has also been reported that agmatine is a secretagogue effecting the release of β-endorphin from the adrenal medulla [8] and that imidazoline receptors are also widely expressed outside the brain, including in the adrenal gland and kidney [12]. Therefore, the present study examined the possible action of agmatine on plasma glucose via peripheral I2-imidazoline receptors in diabetic rats, and analysed the role of β-endorphin in this context.
Materials and methods
Animals
Male Wistar rats (200–250 g) obtained from the Animal Center of National Cheng Kung University Medical College were housed in a temperature-controlled room (25±1°C) under a 12:12-h light:dark cycle (light on at 06.00 h). Water and standard laboratory diet were freely available throughout. STZ-diabetic rats were produced by intravenous injection of STZ (Sigma-Aldrich, St. Louis, MO, USA) at 60.0 mg/kg into Wistar rats. Rats with a plasma glucose level of 20.0 mmol/l or greater, in addition to polyuria and other symptoms of diabetes, were considered to have type 1 diabetes mellitus. Plasma insulin levels in STZ-diabetic rats declined to 1.28±0.51 pmol/l (n=8) vs 163.5±4.4 pmol/l (n=8) in normal rats. Studies were carried out 2 weeks after the injection of STZ. All animal procedures were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, as well as the guidelines of the Animal Welfare Act.
Effect of agmatine on plasma glucose and β-endorphin-like immunoreactivity levels in STZ-diabetic rats
After fasting overnight, rats received an i.v. injection of agmatine sulphate (Sigma-Aldrich) into the tail vein at the desired doses, and blood samples (0.1 ml) were collected under sodium pentobarbital anaesthesia (30.0 mg/kg, i.p.) from the tail vein. In preliminary experiments, agmatine at 1.0 mg/kg was found to reach a maximal plasma glucose-lowering effect 30 min after the i.v. injection. The effects of agmatine on plasma levels of glucose, β-endorphin-like immunoreactivity and insulin-like immunoreactivity were determined using blood samples collected from the tail vein with a chilled syringe containing 10 IU heparin 30 min after the i.v. injection. Changes of systolic blood pressure (SBP) were compared in STZ-diabetic rats receiving the same agmatine treatment. The same i.v. injection of agmatine was also performed on normal rats. Control animals received an injection of the same volume of sterile saline as that used to dissolve the testing drugs in the STZ-diabetic rats. The effects of clonidine hydrochloride (Sigma-Aldrich) were also determined by an i.v. injection into STZ-diabetic rats. The inhibitors, including the opioid μ-receptor antagonist naloxone or naloxonazine (Research Biochemical, Natick, MA, USA) and the I2-i imidazoline receptor blocker BU-224 (Tocris Cookson, Bristol, UK), were injected into the vein of animals 30 min before treatment with agmatine.
Isolation and incubation of adrenal medulla
The adrenal glands were obtained from STZ-diabetic rats after they had been killed, and the medullae were immediately dissected as described previously [13]. Tissues were placed in an incubator for 15 min pre-incubation at 37°C and bubbled with air (95% O2 and 5% CO2) under continuous agitation with 2 ml modified Krebs solution as in the previous method [13]. Next, tissues were transferred to fresh incubation tubes, with or without an I2-i imidazoline receptor antagonist, for 15 min at 37°C and further incubated with agmatine for another 30 min under continuous agitation (40 cycles/min). Incubation was terminated by placing the tubes on ice. The medium from each incubation was then collected and frozen at −70°C until the assay of β-endorphin-like immunoreactivity.
Adrenalectomised rats
Bilateral adrenalectomy was performed in Wistar rats under pentobarbital anaesthesia (30.0 mg/kg, i.p.) using the dorsal approach as described previously [14]. The adrenalectomised Wistar rats had free access to standard rat chow and 0.9% NaCl in drinking water [14]. Sham-operated Wistar rats served as controls and had free access to the same chow and water [14]. Animals were allowed to recover for 2 weeks after the operation. The animals appeared alert and in good health. Type 1 diabetes was induced by an injection of STZ as described above and the effects of agmatine were then determined.
Laboratory measurements
Concentrations of plasma glucose were measured by the glucose oxidase method via an analyser (Quik-Lab; Ames, Miles, Elkhart, IN, USA). The ELISA for determination of β-endorphin-like immunoreactivity in plasma and adrenal medulla or the assay of plasma insulin was performed using a commercial kit (Penisula Lab, San Carlos, CA, USA). SBP was estimated by a non-invasive tail-cuff monitor (UR-5000; Ueda Company, Tokyo, Japan) in conscious diabetic rats. For measuring SBP, rats were placed in a warm chamber maintained at 37°C for 1 h in individual plexiglas restrainers. The tail-cuff device was placed around the rat’s tail; four measurements were recorded for each rat and the average SBP was calculated.
Effect of agmatine on gene expression
STZ-diabetic rats were given an i.v. injection of vehicle or agmatine (1.0 mg/kg) into the tail vein three times daily. In preliminary experiments, agmatine was found to modify mRNA and protein levels for subtype 4 form of glucose transporter (GLUT4) and phosphoenolpyruvate carboxykinase (PEPCK) in STZ-diabetic rats after 4 days of treatment. Thus, the effects of agmatine on the gene expression of these were determined in samples after 4 days of treatment. Normal rats receiving a similar treatment with vehicle were used as controls. After the final treatment, animals were killed without fasting. The liver and soleus muscle were immediately removed, frozen in liquid nitrogen and stored at −70°C for analysis. Each sample was divided into two parts equally for northern and Western blot analysis. Blood samples were collected from the femoral vein of these rats before death to evaluate plasma levels of glucose, insulin and β-endorphin-like immunoreactivity as described above.
Northern blot analysis
Total RNA was extracted from liver or soleus muscle using the Ultraspec-II RNA extraction system (Biotech, Houston, TX, USA) according to the manufacturer’s instructions. Northern blot analysis was carried out as previously described [15]. In brief, RNA was denatured and aliquots of total RNA were then size-fractionated in a 1.2% agarose/formaldehyde gel. The RNA was transferred to a Hybond-N membrane (Amersham Biosciences, Bucks, UK). GLUT4 and PEPCK mRNA levels were detected using prime-labelled full-length cDNA under stringent hybridisation conditions. The intensity of the mRNA blot was quantified by scanning densitometry (Hoefer, San Francisco, CA, USA). The response of β-actin was used as internal standard.
Western blot analysis
After homogenisation, the protein content was determined by protein dye binding assay (BioRad, Richmond, CA, USA). Tissue samples (50 μg) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (10% acrylamide gel) using Bio-Rad’s Mini-Protein II system. Protein was transferred to polyvinylidene difluoride membrane. Then, the membrane was washed with PBS and blocked for 1 h at room temperature with 5% (w/v) skimmed milk powder in PBS. Western blot analysis was performed as previously described [15] using either anti-rat antibody to bind GLUT4 (1:1000) (Genzyme Diagnostics, Cambridge, MA, USA) in soleus muscle, or sheep anti-rat liver to bind PEPCK (1:1000) (kindly supplied by Professor D. K. Granner). The intensity of blots incubated with goat polycolonal antibody (1:500) to bind actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse monoclonal antibody (1:500) to bind β-tubulin (Zymed Laboratories, San Francisco, CA, USA) was used as internal control. Blots were developed by autoradiography using the ECL-western blot system (Amersham, Braunschweig, Germany). Densities of the obtained immunoblots were quantified using laser densitometry.
Statistical analysis
Data are expressed as means±SEM from the number (n) of animals in each group. Repeated measures ANOVA was used to analyse the changes in plasma glucose and other parameters. Dunnett post-hoc comparisons were used to determine the source of significant differences where appropriate. A p value of less than 0.05 was considered statistically significant.
Results
Effects of agmatine on plasma glucose and β-endorphin-like immunoreactivity in STZ-diabetic rats
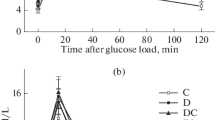
Thirty minutes after an i.v. injection of agmatine, plasma glucose was reduced in STZ-diabetic rats. Agmatine dose-dependently decreased plasma glucose (Fig. 1a) and reached its maximal effect at 1.0 mg/kg; plasma glucose was reduced to 18.2±2.5 mmol/l (n=8) with a lowering activity of 22.2±2.1%. An increased dose of agmatine (5.0 mg/kg) had a plasma glucose-lowering activity of 23.5±3.2%, which was not any more effective than 1.0 mg/kg.
The plasma glucose-lowering activity (a) produced by an intravenous injection of agmatine into STZ-diabetic rats. b The plasma level of β-endorphin-like immunoreactivity determined in the same group after agmatine treatment. Values (means±SEM) were obtained from each group of eight animals. The saline vehicle used to dissolve agmatine was given at the same volume as in vehicle alone. *p<0.05 and ** p<0.01 versus data from animals treated with vehicle (0)
Plasma glucose was not modified in normal rats 30 min after a similar injection of agmatine (1.0 mg/kg); plasma glucose remained at 5.2±0.7 mmol/l (n=8), near the value (5.3±0.6 mmol/l) of vehicle-treated animals (n=8). Plasma glucose decreased in normal rats 40 min after injection of agmatine, showing a lowering activity of 6.4±1.1, 10.3±1.4, and 13.2±1.7% after dosing with 0.1, 0.5 and 1.0 mg/kg, respectively (n=8).
Basal plasma β-endorphin-like immunoreactivity in STZ-diabetic rats was 43.2±2.9 pg/ml. A dose-dependent increase of this was also observed in STZ-diabetic rats after i.v. injection of agmatine with doses ranging from 0.1 to 5.0 mg/kg, the doses effective for lowering plasma glucose (Fig. 1b). Agmatine at 1.0 mg/kg increased plasma β-endorphin-like immunoreactivity in STZ-diabetic rats from 43.2±4.7 to 84.2±4.5 pg/ml. However, no further increase of plasma β-endorphin-like immunoreactivity was observed at higher doses of agmatine. Thus, 1.0 mg/kg of agmatine was employed in subsequent experiments.
Plasma insulin levels in normal rats that received an i.v. injection of agmatine at 1.0 mg/kg for 30 min were 165.2±6.1 pmol/l (n=8), which was not statistically different (p>0.05) from the vehicle-treated group (164.5±5.4 pmol/l; n=8). Plasma insulin in the agmatine-treated (1.0 mg/kg) STZ-diabetic rats (1.29±0.43 pmol/l; n=7) was also not different from the vehicle-treated group (1.25±0.38 pmol/l; n=7).
Effect of repeated injections of agmatine on plasma glucose and β-endorphin-like immunoreactivity levels in STZ-diabetic rats
Plasma glucose levels of STZ-diabetic rats were significantly (p<0.01) decreased to 17.8±0.8 mmol/l after i.v. injections of agmatine (1.0 mg/kg) three times daily for 4 days, as compared with vehicle-treated STZ-diabetic rats (23.5±1.3 mmol/l). This repeated i.v. injection of agmatine had a plasma glucose-lowering activity of 24.2±2.1% in STZ-diabetic rats. Elevation of plasma β-endorphin-like immunoreactivity occurred in the same group of STZ-diabetic rats, with levels increasing to 88.5±4.7 pg/ml (vehicle-treated group: 43.6±2.8 pg/ml, p<0.05). The 4 days of treatment with agmatine (1.0 mg/kg, i.v.) did not influence the feeding behavior and/or body weight of STZ-diabetic rats. Also, plasma insulin in the agmatine-treated group (1.30±0.39 pmol/l; n=7) was not different (p>0.05) from the vehicle-treated group (1.26±0.52 pmol/l; n=7).
Effect of agmatine on systolic blood pressure in STZ-diabetic rats
Two weeks after injection of STZ, SBP in rats rose from 98.8±4.1 to 141.9±5.1 mmHg (n=8) (p<0.05). But SBP in STZ-diabetic rats was not markedly (p>0.05) modified by an i.v. injection of agmatine for 30 min (138.4±3.7 mmHg; n=8).
Effects of clonidine on plasma glucose and systolic blood pressure in STZ-diabetic rats
Plasma glucose in STZ-diabetic rats was raised by an i.v. injection of clonidine for 30 min, increasing from 23.5±1.8 mmol/l to 25.3±2.1, 26.7±1.8 and 27.4±1.9 mmol/l, respectively, after the following doses of clonidine: 0.1, 0.5 and 1.0 mg/kg (n=8). SBP in STZ-diabetic rats was also significantly (p<0.05) reduced from 142.5±3.4 mmHg (n=8) to 105.4±3.5 mmHg by an i.v. injection of clonidine (1.0 mg/kg) for 30 min.
Effects of I2-imidazoline receptor blocker, BU-224, on the actions of agmatine in STZ-diabetic rats
As shown in Table 1, the increase of plasma β-endorphin-like immunoreactivity by agmatine (1.0 mg/kg, i.v.) in STZ-diabetic rats was attenuated in a dose-dependent manner by the i.v. injection of BU-224 given 30 min before agmatine treatment. BU-224 (1.0 mg/kg, i.v.) completely reversed the effect of agmatine, but did not modify the basal level of plasma β-endorphin-like immunoreactivity.
Similarly, the plasma glucose-lowering effect of agmatine (1.0 mg/kg, i.v.) was inhibited by pretreatment with BU-224 in a dose-dependent manner. BU-224 at the highest dose (1.0 mg/kg, i.v.) abolished the plasma glucose-lowering action of agmatine, but did not modify the basal plasma glucose of STZ-diabetic rats (Table 1).
Effect of agmatine on STZ-diabetic rats with bilateral adrenalectomy
Bilateral adrenalectomy did not markedly change basal plasma β-endorphin-like immunoreactivity in STZ-diabetic rats as compared to the sham-operated group (Table 2). However, the effects of agmatine (1.0 mg/kg, i.v.), i.e. the lowering of plasma glucose and increase of plasma β-endorphin-like immunoreactivity that were markedly produced in sham-operated STZ-diabetic rats, did not occur in adrenalectomised STZ-diabetic rats (Table 2).
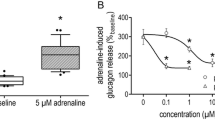
Effect of agmatine on the secretion of β-endorphin-like immunoreactivity from isolated adrenal medulla of STZ-diabetic rats
The spontaneous secretion of β-endorphin-like immunoreactivity from the isolated adrenal medulla of STZ-diabetic rats was 82.3±5.5 pg/mg protein. The amount of β-endorphin-like immunoreactivity in the medium was markedly raised by agmatine in a concentration-dependent manner (Fig. 2). Agmatine at 1.0 μmol/l increased the amount of β-endorphin-like immunoreactivity in the medium to 159.3±5.2 pg/mg protein and no further increase was observed at higher concentrations of agmatine (Fig. 2).
Effect of I2-imidazoline receptor antagonist on agmatine-stimulated β-endorphin-like immunoreactivity secretion from isolated adrenal medulla of STZ-diabetic rats
A shown in Table 3, BU-224 blocked agmatine-stimulated β-endorphin-like immunoreactivity secretion from STZ-diabetic rat adrenal medulla in a concentration-dependent manner. BU-224 at 1.0 μmol/l reduced the β-endorphin-like immunoreactivity secretion induced by 1.0 μmol/l agmatine from 160.3±5.8 to 90.2±6.2 pg/mg protein, which was not markedly different from the basal value (84.2±6.1 pg/mg protein) in vehicle-treated samples. BU-224 at maximal concentration (1.0 μmol/l) did not modify the spontaneous secretion of β-endorphin-like immunoreactivity (Table 3).
Effects of opioid μ-receptor antagonists on agmatine-induced plasma glucose-lowering action in STZ-diabetic rats
Table 4 shows the dose-dependent inhibitory effect of naloxone or naloxonazine on the plasma-glucose-lowering action of agmatine (1.0 mg/kg, i.v.) in STZ-diabetic rats. In the presence of naloxone (10.0 μg/kg, i.v.), plasma glucose levels in STZ-diabetic rats treated with agmatine (1.0 mg/kg, i.v.) were 22.9±2.8 mmol/l, which was not statistically different from the basal level (23.4±2.1 mmol/l). Similarly, naloxonazine (10.0 μg/kg, i.v.) prevented the agmatine-induced (1.0 mg/kg, i.v.) decrease of plasma glucose in STZ-diabetic rats to 23.2±1.8 mmol/l, which was near the basal level. Neither naloxone nor naloxonazine modified the basal plasma glucose of STZ-diabetic rats, even at the highest dosage.
Effect of agmatine on gene expression of GLUT4 in soleus muscle of STZ-diabetic rats
The mRNA level of GLUT4 in isolated soleus muscle from the vehicle-treated STZ-diabetic rats declined to about 38% of that in the vehicle-treated normal rats (Fig. 3a). Treatment of STZ-diabetic rats with agmatine (1.0 mg/kg, i.v.) three times daily for 4 days increased GLUT4 mRNA in soleus muscle to nearly 90% of that in vehicle-treated normal rats.
Representative response (a) of the mRNA level for GLUT4 or β-actin in soleus muscle isolated from normal or STZ-diabetic rats after treatment with agmatine (1.0 mg/kg, i.v.) or the same volume of vehicle (saline) three times daily for 4 days. b Identification of protein level of GLUT4 or actin using immunoblot analysis. Lane 1, vehicle-treated normal rats; lane 2, vehicle-treated STZ-diabetic rats; lane 3, agmatine-treated STZ-diabetic rats. Similar results were obtained in four other determinations. For quantification of the data, see Table 5
The protein level of GLUT4 in soleus muscle of vehicle-treated STZ-diabetic rats was also reduced to 41% of that in vehicle-treated normal rats (Fig. 3b). Similarly, the injection of agmatine for 4 days increased GLUT4 protein levels in soleus muscle of STZ-diabetic rats to about 88% of that in vehicle-treated normal rats. The quantification of the mRNA and protein level for GLUT4 is indicated in Table 5.
This treatment with agmatine (1.0 mg/kg, i.v.) in normal rats did not modify the mRNA and protein levels of GLUT4 in soleus muscle as compared with the vehicle-treated group (p>0.05; data not shown).
Effect of agmatine on gene expression of hepatic PEPCK in STZ-diabetic rats
Figure 4a shows that the mRNA level of PEPCK was nearly three times higher in liver of STZ-diabetic rats than in that of normal rats. This increase was reduced by the injection of agmatine (1.0 mg/kg, i.v.) three times daily for 4 days, but levels were still higher than in vehicle-treated normal rats. However, the same repeated treatment with agmatine in normal rats did not alter mRNA levels of hepatic PEPCK (data not shown).
Representative response (a) of the mRNA level for PEPCK or β-actin in liver isolated from normal or STZ-diabetic rats receiving treatment with agmatine (1.0 mg/kg, i.v.) or the same volume of vehicle (saline) three times daily for 4 days. b Identification of protein levels of PEPCK or β-tubulin using immunoblot analysis. Lane 1, vehicle-treated normal rats; lane 2, vehicle-treated STZ-diabetic rats; lane 3, agmatine-treated STZ-diabetic rats. Similar results were obtained in four other determinations. For quantification of the data, see Table 5
Also, protein levels of PEPCK in liver of vehicle-treated STZ-diabetic rats was about 3.2-fold that of vehicle-treated normal rats. Treatment of STZ-diabetic rats with agmatine (1.0 mg/kg, i.v.) three times daily for 4 days decreased the protein level of PEPCK to about 60% of that in vehicle-treated STZ-diabetic rats (Fig. 4b). The quantification of protein levels for PEPCK is indicated in Table 5. Hepatic PEPCK protein levels in normal rats were not modified by the same treatment with agmatine (data not shown).
Discussion
Agmatine is a novel neurotransmitter in brain and peripheral tissues [16, 17]. In the present study, agmatine was injected into STZ-diabetic rats to clarify the role of peripheral imidazoline receptors in plasma glucose regulation. We found that agmatine induced a dose-dependent plasma glucose reduction in STZ-diabetic rats. Although imidazoline receptor activation can enhance insulin secretion [18], plasma levels of glucose and insulin in normal rats were not modified by an i.v. injection of agmatine, indicating that the action of agmatine seems unrelated to insulin secretion. Actually, agmatine showed a higher affinity for α2-adrenoceptor, I1- and I2-binding sites, while the selectivity between α2-adrenoceptor and imidazoline receptor was poor [8]. Both α2-adrenoceptor and I1-binding site are involved in blood pressure regulation [1]. Thus, we measured blood pressure to identify the effect of agmatine and used clonidine, an agonist of α2-adrenoceptors and I1-imidazoline ligand [19], as a positive control. Agmatine at a dose sufficient to lower plasma glucose failed to modify blood pressure in STZ-diabetic rats. In contrast, clonidine increased plasma glucose in STZ-diabetic rats at a dose sufficient to reduce blood pressure. Thus, it seems unlikely that α2-adrenoceptor and/or I1-imidazoline receptor mediate the action of agmatine. Otherwise, the action of agmatine was blocked by BU-224, a specific antagonist of I2-imidazoline receptors [20]. The sulphate salt of agmatine was used in this study to prevent agmatine from penetrating the blood–brain barrier. Also, peripheral I2-imidazoline receptor has been characterised [12]. Therefore, the activation of peripheral I2-imidazoline receptors seems to be related to the action of agmatine in STZ-diabetic rats. If one follows the previous definition of the I2-imidazoline receptor as the mitochondrial subtype [12], which is unlikely to mediate this effect, it would be more precise to put this into a category of I2-like non-mitochondrial responses.
Although the decrease in plasma glucose after peripheral agmatine injection was similar to that found previously in response to central agmatine administration [9], the mediating mechanisms were obviously different. Regulation of plasma glucose by agmatine seems related to endogenous β-endorphin, which can activate opioid μ-receptor [21]. Actually, activation of opioid μ-receptors increased glucose utilisation to lower plasma glucose in type 1-like diabetic animals [11, 15]. But morphine led to hyperglycaemia [22], mainly due to the effect on all subtypes of opioid receptors. In healthy volunteers and insulin-dependent diabetic patients, infusion of β-endorphin increased plasma glucagon with an increase of plasma glucose [23]. However, injection of β-endorphin decreased plasma glucose in STZ-diabetic rats [24]. This difference might be due to the purity and/or concentration of synthetic peptides used, which might affect the variable subtype of opioid receptors. The affinity of the β-endorphin used for opioid receptors seems not so ideal, since the effect of β-endorphin on islet beta cell function in these subjects was resistant to naloxone [23]. Actually, the plasma glucose-lowering action of β-endorphin was abrogated in opioid μ-receptor knockout diabetic mice [25]. Thus, endogenous β-endorphin and/or opioid μ-receptor activation seem important in glucose homeostasis. The action of agmatine was blocked by BU-224, an antagonist of I2-imidazoline receptors, and inhibited by naloxone or naloxonazine at a dose sufficient to block opioid μ-receptors. A similar integration of opioid receptors and I2-binding sites has been mentioned before [26, 27].
Similar to the pituitary-gland-independent release of opioid in other organs [28], the secretion of opioid from the adrenal gland is thought to relate with plasma glucose reduction in STZ-diabetic rats [13]. In the present study, the action of agmatine was abolished by bilateral adrenalectomy and agmatine increased β-endorphin secretion from isolated adrenal medulla of STZ-diabetic rats. Thus, secretion of β-endorphin from the adrenal gland appears to be responsible for the action of agmatine in STZ-diabetic rats. The release of opioid peptides is mainly regulated by receptors located in the adrenal medulla [29]. In fact, imidazoline receptors also occur in adrenal medulla [30]. BU-224 inhibited agmatine-stimulated secretion of β-endorphin, indicating the mediation of I2-imidazoline receptors. Therefore, the activation of I2-imidazoline receptors in the adrenal gland by agmatine may result in an increase of β-endorphin-like immunoreactivity in STZ-diabetic rats.
In diabetes mellitus, hyperglycaemia is a consequence of increased hepatic glucose output and/or reduced peripheral glucose utilisation [31]. Skeletal muscle is a major site for glucose disposal [32]. Glucose transporters (GLUT) mediate glucose transport across the cell membrane and GLUT4 is predominant in skeletal muscle [33]. Decreased gene expression of GLUT4 in diabetes has been demonstrated [34]. In STZ-diabetic rats, we found that the mechanism(s) by which repeated agamatine administration lowers plasma glucose may involve the increase of GLUT4 gene expression. Enhanced skeletal muscle glucose uptake is associated with an increase of GLUT4 gene expression [35]. The molecular effect of agmatine on glucose utilisation in peripheral tissue needs to be further studied in the future.
In diabetic animals, increased gluconeogenesis is a major cause of hyperglycaemia in the fasting and postabsorptive states [31]. In liver, PEPCK is the key enzyme of gluconeogenesis [36]. It has already been documented that the activation of opioid μ-receptors is a negative regulator to modify PEPCK gene expression [11, 15]. We also observed that a decrease of PEPCK gene expression is related to plasma glucose reduction following repeated agamatine administration. The gene expression of PEPCK in liver is regulated by multiple hormones [36]. The mechanism(s) leading to a change of hepatic PEPCK gene expression in STZ-diabetic rats by agmatine should be clarified in future.
In conclusion, our results suggest that the activation of peripheral I2-imidazoline receptors in adrenal medulla by agmatine may enhance the secretion of β-endorphin in STZ-diabetic rats. the plasma glucose-lowering action of agmatine appeared to result from the release of β-endorphin, via activation of opioid receptors in peripheral tissues, and in the long term may also involve enhanced GLUT4 and reduced PEPCK gene expression, without an insulin-mediated mechanism. The development of agonists for I2-imidazoline receptors or opioid μ-receptors could be helpful for treating diabetic disorders.
Abbreviations
- GLUT4:
-
subtype 4 form of glucose transporter
- PEPCK:
-
phosphoenolpyruvate carboxykinase
- SBP:
-
systolic blood pressure
- STZ-diabetic rats:
-
streptozotocin-induced diabetic rats
References
Reid JL, Panfilov V, MacPhee G, Elliott HL (1995) Clinical pharmacology of drugs acting on imidazoline and adrenergic receptors. Studies with clonidine, moxonidine, rilmenidine, and atenolol. Ann NY Acad Sci 763:673–678
Michel MC, Insel PA (1989) Are there multiple imidazoline binding sites? Trends Pharmacol Sci 10:342–344
Michel MC, Ernsberger P (1992) Keeping an eye on the I site: imidazoline-preferring receptors. Trends Pharmacol Sci 13:369–370
Raasch W, Schafer U, Chun J, Dominiak P (2001) Biological significance of agmatine, an endogenous ligand at imidazoline binding sites. Br J Pharmacol 133:755–780
Schulz A, Hasselblatt A (1989) An insulin-releasing property of imidazoline derivatives is not limited to compounds that block alpha-adrenoceptors. Naunyn-Schmiedeberg’s Arch Pharmacol 340:321–327
Schulz A, Hasselblatt A (1989) Dual action of clonidine on insulin release: suppression, but stimulation when alpha 2-adrenoceptors are blocked. Naunyn-Schmiedeberg’s Arch Pharmacol 340:712–714
Dunne MJ, Harding EA, Jaggar JH et al (1995) Potassium channels, imidazolines, and insulin-secreting cells. Ann NY Acad Sci 763:243–261
Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ (1994) Agmatine: an endogenous clonidine-displacing substance in the brain. Science 263:966–969
Jou SB, Liu IM, Cheng JT (2004) Activation of imidazoline receptor by agmatine to lower plasma glucose in streptozotocin-induced diabetic rats. Neurosci Lett 358:111–114
Curry DL, Li CH (1987) Stimulation of insulin secretion by beta-endorphin (1-27 and 1-31). Life Sci 40:2053–2058
Cheng JT, Liu IM, Tzeng TF, Tsai CC, Lai TY (2002) Plasma glucose-lowering effect of beta-endorphin in streptozotocin-induced diabetic rats. Horm Metab Res 34:570–576
Olmos G, Alemany R, Boronat MA, Garcia-Sevilla JA (1999) Pharmacologic and molecular discrimination of I2-imidazoline receptor subtypes. Ann NY Acad Sci 881:144–160
Cheng JT, Liu IM, Kuo DH, Lin MT (2001) Stimulatory effect of phenylephrine on the secretion of β-endorphin from rat adrenal medulla in vitro. Auton Neurosci 93:31–35
Suzuki H, Zweifach BW, Forrest MJ, Schmid-Schönbein GW (1995) Modification of leukocyte adhesion in spontaneously hypertensive rats by adrenal corticosteroids. J Leukoc Biol 57:20–26
Cheng JT, Liu IM, Chi TC, Tzeng TF, Lu FH, Chang CJ (2001) Plasma glucose lowering effect of tramadol in streptozotocin-induced diabetic rats. Diabetes 50:2815–2821
Raasch W, Regunathan S, Li G, Reis DJ (1995) Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sci 56:2319–2330
Feng Y, Halaris AE, Piletz JE (1997) Determination of agmatine in brain and plasma using high-performance liquid chromatography with fluorescence detection. J Chromatogr 691:277–286
Morgan NG, Chan SL, Mourtada M, Monks LK, Ramsden CA (1999) Imidazolines and pancreatic hormone secretion. Ann NY Acad Sci 881:217–228
Eglen RM, Hudson AL, Kendall DA et al (1998) ‘Seeing through a glass darkly’: casting light on imidazoline ‘I’ sites. Trends Pharmacol Sci 19:381–390
Hudson AL, Gough R, Tyacke R et al (1999) Novel selective compounds for the investigation of imidazoline receptors. Ann NY Acad Sci 881:81–91
Goldstein A (1987) Binding selectivity profiles for ligands of multiple receptor types: focus on opioid receptors. Trends Pharmacol Sci 8:456–459
Johansen O, Tønnesen T, Jensen T, Burhol PG, Jorde R, Reikerås O (1993) Morphine and morphine/naloxone modification of glucose, glucagon and insulin levels in fasted and fed rats. Scand J Clin Lab Invest 53:805–809
Feldman M, Kiser RS, Unger RH, Li CH (1983) Beta-endorphin and the endocrine pancreas. Studies in healthy and diabetic human beings. N Engl J Med 308:349–353
Liu IM, Niu CS, Chi TC, Kuo DH, Cheng JT (1999) Investigations of the mechanism of the reduction of plasma glucose by cold-stress in streptozotocin-induced diabetic rats. Neurosci 92:1137–1142
Liu IM, Chi TC, Shiao GC, Lin MT, Cheng JT (2001) Loss of plasma glucose-lowering response to cold stress in opioid mu-receptor knock-out diabetic mice. Neurosci Lett 307:81–84
Sanchez-Blazquez P, Boronat MA, Olmos G, Garcia-Sevilla JA, Garzon J (2000) Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br J Pharmacol 130:146–152
Boronat MA, Olmos G, Garcia-Sevilla JA (1998) Attenuation of tolerance to opioid-induced antinociception and protection against morphine-induced decrease of neurofilament proteins by idazoxan and other I2-imidazoline ligands. Br J Pharmacol 125:175–185
Hanbauer I, Kelly GD, Saiani L, Yang HY (1982) [Met5]-enkephalin-like peptides of the adrenal medulla: release by nerve stimulation and functional implications. Peptides 3:469–473
Hexum TD, Russett LR (1989) Stimulation of cholinergic receptor mediated secretion from the bovine adrenal medulla by neuropeptide Y. Neuropeptides 13:35–41
King PR, Gundlach AL, Louis WJ (1999) Identification of imidazoline-receptor binding sites in cortex and medulla of the bovine adrenal gland. Colocalization with MAO-A and MAO-B. Ann NY Acad Sci 881:161–170
Consoli A, Nurjhan N, Capani F, Gerich J (1989) Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes 38:550–557
Ziel FH, Venkatesan N, Davidson MB (1988) Glucose transport is rate-limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes 37:885–890
Pessin JE, Bell GI (1992) Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol 54:911–930
Berger J, Biswas C, Vicario PP, Strout HV, Saperstein R, Pilch PF (1989) Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature 340:70–72
Mountjoy KG, Housey GM, Flier JS (1989) Overproduction of the beta 1 form of protein kinase C enhances phorbol ester induction of glucose transporter mRNA. Mol Endocrinol 3:2018–2027
Hanson RW, Reshef L (1997) Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem 66:581–611
Acknowledgements
We appreciate the research assistance of Mr T. C. Chi. We are also grateful to Professor R. W. Hanson (Department of Biochemistry School of Medicine, Case Western Reserve University, Cleveland, OH, USA), Professor C. Makepeace (Department of Cell Biology and Physiology, School of Medicine, Washington University, St. Louis, MO, USA) and Professor S. S. Liu (Department of Microbiology and Immunology, National Cheng Kung University, Tainan City, Taiwan, ROC) for kindly providing us with the plasmid containing cDNA. Thanks are also due to Professor D. K. Granner (Department of Molecular Physiology and Biophysics, Vanderbilt University School of Medicine, Nashville, TN, USA) for the supply of antibodies specific to PEPCK. The present study was supported in part by a grant from the National Science Council (NSC 91-2320-B006-099) of the Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, SL., Liu, IM., Tzeng, TF. et al. Activation of imidazoline receptors in adrenal gland to lower plasma glucose in streptozotocin-induced diabetic rats. Diabetologia 48, 767–775 (2005). https://doi.org/10.1007/s00125-005-1698-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1698-2