Abstract
Key message
Catalytically active indica SSIIa allele in high amylose rice with down-regulated japonica SBEIIb can increase starch content and modify the starch structure and properties without changing its amylose content.
Abstract
Rice (Oryza sativa) genotypes with inactive starch synthase IIa (SSIIa) with recessive variants of starch branching enzyme IIb (SBEIIb) exhibit a range of alterations in grain phenotype, starch granule morphology, starch granule bound proteins, starch structure, and functional properties. However, the interactions between the two enzymes have not been thoroughly investigated yet. We analysed recombinant rice lines having down-regulated SBEIIb expression (SBEIIbDR) with either indica or japonica type SSIIa (SSIIaind or SSIIajap). In SBEIIbDR rice starch granules, the increased abundance of two protein bands (SSI and SSIIa) was found with eight additional protein bands not generally associated with starch granules. The amount of SSIIa was higher in SSIIaindSBEIIbDR than SSIIajapSBEIIbDR, which indicated that indica type SSIIa, possibly in the monomer form, was extensively involved in starch biosynthesis in the SBEIIbDR endosperm. Furthermore, SSIIaindSBEIIbDR grains had higher total starch content and higher starch swelling power than SSIIajapSBEIIbDR lines, but the amylopectin gelatinization temperatures and enthalpy and the apparent amylose content remained similar. In summary, this work suggests that SSIIaind can partly compensate for the alteration of starch synthesis resulting from the SBEIIb down-regulation in japonica background without reducing its amylose content. The study provides insight into the starch structural and textural improvements of high amylose starch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Amylose and amylopectin are the two principal components of starch. The former molecule (20–30% dry weight of starch) is a long linear glucose polymer with few branches. It is mainly elongated by adding glucose from ADP-glucose to existing α-(1,4)-glucan chains catalysed by granule bound starch synthase I (GBSSI). GBSSI is encoded by Wx, which primarily determines the amylose content of starch in cereals (Nelson and Rines 1962; Tsai 1974; Fedoroff et al. 1983; Shure et al. 1983). Amylopectin typically makes up 70–80% of rice (Oryza sativa) starch and is a much larger molecule with frequent α-(1–6)-branches. Amylopectin is synthesized by multiple enzymes in a coordinated manner, involving starch synthases (SSs), starch branching enzymes (SBEs), debranching enzymes (DBEs), starch phosphorylase and other regulatory proteins (Ball and Morell 2003; Martin and Smith 1995). Distinctive SSs isoforms (SSI, SSII, SSIII, and SSIV) act in succession to elongate the amylopectin linear glucan chains. Isoforms of SBEs (SBEI and SBEII) play distinct roles in the formation of branch linkages in amylopectin (Ball and Morell 2003). DBEs catalyse the debranching of α-(1–6)-glucan chains of amylopectin and removal of improperly linked branches.
Among these enzymes, active SSIIa and SBEIIb are both crucial for maintaining the regular chain length of amylopectin in rice (Oryza sativa) grains. SSIIa is responsible for the elongation of short α-(1,4)-glucan chains (DP < 10) of amylopectin to reach intermediate chain length (11 < DP < 24) (Umemoto et al. 2002, 2004). On the other hand, SBEIIb transfers short chains (DP 6–7) to form α-(1,6)-glucosidic linkages on the outer chains of amylopectin (Nakamura et al. 2010). Previous studies indicated that japonica type SSIIa (SSIIajap, a less-active isoform), in contrast to indica SSIIa (SSIIaind, an active isoform), led to amylopectin with increased short chains and decreased intermediate chains (Umemoto et al. 2002, 2004). Although the variation in amylopectin structure, the apparent amylose content (AAC) of these starches is comparable (Umemoto et al. 2002, 2004). Neither of the two SSIIa isoforms causes significant differences in starch granule or grain morphology in the hybrid rice (Luo et al. 2015a) or near-isogenic lines (Umemoto et al. 2008). On the contrary, SBEIIb recessive rice variants (amylose-extender mutants (ae) and SBEIIb down-regulated transgenic lines) produce fewer short chains and more intermediate chains of amylopectin than wildtypes in rice grains both in indica and japonica backgrounds (Nishi et al. 2001; Butardo et al. 2011, 2012). The increase of starch AAC of rice SBEIIb mutant is due to the consequence of increased long-chain relative proportion in amylopectin molecules, rather than amylose increase (Butardo et al. 2011). The starch granules of rice SBEIIb-recessive and down-regulated grains are round-shaped and loosely present in their compound structure, and the grains are smaller and chalky or opaque (Nishi et al. 2001; Butardo et al. 2011; Sun et al. 2017).
SSIIa and SBEIIb proteins are believed to be involved in amylopectin biosynthesis in a trimeric protein complex together with SSI protein in wheat (Tetlow et al. 2008) and maize (Hennen-Bierwagen et al. 2008). Analysis of the maize sugary-2 mutant demonstrated that SSIIa protein is closely associated with SBEIIb and SSI proteins and links with starch granules during starch synthesis in the endosperm. Therefore, it is believed that SSIIa protein plays the role of the core in the formation of the trimeric protein complex of SSI-SSIIa-SBEIIb in maize (Liu et al. 2012). The sugary-2 starch granules are found to be devoid of amylopectin biosynthetic enzymes (Liu et al. 2012). That study strongly supports the previous reports on significantly decreased amounts of SBEIIb and SSI inside cereal starch granules in the endosperm resulting from either reducing the activity or eliminating the presence of SSIIa (Yamamori et al. 2000; Morell et al. 2003; Umemoto et al. 2004; Umemoto and Aoki 2005; Kosar-Hashemi et al. 2007; Grimaud et al. 2008). It appears that SSIIa deficiency results in an altered granular distribution pattern of SSI and SBEIIb, with both proteins remaining in the amyloplast stroma instead of associating with starch granules after synthesis (Luo et al. 2015b).
The interactions among SSI, SSIIa and SBEIIb in enzyme complexes of cereal endosperm have been widely investigated using co-immunoprecipitation methods in barley (Ahmed et al. 2015), wheat (Tetlow et al. 2004, 2008), maize (Hennen-Bierwagen et al. 2008, 2009; Liu et al. 2009, 2011, 2012), and rice (Crofts et al. 2015, 2018). Interactions among SSI, SSIIa, and SBEIIb in rice have been mainly investigated by analysing multiple-gene mutants. Abe et al. (2014) reported that an SSI and SBEIIb double-null mutant was rarely fertile, but a double mutant with SSI-leaky mutation and SBEIIb-null had opaque seeds. The branching activity of SBEIIb in amylopectin chain formation is followed by the SSI activity of chain elongation (Nakamura 2002). An in vitro study of SSs and SBEs indicated that SBEIIb stimulates SSI activity in glucan chain synthesis by increasing the number of non-reducing ends and functional interaction with SSI in rice endosperm (Nakamura et al. 2014). It was also postulated in a study analysing starch structure that SS (SSI or SSIIIa) deficiency alters the branch points of side chains via the effects on enzyme complex formed by SS and SBE (Hanashiro et al. 2011). It has been demonstrated that ten starch synthetic enzymes are present at larger molecular weights of protein complexes than their respective monomeric sizes through various interactions of different enzymes, including SSI, SSIIa, and SBEIIb (Crofts et al. 2015, 2018). However, the functional interaction between SSIIa and SBEIIb and corresponding starch properties have not been reported in a double gene mutant so far.
In this study, we investigated the impacts of SSIIaind and SSIIajap alleles on SBEIIb down-regulated allele (SBEIIbDR) in rice grain on starch structures and properties. Rice grain with SSIIaind and SBEIIbDR alleles produced starch with novel starch structure and properties. The role of SSIIa alleles is discussed with regards to rice starch quality and functional property improvement.
Materials and methods
Plant materials
All plants were grown in the glasshouse of plant growth facility of CSIRO Agriculture and Food (Canberra) at 27 °C under natural light conditions. The experimental rice lines were derived from a cross-population between two parental rice lines (A203 and 3–12). The line A203 was a rice line with down-regulated SBEIIb gene expression (SBEIIbDR) in a Nipponbare background through the artificial microRNA technology (Butardo et al. 2011). The line 3–12 was an inbred line having heterozygous SSIIa allele (SSIIaindSSIIajap) and homozygous SBEIIbjapallele (SBEIIbjapSBEIIbjap), which was derived from a crossing population between a japonica variety (Nipponbare) and an indica variety (IR64) (Luo et al. 2015a). Both A203 and 3–12 lines contained japonica type Wx locus to ensure the same effect of Wx locus on amylose content in all progeny lines.
The derivation of recombinant lines is shown in Fig. 1. The F2 seeds were separated into two groups according to the phenotype. The opaque grains were verified to be homozygous SBEIIbDR allele (SBEIIbDRSBEIIbDR) or heterozygous SBEIIbDR allele (SBEIIbWTSBEIIbDR). In contrast, the translucent grains were homozygous wild type SBEIIb allele (SBEIIbWTSBEIIbWT) using gene-specific markers (Butardo et al. 2011). The DNA markers used to determine the genotypes of SSIIa were published in our previous work (Luo et al. 2015a). After 3–4 generations of phenotyping and genotyping selections, the homozygote lines were segregated into four groups (G1 to G4) by two alleles of SSIIa and SBEIIb genes: G1, SSIIaindSSIIaind/SBEIIbDRSBEIIbDR; G2, SSIIajapSSIIajap/SBEIIbDRSBEIIbDR; G3, SSIIaindSSIIaind/SBEIIbWTSBEIIbWT; G4, SSIIajapSSIIajap/SBEIIbWTSBEIIbWT. Grains of three independent F4 lines were used as triplicates for each group in the following analysis.
Generation of four genotype groups of rice lines for SBEIIb and SSIIa alleles. The parents are ami-SBEIIb (A203) and L3-12. The derivation of self-pollinated progenies is indicated by lines with arrowheads. The genotype of SSIIa and SBEIIb in each line is indicated, and the grain phenotype in brackets. Generations (from Parents to F3) are labeled on the right, and four groups are labeled underneath. Ind: indica; Jap: japonica; DR: down-regulated; WT: wildtype
Grain weight
Grain weight for one line of each four groups was determined as the average grain weight of 20 grains with three replicates.
Microscopic examination of rice grains
The dehulled rice grains from one line of each four groups were photographed with a Leica MZFLIII (Leica Microsystems, Germany) at 12.5 amplification.
Starch preparation
Mature panicles were harvested and dried at 37 °C. The seeds were threshed manually and dehulled with a Satake dehuller. Brown rice grains were then polished using a fabricated circulating abrasive polishing machine. Polished grains (approximately 100–150 mg) were ground in a capsule with ball bearing of an ESPE CapMix™ (model 3 M, AU) for 30 s three times to produce flour samples. The flour samples were then washed with 0.005% NaOH by vigorous vortexing for 2 min, followed by filtration through 0.5 mm nylon sieves. Each sample was then washed three times by vortex mixing and centrifugation at 5000 g for 5 min. The starch pellet was resuspended in a phosphate buffer (50 mM, pH7.5) containing proteinase K (50 μg/ml) and incubated at 37 °C for 2 h. After 5 min centrifugation at 5000 g, the pellet was suspended in water and centrifuged, and the water washing process was repeated three times. The starch pellet was then washed with acetone once and centrifuged for 5 min centrifugation at 5000 g. The residual starch was air-dried at 37 °C overnight.
Starch granule bound protein analyses
Starch samples were used for granule bound proteins (GBPs) extraction, as previously described (Luo et al. 2015a). The total crude proteins were then separated by SDS-PAGE, and the gels were stained for visualization. Afterwards, the protein bands were excised for mass spectrometry (MS) analysis (Luo et al. 2015a). The gels were also immunoblotted using specific antisera, as described in our previous work (Luo et al. 2015a).
Starch structural analyses
Total starch content (TSC) of rice flour samples was determined using a Megazyme total starch analysis kit. The analyses were adapted to a 96-well microplate following the manufacturer’s procedure (AACC Method 76.13). Rice flour samples were also used for the following analyses: apparent amylose content (AAC) by iodometric estimation (Konik-Rose et al. 2007), chain length distribution (CLD) of debranched starch by fluorophore-assisted carbohydrate electrophoresis (FACE) (O’Shea et al. 1998) and size-exclusion chromatography (SEC). The procedures were conducted following published methods (Luo et al. 2015a).
Starch property analyses
Flour samples were used to measure the swelling power of starch following the method of Konik-Rose et al. (2001). A Differential Scanning Calorimeter (DSC 8000, PerkinElmer, USA) was used to determine the thermal profiles of triplicate flour samples for each line. The procedures were conducted following published methods (Luo et al. 2015a). Data were analysed using the instrument software provided by the manufacturer.
Microscopic and particle size analysis of separated starch granules
Starch samples of each group were spread on the surface of a carbon stub and coated with gold. The morphology of the starch granules was observed under an environmental scanning electron microscope (SEM) (Zeiss EVO LS15) at room temperature and 10 Pa pressure.
Granule size distribution (by volume) of the starch slurries was determined using a laser-diffraction particle size analyser (Mastersizer 2000, Malvern Instruments, Malvern, England). The percentage of starch granules was calculated using a cut-off diameter of starch granules at < 1.9 µm, < 10 µm, and < 20 µm.
Statistical analyses
Statistical analyses were performed using one-way Analysis of Variance of Genstat version 9. Analysis of variance was performed to obtain the least significant differences at p < 0.05, looking at variations among groups.
Results
Starch granule bound protein analyses
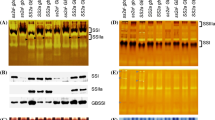
SDS-PAGE analysis showed that two parental lines (Nipponbare and A203) had different protein banding patterns. Using crude protein extract from Nipponbare starch, four protein bands were separated by SDS-PAGE at 60 kDa and above (Fig. 2). These protein bands were identified by MS analysis as SSIIa, SBEIIb, SSI, and GBSSI, from high to low molecular weight, respectively. This result was corroborated by our previous observation (Luo et al. 2015a). In contrast, other new granule bound proteins were detected in the SBEIIbDR line (A203). The MS analysis identified phosphatidylinositol 4-kinase (PI 4-kinase), pullulanase, SBEI, and 70 kDa heat shock (Hsp70) proteins (Fig. 2; Supplemental Fig. 1). Three protein bands could not be identified because of their low abundance and/or absence in the Uniprot database (band 1, 4, and 9 indicated by question marks, Fig. 2).
Analysis of starch granule bond proteins in mature rice grain starch of ami-SBEIIb (A203) and Nipponbare (Nip) by SDS-PAGE. The molecular sizes are labeled on the left of the gel in kDa. The bands are numbered as 1–10 based on their positions from top to bottom of the gel. The identity of each protein bands in each sample is indicated by lines on the right side, followed by Uniprot ID
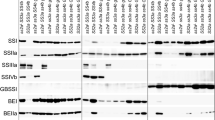
Immunoblot and SDS-PAGE analyses showed that four genotype groups of grains contained different protein banding patterns in starch granules. Immunoblot analysis using specific antisera showed that G1 genotype group had a high abundance of SSIIa protein. In contrast, the SSIIa protein abundance was low in G2 and G3, and absent in G4 (Fig. 3a). Similarly, SBEI and SBEIIa proteins were observed in both G1 and G2 samples using specific antibodies, and their protein abundance was just slightly higher in G1 than G2. The SBEI protein bands in G2 were very low abundance, which was hard to be observed. SBEI protein could not be detected in both G3 and G4, while SBEIIa protein was present at a low level. In contrast, SBEIIb protein was observed in G3 and G4, and G3 had higher SBEIIb abundance than G4. The weak SBEIIb protein bands in G1 and G2 may be due to an effect of various levels of SBEIIb down-regulation, which occurred in the independent lines. The results of SDS-PAGE analysis on total GBPs were also consistent with protein band patterns from two parental lines (Fig. 3b). Also, the abundance of SSI and GBSSI in G1 and G2 was higher than that in G3 and G4. Three protein bands were also detected at 100–120 kDa in both G1 and G2 (Fig. 3b) as observed in A203 lane (Fig. 2).
Comparative analysis of starch granule bond proteins in mature rice grain starch of four genotype groups by immunoblot (a) and SDS-PAGE (b). Samples from three independent lines of each genotype group were used. The names of genotype groups are labeled on the top of each figure. G1: SSIIaindSBEIIbDR, G2: SSIIajapSBEIIbDR, G3: SSIIaindSBEIIbWT, G4: SSIIajapSBEIIbWT. Panel A: The protein bands detected by various antibodies are indicated on the left. The molecular sizes are labeled on the right of the gel in kDa. Panel B: The identity of each protein band is indicated by an arrowhead on both sides
Grain weight and morphology
There were no statistically significant differences in grain weight among four genotype groups (p < 0.05) (Table 1), although there were some variations of the grain weight among them. The range of grain weights was from 19.5 mg (G1) to 23.8 mg (G3).
Intact grains from four representative lines for the four genotype groups were examined by stereoscopic microscopy. Grain appearances were opaque for G1 and G2 groups, and translucent for G3 and G4 groups (Fig. 4). The grain shapes were also varied among four lines as one of parental line 3–12 originally was derived from the crosses an indica rice, IR64, and japonica rice, Nipponbare (Luo et al. 2015a).
Two types of rice grain morphology were observed among the four genotype groups. Polished grains of G1 and G2 lines were opaque throughout similar to the SBEIIb down-regulated parent, A203, while polished grains of G3 and G4 showed uniformly translucent endosperms similar to Nipponbare. No significant differences were observed either between G1 and G2 or between G3 and G4 (data not shown).
Starch granule phenotypic analyses
SEM analysis showed that starch of G1 and G2 genotype groups contained more round starch granules, some large round starch granules, and a few starch granules with polyhedral structure (Fig. 5a, b). After the isolation and purification process, large starch granules of G1 and G2 were structurally intact, while those of G3 and G4 were completely disassociated into polygonal granules. Compared with those angular typical rice starch granules in G3 and G4 (Fig. 5c, d), G1 and G2 exhibited non-angular and rounded starch granules. The edges of numerous starch granules in G1 and G2 became less apparent. There were a few very large granules in G1 and G2 genotype groups, the surface of which was irregularly undulating with protrusions and crack lines on the surface (Fig. 5a, b). The differences in starch granule appearance between G1 and G2 groups, and G3 and G4 groups were similar to those between starch granules from the parents (Nipponbare and A203) as shown in Fig. 5e, f. However, A203 did not have any large round starch granules.
Starch granule morphology analysis of purified rice starch of four genotype groups using SEM. The starch samples used for analysis are as follows: a G1 genotype group (SSIIaindSBEIIbDR), b G2 genotype group (SSIIajapSBEIIbDR), c G3 genotype group (SSIIaindSBEIIbWT), d G4 genotype group (SSIIajapSBEIIbWT), e Nipponbare, f ami-SBEIIb (A203). Bars on the low right corner of the photos represent 10 µm
Particle size analysis revealed that the percentage of starch granules sizes of purified starch was different in four genotype groups. The portion of three selected sizes of starch granules of G1 and G2 groups was significantly lower than those in G3 and G4 groups in the fractions (< 1.9 µm, < 10 µm and < 20 µm) (p < 0.05) (Table 1). Generally, the majority of starch granules were 1.9–10 µm in all four genotype groups (Fig. 6). G1 and G2 groups had around 3% fewer very small granules (< 1.9 µm) than that in G3 and G4 groups (Table 1). In < 10 µm fraction, the difference was profound among four groups with the order from less to more starch granules with the size at < 10 µm as G1 < G2 < G3 < G4. Approximately 3–5% of starch granules were over 20 µm in G1 and G2 genotype groups, whereas all starch granules in G3 and G4 groups were under 20 µm (Table 1).
Starch structural analyses
Variations in TSC and AAC were observed among four genotype groups of grains. All four genotype groups contained over 90% in TSC. The TSC of G1, G3, and G4 groups were comparable between 93 and 95% (Table 1). But, the G2 group having just over 90% TSC was significantly lower than the other three groups. The reduction of TSC was also observed with a 5% lower in A203 compared with its corresponding wildtype (Butardo et al. 2011). The AAC of G1 and G2 was comparable and remarkably higher than G3 and G4, which nearly doubled the figures of G3 and G4 (Table 1).
The SEC analysis of debranched starch revealed two types of starch chain length distribution patterns for the four genotype groups (Fig. 7). The proportion of amylose fraction (approximately 400–4000 DP) was comparable in all groups, while the difference appeared in the amylopectin fraction (about 4–400 DP). Compared with G3 and G4, G1 and G2 exhibited a marked decrease in 4–40 DP (peak 1) and an increase in chains of 40–400 DP (peak 2).
Comparison analysis of debranched rice starch of four genotype groups using SEC. Distribution of normalized signals of debranched starch is plotted in the picture for each sample. Three independent lines of each group were used for data integration. The different samples are shown on the right. The two peaks of amylopectin and amylose are marked on the top (color figure online)
Further analysis of amylopectin chain length distribution (CLD) using FACE showed a peak change from DP 12 in G3 and G4 to DP 15 in the G1 and G2 (Fig. 8a). By the CLD difference plot, it was shown that the profiles of G1 and G2 were very similar with a remarkable decrease in DP 6–14 and an increase in DP 16–37 chains in comparison to G4 (Fig. 8b). G3 carried a different type of SSIIa to G4, showing a different profile with a decrease in DP 6–11 and an increase in DP 12–DP 23 (Fig. 8b).
Comparison of chain length distribution (CLD) of debranched starch of four genotype groups using CE. a shows the Mol% plots of CLD profiles of four different genotype groups and four genotypes, and two peaks are labeled on the top. b shows the Mol% difference of CLD profiles of G1, G2, and G3 genotype groups compared with the G4 genotype group. The indication of different samples is shown on the right (color figure online)
Starch swelling power, gelatinization, and thermal properties
The starch swelling power test found that the four genotype groups had various capacities for holding water in their gelatinized starch. The order of the starch swelling power of the four groups was G4 > G3 > G1 > G2 (Table 1). Although the difference of the starch swelling power between G1, G3, and G4 was up to 3, it was not determined to be statistically significantly different (p < 0.05). Only G2 showed significantly lower swelling power than the other groups (p < 0.05). The difference between G2 and G4 groups was 5.5, while the G3 group was 2.3 lower than the G4 group.
The DSC analysis revealed the variation of starch thermal property among the four genotype groups. The amylopectin gelatinization temperatures (GTs) of G1 and G2 were higher than those of G3 and G4, respectively. In particular, the G4 was significantly lower than all the other groups for GTs (p < 0.05), whereas G1 and G2 were comparable (Table 2). Regarding the enthalpy of amylopectin gelatinization (ΔH1), G1 and G2 were at the same level, but G3 (1.7 J/g) was significantly lower compared with G4 (2.4 J/g) and the other two groups (over 2.5 J/g) (p < 0.05). The lipid-amylose complex dissociation, the onset, peak and end temperatures, respectively, were not significantly different among the four groups (Table 2). However, G1 and G3 exhibited a remarkable decrease in the dissociation enthalpy of amylose–lipid complexes (ΔH2) compared with G2 and G4, respectively (Table 2). The reduction in ΔH2 was also obtained when comparing G1 with G3 or G2 with G4, respectively, though the differences were not determined to be statistically significant.
Discussion
Pleiotropic effects of different SSIIa alleles on starch granule bound proteins in SBEIIb down-regulated starch in rice
The first objective of this work was to study the roles of the two SSIIa alleles on the deposition of starch biosynthetic enzymes within the starch granule of SBEIIb down-regulated rice grains. The downregulation of SBEIIb in line A203 caused a substantial change in the number of GBP compared to the parent Nipponbare. Aside from starch synthases (SSIIa, SSI, and GBSSI), which are typically present in Nipponbare, other proteins including PI-kinase, pullulanase, SBEI, SBEIIa, HSP70, and three uncharacterized proteins were additionally detectable in line A203. As starch granules were treated with proteinase K during starch preparation in this study, these additional proteins were present inside the starch granules. The result is supported by a recent report of SBEIIb inactive or null mutant with the same distribution pattern of the interior starch granule associated proteins in rice (Crofts et al. 2018). Two of the uncharacterized additional protein bands here were identified as SSIIIa (~ 250 kDa) and PPDK (pyruvate phosphate dikinase, ~ 100 kDa) in that study (Crofts et al. 2018). PI 4-kinase and HSP70 were reported indirectly involve in translocating, correctly folding, and solubilizing starch synthetic enzymes during import into the amyloplast (Yu et al. 1998), as well as in regulating physiological process (Ischebeck et al. 2010). HSP70s are important for protein translocation into amyloplast stroma (Yu et al. 1998), mitochondria and the endoplasmic reticulum (Su and Li 2010). SBEI and SSIIIa were previously reported as tightly granule-bound protein in developing endosperms of an SBEIIb-null rice mutant (Abe et al. 2014; Asai et al. 2014). Studies focused on SBEIIb-null mutant of maize discovered that SBEI, SBEIIa, and starch phosphorylase (SP) are associated with starch granules (Grimaud et al. 2008; Liu et al. 2009). It was suggested that SBEI, SBEIIa, and SP are included in protein complexes to compensate for the loss of the former component SBEIIb in the endosperms of the mutant. Thus, in its absence, the role of SBEIIb in amylopectin cluster synthesizing machinery is executed by SBEI, SBEIIa, and SP.
The starch synthetic enzyme complexes have been reported in rice, suggesting the mechanism of the functional protein complexes in developing endosperm is generally accepted for all cereals (Tetlow et al. 2004; Crofts et al. 2015). The deposition of SSIIIa, SBEI, SBEIIa, and pullulanase in SBEIIb down-regulated starches may be a consequence of involving multiple amyloplast stromal protein complexes at different developmental stages of cereal endosperm when SBEIIb is absent or inactive inside starch granules. Nevertheless, the function of SBEIIb in SBEIIbDR lines cannot be fully recovered by SBEI, and SBEIIa indicated by the lack of short chains of DP6 ~ 13 in amylopectin CLD. This is also supported by a more recent study, in which the impact of the loss of SBEIIb activity on starch structure and properties was most remarkably compared with SBEI and SBEIIa using mutants of multiple SBE genes in different combinations (Sawada et al. 2018). The increase of SSI and SSIIa abundance in SBEIIbDR starch granules suggests the two enzymes are more extensively involved in enzyme complexes in SBEIIb down-regulated endosperms than wildtype. The additional amount of SSI and SSIIa may help the elongation of the short chains (DP < 6) formed by SBEIIa to reach DP ≤ 16 more efficiently to compensate for the lack of branches in this range in SBEIIb down-regulated starches.
As mentioned previously, SSIIa plays a central role in directing the association of other components of protein complexes to starch granules (Liu et al. 2012). The amount of SSI and SBEIIb in starch granules is correlated with that of granule-bound SSIIa which has been widely reported in cereals (Yamamori et al. 2000; Morell et al. 2003; Umemoto et al. 2004; Umemoto and Aoki 2005; Kosar-Hashemi et al. 2007; Grimaud et al. 2008; Luo et al. 2015b; Crofts et al. 2018). Our result here again shows such a pleiotropic effect in starches of SSIIaind and SSIIajap alleles in the SBEIIbWT background but not in the SBEIIbDR background. The abundance of SSIIa in starch granules is much higher in SSIIaindSBEIIbDR than SSIIajapSBEIIbDR, whereas the variation of SBEI, SBEIIa, and SSI are not so remarkable. This result leads to a hypothesis that SSIIa associates to starch granules possibly in two different forms during rice endosperm development: one is in monomer and/or oligomer form, the other is in the form of protein complex associating with other enzymes. Oligomerization of SSII was recently observed using bioinformatic and in vitro analyses of recombinant Arabidopsis SSII (Patterson et al. 2018). Although that study indicated that the formation of homodimers required the N-terminal region of the protein, which is highly variable among plant species, it still strongly supports the hypothesis in rice in this study. Based on this hypothesis, one explanation for the high abundance of SSIIa proteins in mature starch granules may be that the higher affinity of indica type SSIIa directed more SSIIa monomers and/or oligomers involved in starch biosynthesis and attached to starch granules in SSIIaindSBEIIbWT than SSIIajapSBEIIbWT endosperm. But the indica type SSIIa cannot direct more of those additional proteins in an equivalent additional amount of SSIIa protein to deposit in starch granules of SBEIIbDR background. Therefore, the ability of indica type SSIIa may be comparable with japonica type SSIIa, in terms of protein-starch interaction, during starch synthesis in SBEIIbDR background.
Effects of different SSIIa alleles on starch granule morphology, apparent amylose content and starch content of SBEIIb down-regulated starch in rice
SBEIIbDR allele also affected the grain appearance to opaque in both SSIIaindSBEIIbDR and SSIIajapSBEIIbDR grains, as described in early publications (Tanaka et al. 2004; Butardo et al. 2011). The high expression of SSIIa in SSIIaind allele could not recover such opaque appearance of the rice grain in SBEIIbDR grains.
The early publications showed that down-regulating SBEIIb in rice grain changes the higher level of organization of starch granules, forming compound starch granules in wildtype rice grain, which then changes the morphology of starch granules to spherical shape (Tanaka et al. 2004; Wei et al. 2010; Butardo et al. 2011). It was thought that the activity of SBEs affects the cluster size of amylopectin during endosperm development (Tetlow et al. 2004). Our results showed that a portion of starch granules in SBEIIbDR background are larger in size based on our SEM analysis. This was also supported by the particle size distribution analysis, which revealed that about 5% of starch granules were over 20 µm in diameter in SBEIIbDR starch. In contrast, SBEIIbWT starch did not contain any large granules (> 20 µm). The bigger starch granules were present in both SSIIaindSBEIIbDR and SSIIajapSBEIIbDR grains, suggesting that SSIIaind cannot recover the phenotypic changes in starch granules induced by SBEIIb down-regulation.
In the current study, the association of increased AAC and altered CLD with reduced angular starch granule proportion was observed in SBEIIbDR groups as previously reported on SBEIIb down-regulated lines of rice (Butardo et al. 2011). In comparison, the variation of AAC was not observed between SSIIaind and SSIIajap alleles in neither SBEIIbWT nor SBEIIbDR background, even though the CLD patterns were different. Such AAC results confirmed that the mutation in SSIIa does not affect amylose content. High AAC in G1 and G2 grains was derived from long-chain of amylopectin, not true amylose. In contrast to the mutation of SSIIIa in the SSIIIa and SBEIIb double-null mutant, the knockout of SSIIIa increased the amylose content of SBEIIb mutant to 45% from under 30% (Asai et al. 2014). It could be that SSIIIa possesses the major SS activity in the soluble fraction (Fujita et al. 2006, 2007). Although the SSs (SSI, SSIIa, and SSIIIa) share overlapping chain-length preferences, they cannot fully complement each other (Crofts et al. 2017). Knocking-out SSIIIa causes a remarkable reduction of amylopectin synthesis and increases the AAC level as a consequence.
SSIIajapSBEIIbDR showed a reduction of about 4% TSC compared with SSIIajapSBEIIbWT consistent with previous reports on SBEIIb knockout mutant EM10 (Abe et al. 2014; Asai et al. 2014). The decrease in TSC indicates that loss of SBEIIb or down-regulation of SBEIIb induces the decrease in starch biosynthesis due to a reduction in amylopectin and amylose biosynthesis as evidenced by SEC analysis and the unchanged amylose content. However, the TSCs of SSIIaindSBEIIbDR was comparable with SSIIaindSBEIIbWT, and higher than that of SSIIajapSBEIIbDR, which indicated that indica type SSIIa could increase starch content in SBEIIbDR background, including both amylose and amylopectin. However, the grain weight of SSIIaindSBEIIbDR did not have a significant difference with that of SSIIajapSBEIIbDR. As the availability of the grains from each line of four genotypes was the limitation for current study, the relationship between the increase of the starch content and no-change of grain weight for SSIIaindSBEIIbDR could not be studied in detail. The mechanisms of the increase of starch content for the indica type SSIIa in SBEIIbDR background remain to be further studied. The proportion of long amylopectin debranched chains (DP40–400) of SSIIaindSBEIIbDR was slightly lower than SSIIajapSBEIIbDR while that of short chains (DP 4–40) was marginally higher. The precise structural changes in amylopectin CLD were mild, with only a slight increase in DP16–21 and decrease in DP26–36 between SSIIaindSBEIIbDR and SSIIajapSBEIIbDR, which indicated that the increased abundance of SSIIa in SBEIIbDR starch granules has only limited effect on amylopectin structure. These results suggest the combination of SSIIaind and SBEIIbDR in rice only slightly modifies the amylopectin structure and stimulates starch accumulation in comparison to SSIIajap and SBEIIbDR combination. An early publication also reported a similar effect of SSIIaind on the starch structure in rice grain containing the SBEIIbDR and an indica allele of GBSSI (Itoh et al. 2017). Therefore, a more active isoform than the native SSIIa is required for efficient starch accumulation in rice when amylopectin biosynthesis has been significantly perturbed.
Effects of different SSIIa alleles on starch physical and thermal properties of SBEIIb down-regulated starch in rice
It was previously observed that SBEIIb knockout rice starch granules have more long glucan chains, due to the defect in amylopectin short chains, were more resistant to thermal gelatinization than wild type starch granules (Tanaka et al. 2004; Kubo et al. 2010). This variation in amylopectin CLD leads to high GTs and ΔH1 (Jane et al. 1999). Downregulation of SBEIIb generates more double-helical content and longer its length, which results in higher GTs and ΔH1 in rice (Dhital et al. 2015). In contrast, more short external chains of amylopectin lead to the weakening of the interaction among amylopectin molecules in crystal lamellae, which results in the low GTs and ΔH1 in wheat SSIIa mutant (Konik-Rose et al. 2007). As a more active form of SSIIa was present in SSIIaindSBEIIbDR starch than SSIIajapSBEIIbDR, it would be considered to be more efficient in synthesizing intermediate chains with DP13-24, and it may then affect the GTs and ΔH1. However, only subtle alterations in amylopectin structure groups were observed in the SSIIaindSBEIIbDR starch with no variation of GTs and ΔH1 detected between SSIIaindSBEIIbDR and SSIIajapSBEIIbDR. Increased swelling power of SSIIaindSBEIIbDR starch was found compared to that of SSIIajapSBEIIbDR, which may be a result of the slight increase of the amylopectin intermediate chains (DP16 to DP21).
The variation of amylopectin CLD, shown in SBEIIbWT vs. SBEIIbDR and SSIIaind vs. SSIIajap, has an impact on the ΔH2 of amylose–lipid complexes. The current study showed that mean ΔH2 values were differentiated at 1/3 in both SSIIajapSBEIIbDR vs. SSIIajapSBEIIbWT, and SSIIaindSBEIIbDR vs. SSIIaindSBEIIbWT. Even though the differences were not statistically significant, the evidence probably indicated that the SBEIIb down-regulated starch had an increased proportion of the chain length of DP40–400, a reduced portion of DP4–40 in amylopectin (analysed by SEC), and a decreased amylose–lipid association enthalpy (ΔH2) of rice starch. With more intermediate chains (DP12 to 24) in amylopectin (analysed by CE) of SSIIaind starch than SSIIajap, ΔH2 value is significantly lower in SSIIaindSBEIIbWT vs. SSIIajapSBEIIbWT, and SSIIaindSBEIIbDR vs. SSIIajapSBEIIbDR. Therefore, these results showed that the change of ΔH2 values was mainly controlled by the SSIIa alleles with less contribution from SBEIIb alleles. The SBEIIbDR allele reduced ΔH2 values in the SSIIaindSBEIIbDR vs. SSIIaindSBEIIbWT, and SSIIajapSBEIIbDR vs. SSIIajapSBEIIbWT, but they were not a significant difference in the population. The result was also consistent with the study in wheat, in which ΔH2 value is significantly increased when SSIIa is knocked out (Konik-Rose et al. 2007).
Implications for starch quality improvement in rice
Rice is a staple food in many developing countries and improving the nutritional quality of rice is an important research focus (Butardo and Sreenivasulu 2016), including the vital objective of raising the resistant starch (RS) level of rice grains (Butardo et al. 2011; Matsumoto et al. 2012). Increasing the intake of RS-rich food products benefits to human health by lowering the risk of cardiovascular disease and diabetes due to the characteristic of slow release of glucose, but also provides protection against colorectal cancer due to the high content of short-chain fatty acid produced from non-digestible starch fermentation (Topping and Clifton 2001; Champ et al. 2003; Bird et al. 2010; Fuentes-Zaragoza et al. 2011; Higgins and Brown 2013). Starch with high amylose content is positively correlated with high RS (Ring et al. 1988; Faisant et al. 1993) due to the reduction of starch granule amylolysis (Dhital et al. 2015). Cooking and sensory qualities of rice, including starch textural properties, have major impacts on consumer preferences in different food cultures (Fitzgerald et al. 2009; Calingacion et al. 2014). Rice of high amylose content often has a firm texture, which is not preferred by consumers in many regions (Juliano 2001). The texture of cooked rice grain is related to not only amylose but the fine structure of amylopectin (Li et al. 2016). Improvement in the starch textural quality of rice, as well as increasing the RS content, will be a target in further breeding programs. Our study on SSIIa and SBEIIb interactions indicates that it is possible to improve rice grain quality, yet maintain their amylose content in SBEIIb down-regulation lines through manipulating the amylopectin chain length distribution. Genetic introduction of allelic variants of amylopectin biosynthetic genes is one of the applicable strategies.
Conclusions
Down-regulation of SBEIIb in rice increases the number of starch granule bound proteins, including PI 4-kinase, pullulanase, SBEI, SBEIIa, Hsp70, and three other unidentified proteins. The impacts of SBEIIb down-regulation in the japonica background on starch granule morphology, starch structure, and properties can be partly recovered by molecular breeding with an indica SSIIa allele. The active allele of SSIIa increases total starch content and starch swelling power, mildly modifies chain length distribution of amylopectin, and reduces the amylose–lipid complexes dissociation enthalpy of SBEIIb down-regulated starch without reducing the amylose content. This study sheds light on the improvement of the texture of rice grains with high resistant starch.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information file.
References
Abe N, Asai H, Yago H, Oitome NF, Itoh R, Crofts N, Nakamura Y, Fujita N (2014) Relationships between starch synthase I and branching enzyme isozymes determined using double mutant rice lines. BMC Plant Biol 14:80–91
Ahmed Z, Tetlow IJ, Ahmed R, Morell MK, Emes MJ (2015) Protein-protein interactions among enzymes of starch biosynthesis in high-amylose barley genotypes reveal the differential roles of heteromeric enzyme complexes in the synthesis of A and B granules. Plant Sci 233:95–106
Asai H, Abe N, Matsushima R, Crofts N, Oitome NF, Nakamura Y, Fujita N (2014) Deficiencies in both starch synthase IIIa and branching enzyme IIb lead to a significant increase in amylose in SSIIa-inactive japonica rice seeds. J Exp Bot 65:5497–5507
Ball SG, Morell MK (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu Rev Plant Biol 54:207–233
Bird AR, Conlon MA, Christophersen CT, Topping DL (2010) Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Benef Microbes 1:423–431
Butardo VM, Fitzgerald MA, Bird AR et al (2011) Impact of down-regulation of starch branching enzyme IIb in rice by artificial microRNA- and hairpin RNA-mediated RNA silencing. J Exp Bot 62:4927–4941
Butardo VM, Arrowhead DV, Colgrave ML et al (2012) Analysis of starch and starch granule proteins in the high amylose rice mutant Goami 2. J Agri Food Chem 60:11576–11585
Calingacion M, Laborte A, Nelson A et al (2014) Diversity of global rice markets and the science required for consumer-targeted rice breeding. PLoS ONE 9:e85106
Champ M, Langkilde AM, Brouns F, Kettlitz B, Bail-Collet YL (2003) Advances in dietary fibre characterisation. 2. Consumption, chemistry, physiology and measurement of resistant starch; implications for health and food labelling. Nutr Res Rev 16:143–161
Crofts N, Abe N, Oitome NF, Matsushima R, Hayashi M, Tetlow IJ, Emes MJ, Nakamura Y, Fujita N (2015) Amylopectin biosynthetic enzymes from developing rice seed form enzymatically active protein complexes. J Exp Bot 66:4469–4482
Crofts N, Sugimoto K, Oitome NF, Nakamura Y, Fujita N (2017) Differences in specificity and compensatory functions among three major starch synthases determine the structure of amylopectin in rice endosperm. Plant Mol Biol. https://doi.org/10.1007/s11103-017-0614-8
Crofts N, Iizuka Y, Abe N, Miura S, Kikuchi K, Matsushima R, Fujita N (2018) Rice mutants lacking starch synthase I or branching enzyme IIb activity altered starch biosynthetic protein complexes. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01817
Dhital S, Butardo VM Jr, Jobling SA, Gidley MJ (2015) Rice starch granule amylolysis—differentiating effects of particle size, morphology, thermal properties and crystalline polymorph. Carbohydr Polym 115:305–316
Faisant N, Champ M, Colonna P, Buleon A, Molis C, Langkilde AM, Schweizer T, Flourie B, Galmiche JP (1993) Structural features of resistant starch at the end of the human small-intestine. Eur J Clin Nutr 47:285–296
Fedoroff N, Wessler S, Shure M (1983) Isolation of the transposable maize controlling elements Ac and Ds. Cell 35:235–242
Fitzgerald MA, McCouch SR, Hall RD (2009) Not just a grain of rice: the quest for quality. Trends Plant Sci 14:133–139
Fuentes-Zaragoza E, Sanchez-Zapata E, Sendra E, Sayas E, Navarro C, Fernandez-Lopez J, Perez-Alvarez JA (2011) Resistant starch as prebiotic: a review. Starch-Stärke 63:406–415
Fujita N, Yoshida M, Asakura N, Ohdan T, Miyao A, Hirochika H, Nakamura Y (2006) Function and characterization of starch synthase I using mutants in rice. Plant Physiol 140:1070–1084
Fujita N, Yoshida M, Kondo T et al (2007) Characterization of SSIIIa-Deficient mutants of rice: the function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol 144:2009–2023
Grimaud F, Rogniaux H, James MG, Myers AM, Planchot V (2008) Proteome and phosphoproteome analysis of starch granule-associated proteins from normal maize and mutants affected in starch biosynthesis. J Exp Bot 59:3395–3406
Hanashiro I, Higuchi T, Aihara S, Nakamura Y, Fujita N (2011) Structures of starches from rice mutants deficient in the starch synthase isozyme SSI or SSIIIa. Biomacromol 12:1621–1628
Hennen-Bierwagen TA, Liu F, Marsh RS, Kim S, Gan Q, Tetlow IJ, Emes MJ, James MG, Myers AM (2008) Starch biosynthetic enzymes from developing maize endosperm associate in multisubunit complexes. Plant Physiol 146:1892–1908
Hennen-Bierwagen TA, Lin Q, Grimaud F, Planchot V, Keeling PL, James MG, Myers AM (2009) Proteins from multiple metabolic pathways associate with starch biosynthetic enzymes in high molecular weight complexes: a model for regulation of carbon allocation in maize amyloplasts. Plant Physiol 149:1541–1559
Higgins JA, Brown IL (2013) Resistant starch: a promising dietary agent for the prevention/treatment of inflammatory bowel disease and bowel cancer. Curr Opin Gastroenterol 29:190–194
Ischebeck T, Seiler S, Heilmann I (2010) At the poles across kingdoms: phosphoinositides and polar tip growth. Protoplasma 240:13–31
Itoh Y, Crofts N, Abe M, Hosaka Y, Fujita N (2017) Characterization of the endosperm starch and the pleiotropic effectsof biosynthetic enzymes on their properties in novel mutant rice lineswith high resistant starch and amylose content. Plant Sci 258:52–60
Jane J, Chen YY, Lee LF, McPherson AE, Wong KS, Radosavljevic M, Kasemsuwan T (1999) Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem 76:629–637
Juliano BO (2001) Asian perspective on rice sensory quality. Cereal Food World 46:531–535
Konik-Rose C, Moss R, Rahman S, Appels R, Stoddard F, McMaster G (2001) Evaluation of the 40 mg swelling test for measuring starch functionality. Starch Stärke 53:14–20
Konik-Rose C, Thistleton J, Chanvrier H et al (2007) Effects of starch synthase IIa gene dosage on grain, protein and starch in endosperm of wheat. Theor Appl Genet 115:1053–1065
Kosar-Hashemi B, Li Z, Larroque O, Regina A, Yamamori M, Morell MK, Rahman S (2007) Multiple effects of the starch synthase II mutation in developing wheat endosperm. Func Plant Biol 34:431–438
Kubo A, Akdogan G, Nakaya M, Shojo A, Suzuki S, Satoh H, Kitamura S (2010) Structure, physical, and digestive properties of starch from wx ae double-mutant rice. J Agr Food Chem 58:4463–4469
Li H, Prakash S, Nicholson TM, Fitzgerald MA, Gilbert RG (2016) The importance of amylose and amylopectin fine structure for textural properties of cooked rice grains. Food Chem 196:702–711
Liu F, Makhmoudova A, Lee EA, Wait R, Emes MJ, Tetlow IJ (2009) The amylose extender mutant of maize conditions novel protein-protein interactions between starch biosynthetic enzymes in amyloplasts. J Exp Bot 60:4423–4440
Liu F, Ahmed Z, Lee EA, Donner E, Liu Q, Ahmed R, Morell MK, Emes MJ, Tetlow IJ (2011) Allelic variants of the amylose extender mutation of maize demonstrate phenotypic variation in starch structure resulting from modified protein-protein interactions. J Exp Bot 63:1167–1183
Liu F, Romanova N, Lee EA, Ahmed R, Evans M, Gilbert EP, Morell MK, Emes MJ, Tetlow IJ (2012) Glucan affinity of starch synthase IIa determines binding of starch synthase I and starch branching enzyme IIb to starch granules. The Biochem J 448:373–387
Luo J, Jobling SA, Millar A, Morell MK, Li Z (2015a) Allelic effects on starch structure and properties of six starch biosynthetic genes in a rice recombinant inbred line population. Rice 8:15
Luo J, Regina A, Kosar-Hashemi B et al (2015b) The different effects of starch synthase IIa mutations or variation on endosperm amylose content of barley, wheat and rice are determined by the distribution of starch synthase I and starch branching enzyme IIb between the starch granule and amyloplast stroma. Theor Appl Genet 128:1407–1419
Martin C, Smith AM (1995) Starch Biosynthesis. Plant Cell 7:971–985
Matsumoto K, Maekawa M, Nakaya M, Takemitsu H, Satoh H, Kitamura S (2012) Wx/ae double-mutant brown rice prevents the rise in plasma lipid and glucose levels in mice. Biosci Biotech Biochem 76:2112–2117
Morell MK, Kosar-Hashemi B, Cmiel M, Samuel MS, Chandler P, Rahman S, Buleon A, Batey IL, Li Z (2003) Barley sex6 mutants lack starch synthase IIa activity and contain a starch with novel properties. Plant J 34:172–184
Nakamura Y (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol 43:718–725
Nakamura Y, Utsumi Y, Sawada T, Aihara S, Utsumi C, Yoshida M, Kitamura S (2010) Characterization of the reactions of starch branching enzymes from rice endosperm. Plant Cell Physiol 51:776–794
Nakamura Y, Aihara S, Crofts N, Sawada T, Fujita N (2014) In vitro studies of enzymatic properties of starch synthases and interactions between starch synthase I and starch branching enzymes from rice. Plant Sci 224:1–8
Nelson OE, Rines HW (1962) The enzymatic deficiency in the waxy mutant of maize. Biochem Biophys Res Commun 9:297–300
Nishi A, Nakamura Y, Tanaka N, Satoh H (2001) Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol 127:459–472
O’Shea MG, Samuel MS, Konik CM, Morell MK (1998) Fluorophoreassisted carbohydrate electrophoresis (FACE) of oligosaccharides: efficiency of labeling and high-resolution separation. Carbohydr Res 307:1–12
Patterson JA, Tetlow IJ, Emes MJ (2018) Bioinformatic and in vitro analyses of Arabidopsis starch synthase 2 reveal post-translational regulatory mechanisms. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01338
Ring SG, Gee JM, Whittam M, Orford P, Johnson IT (1988) Resistant starch: its chemical form in foodstuffs and effect on digestibility in vitro. Food Chem 28:97–109
Sawada T, Itoh M, Nakamura Y (2018) Contributions of three starch branching enzyme isozymes to the fine structure of amylopectin in rice endosperm. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01536
Shure M, Wessler S, Fedoroff N (1983) Molecular-identification and isolation of the waxy locus in maize. Cell 35:225–233
Su P-H, Li H-M (2010) Stromal Hsp70 is important for protein translocation into pea and Arabidopsis chloroplasts. Plant Cell 22:1516–1531
Sun Y, Jiao G, Liu Z et al (2017) Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Front Plant Sci 8:298
Tanaka N, Fujita N, Nishi A, Satoh H, Hosaka Y, Ugaki M, Kawasaki S, Nakamura Y (2004) The structure of starch can be manipulated by changing the expression levels of starch branching enzyme IIb in rice endosperm. Plant Biotech J 2:507–516
Tetlow IJ, Wait R, Lu Z, Akkasaeng R, Bowsher CG, Esposito S, Kosar-Hashemi B, Morell MK, Emes MJ (2004) Protein phosphorylation in amyloplasts regulates starch branching enzyme activity and protein-protein interactions. Plant Cell 16:694–708
Tetlow IJ, Beisel KG, Cameron S, Makhmoudova A, Liu F, Bresolin NS, Wait R, Morell MK, Emes MJ (2008) Analysis of protein complexes in wheat amyloplasts reveals functional interactions among starch biosynthetic enzymes. Plant Physiol 146:1878–1891
Topping DL, Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81:1031–1064
Tsai CY (1974) Function of waxy locus in starch synthesis in maize endosperm. Biochem Genet 11:83–96
Umemoto T, Aoki N (2005) Single-nucleotide polymorphisms in rice starch synthase IIa that alter starch gelatinisation and starch association of the enzyme. Func Plant Biol 32:763–768
Umemoto T, Yano M, Satoh H, Shomura A, Nakamura Y (2002) Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor Appl Genet 104:1–8
Umemoto T, Aoki N, Lin HX, Nakamura Y, Inouchi N, Sato Y, Yano M, Hirabayashi H, Maruyama S (2004) Natural variation in rice starch synthase IIa affects enzyme and starch properties. Func Plant Biol 31:671–684
Umemoto T, Horibata T, Aoki N, Hiratsuka M, Yano M, Inouchi N (2008) Effects of variations in starch synthase on starch properties and eating quality of rice. Plant Prod Sci 11:472–480
Wei CX, Qin FL, Zhu LJ, Zhou WD, Chen YF, Wang YP, Gu MH, Liu QQ (2010) Microstructure and ultrastructure of high-amylose rice resistant starch granules modified by antisense RNA inhibition of starch branching enzyme. J Agri Food Chem 58:1224–1232
Yamamori M, Fujita S, Hayakawa K, Matsuki J, Yasui T (2000) Genetic elimination of a starch granule protein, SGP-1, of wheat generates an altered starch with apparent high amylose. Theor Appl Genet 101:21–29
Yu Y, Mu H-H, Mu-Forster C, Wasserman BP (1998) Polypeptides of the maize amyloplast stroma: stromal localization of starch-biosynthetic enzymes and identification of an 81-kilodalton amyloplast stromal heat-shock cognate. Plant Physiol 116:1451–1460
Acknowledgements
Authors would like to thank Oscar Larroque for SEC and CE analysis and data collection; Hong Wang for amylose content and total starch content assay.
Funding
Jixun Luo was supported by the Chinese Scholarship Council and Australian National University scholarships. This work was funded by CSIRO Food Future National Research Flagship.
Author information
Authors and Affiliations
Contributions
JL carried out the experiments and wrote the draft; QY finished the SDS-PAGE detection; CK did particle size and DSC analyses; MLC did mass spectrometry analysis; VMB contributed to experimental design; AM, SJ and ZL designed the experiments and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Additional information
Communicated by Takuji Sasaki.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luo, J., Butardo, V.M., Yang, Q. et al. The impact of the indica rice SSIIa allele on the apparent high amylose starch from rice grain with downregulated japonica SBEIIb. Theor Appl Genet 133, 2961–2974 (2020). https://doi.org/10.1007/s00122-020-03649-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-020-03649-2