Abstract
Key message
A major QTL on chromosome 2 associated with leptine biosynthesis and Colorado potato beetle resistance was identified in a diploid S. chacoense F2 population using linkage mapping and bulk-segregant analysis.
Abstract
We examined the genetic features underlying leptine glycoalkaloid mediated Colorado potato beetle (Leptinotarsa decemlineata) host plant resistance in a diploid F2 mapping population of 233 individuals derived from Solanum chacoense lines USDA8380-1 and M6. The presence of foliar leptine glycoalkaloids in this population segregated as a single dominant gene and displayed continuous distribution of accumulated quantity in those individuals producing the compound. Using biparental linkage mapping, a major overlapping QTL region with partial dominance effects was identified on chromosome 2 explaining 49.3% and 34.1% of the variance in Colorado potato beetle field resistance and leptine accumulation, respectively. Association of this putative resistance region on chromosome 2 was further studied in an expanded F2 population in a subsequent field season. Loci significantly associated with leptine synthesis colocalized to chromosome 2. Significant correlation between increased leptine content and decreased Colorado potato beetle defoliation suggests a single QTL on chromosome 2. Additionally, a minor QTL with overdominance effects explaining 6.2% associated with Colorado potato beetle resistance donated by susceptible parent M6 was identified on chromosome 7. Bulk segregant whole genome sequencing of the same F2 population detected QTL associated with Colorado potato beetle resistance on chromosomes 2, 4, 6, 7, and 12. Weighted gene co-expression network analysis of parental lines and resistant and susceptible F2 individuals identified a tetratricopeptide repeat containing protein with a putative regulatory function and a previously uncharacterized acetyltransferase within the QTL region on chromosome 2, possibly under the control of a regulatory Tap46 subunit within the minor QTL on chromosome 12.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Colorado potato beetle (Leptinotarsa decemlineata Say, Coleoptera: Chrysomelidae) is the most widespread and destructive insect defoliator of potato crops worldwide. Both larvae and adult beetles consume potato leaves and stems, inflicting yield losses of 30–50% (Alyokhin et al. 2012; Vreugdenhil et al. 2007), depending on the timing of the defoliation within the plant growth cycle (Hare 1980). Control of the Colorado potato beetle historically relied heavily on the use of insecticides (Grafius and Douches 2008), beginning with the use of arsenical compounds in 1871 (Riley 1871). However, Colorado potato beetle resistance is reported for most major classes of synthetic insecticides and over 50 different active ingredients (APRD 2019) necessitating more frequent sprays and higher application rates (Mota-Sanchez et al. 2006). Resistant populations of Colorado potato beetle are found across the entirety of its range but are most prevalent in its North American region of origin (Izzo et al. 2018; Whalon et al. 2008), where greatest insect genetic diversity has been exposed to the longest period of intensive pesticide application (Alyokhin et al. 2008). Availability and effective implementation of Colorado potato beetle resistant varieties could reduce both the environmental impact and the economic burden to growers of repeated insecticide applications.
Cultivated potato Solanum tuberosum Group Tuberosum L (2n = 4x = 48) naturally produces secondary metabolites in the form of glycoalkaloids with antimicrobial and insecticidal properties (Lachman et al. 2001; Tingey 1984). Host plant resistance efficacy is dependent on both total leaf glycoalkaloid accumulation and the production of specific glycoalkaloids. For example, the common glycoalkaloids α-chaconine and α-solanine are present in leaves of Colorado potato beetle susceptible varieties in insufficient amounts to inhibit insect feeding (Friedman and McDonald 1997; Sinden et al. 1980). Accessions of the wild, diploid species S. chacoense produce the potent glycoalkaloids leptines I/II that deter Colorado potato beetle feeding through a cholinesterase inhibiting and cell membrane disruption mechanism (Sanford et al. 1996; Sinden et al. 1980, 1986; Tingey 1984). Unlike α-chaconine and α-solanine, leptines are present only in aerial tissue and thus do not pose a hazard to human health (Mweetwa et al. 2012). While a dose-dependent, neuroreceptor specific to leptine I has been identified in Colorado potato beetle (Hollister et al. 2001), the minimum foliar leptine concentration required to reduce Colorado potato beetle feeding has yet to be established (Deahl et al. 1991; Rangarajan et al. 2000).
Leptines were first implicated in Colorado potato beetle resistance by Sinden et al. (1986) in the S. chacoense accession USDA8380-1 (80-1). Leptines I and II are acetylated forms of the ubiquitous glycoalkaloids α-chaconine and α-solanine, respectively, and are hypothesized to share a common precursor (Ronning et al. 1998). The leptinines I/II also present in 80-1 are proposed to be intermediates between α-chaconine/α-solanine and leptines I/II but do not exhibit strong Colorado potato beetle antifeedant properties (Lorenzen et al. 2001; Stürekow and Löw 1961; Yencho et al. 2000). Glycoalkaloids are composed of a cholesterol-derived skeleton, or aglycone, and a glycosidic group. Cholesterol, produced via the mevalonate pathway, is converted to solanidine which is then glycosylated in two separate reactions to produce α-chaconine and α-solanine (Kumar et al. 2017). It has been proposed that the aglycone solanidine is first modified to generate the leptinine aglycone leptinidine by a hydroxylation at C-23 (Lawson et al. 1993; Osman et al. 1987; Silhavy et al. 1996). Acetylation of the resulting hydroxyl group is proposed to yield the leptine aglycone acetyl-leptinidine (Lawson et al. 1993; Osman et al. 1987). Subsequent glycosylation of each aglycone would give rise to leptinines and leptines.
Several recessive genes are hypothesized to control the presence of leptines (Boluarte-Medina et al. 2002; Hutvágner et al. 2001; Manrique-Carpintero et al. 2014; Ronning et al. 1999; Sagredo et al. 2006). Loci associated with leptine synthesis were identified on potato chromosomes 1, 2, 7, and 8 (Manrique-Carpintero et al. 2014) and two complimentary epistatic loci associated with the synthesis of aglycones leptinidine and acetyl-leptinidine were mapped to chromosomes 2 and 8, respectively (Sagredo et al. 2006). Yet despite decades of research, the genetic underpinnings of leptine biosynthesis and accumulation remain elusive and introgression of this trait into cultivated material has not been achieved (Ginzberg et al. 2009; Grafius and Douches 2008; Manrique-Carpintero et al. 2014). Consequently, there are currently no Colorado potato beetle resistant cultivars with commercial acreage.
Investigation of S. chacoense derived Colorado potato beetle resistance at the diploid level using interspecific populations is difficult (Boluarte-Medina et al. 2002; Veilleux and Miller 1998). Interspecific hybridization may disrupt unique beneficial allelic combinations underlying this trait in S. chacoense and could affect recombination rates as reported by (Manrique-Carpintero et al. 2016). Moreover, diploid potatoes are largely self-incompatible due to a gametophytic system which precludes the generation of large F2 or recombinant inbred line mapping populations. In the S. chacoense diploid inbred line M6, however, the self-incompatibility system is inactivated by the dominant allele of the S-locus inhibitor gene Sli on chromosome 12 (Jansky et al. 2014). We propose that M6-mediated introduction of self-compatibility into Colorado potato beetle resistant germplasm provides an ideal system to study, understand and exploit this mechanism of host-plant insect resistance in potato. To that end, we employed combined bi-parental linkage mapping and whole-genome sequencing bulk segregant analysis in a diploid F2 S. chacoense population. Here we describe the identification of a major QTL region on the long arm of chromosome 2 explaining 49.3% and 34.1% of the variance in Colorado potato beetle field resistance and leptine accumulation, respectively. Minor QTL on chromosomes 4, 6, 7, and 12 associated with Colorado potato beetle field resistance are also discussed.
Materials and methods
Plant material
Twenty F1 hybrids were generated from a cross between the S. chacoense clone USDA8380-1 (PI 458310, 80-1) and the S. chacoense self-compatible inbred line M6 (Jansky et al. 2014). Clone 80-1 is largely homozygous, based on SNP genotyping described below, produces high levels of leptines, comprising 90% of total leaf glycoalkaloids (Sanford et al. 1996) and is resistant to Colorado potato beetle defoliation (Sanford et al. 1997) while M6 does not produce leptines and is susceptible to Colorado potato beetle defoliation (Crossley et al. 2018). A single F1 individual demonstrating robust self-compatibility, Colorado potato beetle resistance and high heterozygosity based on SNP marker genotype was selected for self-pollination to produce 700 diploid F2 seedlings. Of these, 325 individuals grew and developed and 305 were determined to be self-compatible. Self-compatibility was evaluated by a maximum of 50 self-pollinations of each F2 individual under greenhouse conditions for the purpose of creating a recombinant inbred line population for future genetic studies. A total of 233 F2 self-compatible individuals were randomly selected to constitute the mapping population. All plant material was maintained in tissue culture by nodal propagation in modified Murashige and Skoog media (MS salts at 8.8 g/L, 3% sucrose, pH 5.8 and 0.6% plant agar) at 25 °C under a 16-h photoperiod, permitting replicated experiments of these individuals in Michigan because the parental lines, F1 and F2 progeny do not readily tuberize under long day conditions of northern latitudes.
Developmental resistance profiling of parental lines
Host plant resistance to Colorado potato beetle larval feeding was assessed using a detached-leaf no-choice assay. No-choice assays are a tool to measure insect behavior such as feeding, oviposition, and larval survival to maturity when exposed to plant material from a single genotype. Newly hatched Colorado potato beetle larvae have limited mobility and thus their food source is largely dependent on adult maternal oviposition preference. No-choice neonate assays mimic this situation where the insect must feed or starve. Minimal, or a complete lack of, adult feeding in a no-choice setting also provides strong evidence that the genotype is not a suitable host. However, feeding on a sub-optimal host may be artificially inflated by virtue of the containment.
No-choice detached leaf assays of parental lines were conducted using Colorado potato beetle first instars. Eggs from a colony initially collected from Long Island, NY and maintained in culture for use in laboratory bioassays were purchased from French Agricultural Research Incorporated (Lamberton, MN). Eggs and newly hatched neonates were maintained on foliage of S. tuberosum commercial cultivar ‘Atlantic’ plants grown in greenhouse facilities at Michigan State University. Nine plants each of 80-1 and M6 were grown in the greenhouse from in vitro plantlets (N = 18) that had been repropagated on the same day. At 7 weeks post-transplant, the first six fully-expanded leaves were harvested from three randomly chosen plants of each line. Each leaf was placed separately in a 6.35 cm floral tube (Tezula Plants, FL) on filter paper in a 150 mm diameter Petri dish. Five neonates were placed on separate leaflets of each leaf using a paintbrush. Petri dishes were arranged on shelves under fluorescent lights (30 umol m-2 s-1) and 16-h photoperiod. Percent defoliation, larval mortality, and larval development was visually assessed at 5 timepoints: 1, 3, 5, 7, and 9 days after neonates were placed on the leaves. Percent defoliation was estimated visually as the percent of the total foliage consumed by the larvae from 0 to 100% in increments of 5%. For lines with minimal feeding between 0 and 4% estimates were as follows: 0.0% = no feeding, 0.5% = pinhole feeding that did not penetrate the leaf, 1–4% = one or more pinholes of 1 mm diameter that completely penetrated the leaf. Larval mortality was calculated as the number of dead larvae in each Petri dish at the time of observation divided by five and multiplied by 100%. The developmental instar of each living larva was determined according to estimated head width, body length and pronotum coloration. The no-choice assay was repeated at 12- and 15-week post-transplant using three previously unsampled plants for each genotype, using the same method. This resulted in 54 individual assays per line (6 leaf positions × 3 time points × 3 replicates) using a total of 540 larvae. Statistical analyses of differences in larval mortality and defoliation between lines and leaf age were accomplished using Tukey’s HSD (α = 0.05) in JMP® (Version Pro 13. SAS Institute Inc., Cary, NC).
Glycoalkaloid analysis
Sample preparation and extraction
Glycoalkaloid content is strongly influenced by environmental factors (Mondy and Gosselin 1988; Mondy et al. 1987; Mondy and Munshi 1990; Morris and Petermann 1985; Sinden et al. 1984; Sinden and Webb 1972; Slanina 1990; Van Gelder and Dellaert 1988). To mitigate environmentally induced variation, foliar glycoalkaloids were extracted from greenhouse grown plants. One plant each of the two parental lines (M6 and 80-1), their F1 hybrid, and the 233 F2 individuals (N = 236) were grown in the greenhouse under a 16-h photoperiod at 20 °C in 3.8 L pots. Since accumulation of the novel glycoalkaloids leptine I/II in leaves of the resistant parental line, S. chacoense 80-1, increases with maturity (Mweetwa et al. 2012) foliar tissue samples were taken 15-wks post-germination for analysis to ensure glycoalkaloid detection. Five leaflets from the fourth fully-expanded leaf were pooled for each genotype in a 15 mL Corning tube, flash frozen and stored at − 80 °C prior to lyophilization for 72 h. The freeze-dried tissue was ground, and 50 mg of powder was extracted in 1 mL of solution (49% HPLC grade methanol, 49% sterile water, 1% glacial acetic acid, 0.1% formic acid). The samples were briefly vortexed and incubated at 60 °C for 30 min before centrifugation for one minute at 14,000 rpm. The supernatant was filtered through a 0.22 um Corning® Costar® Spin-X® centrifuge tube and diluted 1:100 in extraction solution containing internal standard Telmisartan at a final concentration of 0.5 uM.
Glycoalkaloid quantification
Glycoalkaloids were analyzed using Waters Acquity (Waters Corporation, MA, USA) high performance liquid chromatography (Quattro Micro) coupled with tandem mass spectrometry (HPLC–MS/MS). Compounds were separated on a C-18 reverse-phase column. Glycoalkaloids were eluted in a binary gradient system composed of Solvent A (LC–MS grade water, 0.1% formic acid) and Solvent B (LC–MS grade acetonitrile) at a flow rate of 0.3 mL/min at 25 °C. The following stepwise gradient was implemented: 90% A, 10% B; 2:00 min, 40% A, 60% B; 2:01, 0% A, 100% B; 3:01, 90% A, 10% B. Each sample was injected at a volume of 10 uL in triplicate (N = 678). The mass spectrometer was operated in positive ion mode. Mass spectroscopy data were acquired by the Waters MassLynx software and processed using Waters Quanlynx MS Software. Molar concentrations were determined using standard curves of purified α-solanine and α-chaconine (Sigma-Aldrich) in a range from 0.01 to 40.0 uM. The response factors for α-chaconine and α-solanine were used for leptine I and leptine II, respectively.
Field trial Colorado potato beetle phenotyping
Field trials were conducted in 2017 and 2018 at the Michigan State University Montcalm Research Center (Lakeview, MI) in a field planted annually with untreated, susceptible potato plants and naturally infested with overwintering Colorado potato beetles for at least four decades (Coombs et al. 2003). The beetle population on this research farm has a history of resistance to commonly-used potato insecticides (Ioannidis et al. 1991, 1992; Szendrei 2014). To provide food for emerging beetles prior to transplanting research lines, tuber seed pieces of the commercial cultivar ‘Atlantic’ was planted around, and in alternate rows of, the study area on 19 April 2017 and 25 April 2018. These untreated, susceptible plants also serve to maintain high beetle densities uniformly throughout the field. Adult beetles emerged from the soil within the field the weeks of 21 May 2017 and 28 May 2018. The trial area and borders were fertilized and irrigated according to best management practices, but no insecticides were applied to the field. Damage by other potato insect pests, such as potato leafhopper, were not observed at this field location in 2017 or 2018.
In 2017, nine in vitro plantlets of each research line (2 parental lines, their F1 hybrid, and 151 F2 individuals) and susceptible check ‘Atlantic’ were transplanted to trays in the greenhouse, grown for 6 weeks (16-h photoperiod, 20 °C) and transplanted in the field on 2 June 2017 (N = 1395). At this time, all transplants were intentionally the approximate same age and maturity of the ‘Atlantic’ spreader rows. A randomized complete block design consisting of three replications of three plants was used. Percent defoliation of each three-plant plot was assessed visually each week, beginning on 7 June 2017 and continuing for a total of 7 weeks, at which point the ‘Atlantic’ check was completely defoliated and beetles left the plots. Over the 7 weeks, defoliation was caused by overwintered adults, first-generation larvae, and second-generation adults and larvae.
Defoliation data were used to calculate the area under the defoliation curve (AUDC), comparable to the area under the disease progression curve (Shaner and Finney 1977). To determine the relative AUDC (RAUDC) for each plot over the seven-week observational period, the AUDC for each plot was divided by the maximum defoliation observation for that plot (e.g. 4900 if 100% of the plot was defoliated by the 49th day of the trial). Data were analyzed in JMP® (Version Pro 13. SAS Institute Inc., Cary, NC). Levene’s test revealed unequal variances (P < 0.0001) and accordingly non-parametric Kruskal–Wallis tests were used for pairwise comparison (α = 0.05). Phenotypic extremes (the ten-most susceptible and ten-most resistant F2 individuals) were selected for field evaluation the following year.
In 2018, five replications of five plants each of the parental lines, the F1 hybrid, and the 20 phenotypic extremes from the F2 generation were transplanted to the field on 11 June. ‘Atlantic’ was again included as a susceptible check and ‘Atlantic NewLeaf,’ a deregulated genetically modified clone expressing the Bacillus thuringiensis Cry3A protein, was used as a resistant check (N = 625). Two complete beetle generations were observed during the field season. Beetle pressure in 2018 was observed to be less than in 2017. Beginning on 11 June 2018, defoliation data were collected weekly for 5 weeks, after which beetles were no longer feeding. This shorter experimental duration period was determined by the beetle development pattern and environmental conditions in this year. The RAUDC was calculated for each plot, using a similar method as in 2017.
To account for the non-normality of the data, we used Spearman’s rank correlation coefficients in JMP® (Version Pro 13. SAS Institute Inc., Cary, NC) to test the relationship between measured traits for both years. The mean of technical replicates was used to calculate correlations coefficients for compound concentrations (mg/g DW) of individual measured glycoalkaloids, total measured glycoalkaloids and the ratio of acetylated glycoalkaloids to nonacetylated glycoalkaloids [mean total leptines (mg/g DW)]/[mean α-chaconine (mg/g DW) + mean α-solanine (mg/g DW)]. Means of biological replicates (plots) were used to calculate correlation coefficients for field defoliation (RAUDCx100) for each individual in both years.
SNP genotyping and linkage analysis
Genomic DNA was extracted from freeze-dried leaf tissue of the two parental lines, their F1 hybrid, and the 233 F2 individuals following the Mag-Bind® Plant DNA Plus 96 Kit protocol (Omega Bio-tek, Norcross, GA). SNP genotyping was performed using the Illumina Infinium Potato 22K V3 Array, including a genome wide marker selection from different sources, (Felcher et al. 2012; Hamilton et al. 2011; Vos et al. 2015) at Michigan State University. Filtering removed SNPs that were monomorphic for all individuals, SNPs with > 10% progeny missing genotype, and SNPs with missing parental genotypes. This resulted in 754 segregating markers distributed across the 12 chromosomes. Joinmap® 5.1 (Van Ooijen 2006) was used to create the 12 linkage groups using the F2 population type. The physical position of mapped SNPs from the Illumina V3 Array on the potato doubled monoploid S. tuberosum clone DM1-3 (DM) pseudomolecules (PGSC Version 4.03) was used to compare genetic and physical maps. The physical length of each chromosome was calculated by subtracting the first megabase (Mb) position of mapped loci on each chromosome from the last position. Total physical map length was the sum of the physical map lengths for each of the 12 chromosomes. Map coverage for each chromosome was reported as the total distance in Mb covered by SNP positions divided by the total length of each DM Version 4.03 assembled chromosome. Total map coverage was reported as the total distance (Mb) covered by all 12 chromosomes divided by the total distance of all 12 DM Version 4.03 assembled chromosomes. Average distances between loci mapped in each chromosome was calculated by summing all the individual interlocus intervals in cM and divided by the total number of intervals, and the average from chromosome average intervals for the overall genome. Concordance between the reference DM genome (PGSC Version 4.03) and the linkage map was tested by creating Marey maps comparing the genetic position (cM) to the physical position (Mb) of each SNP in JMP® (Version Pro 13. SAS Institute Inc., Cary, NC). Deviation from the expected 1:2:1 (homozygous:heterozygous:homozygous) genotypic class frequencies was calculated by Chi squared tests of each SNP at three significance levels (P < 0.05, P < 0.01, P < 0.001). The significance of distorted segregation was reported as the minus logarithm of the Chi square test p value.
QTL analysis was performed in MapQTL® 6 software (Van Ooijen and Kyazma 2009) using interval mapping to first identify major QTL for Colorado potato beetle defoliation (mean RAUDC x 100), accumulation of leptine I, leptine II, total leptines, α-solanine, α-chaconine, and total glycoalkaloids as well as the ratio of acetylated to non-acetylated glycoalkaloids. A 1000 × permutation test was run for each trait to establish the LOD threshold corresponding to a genome wide false positive rate of 5%. Markers with LOD scores exceeding this threshold were used as cofactors in multiple-QTL-mapping (MQM). Final reported LOD scores were determined by MQM and MapChart (v 2.32) (Voorrips 2002) was used to visualize the results. To identify QTL associated with leptine synthesis, leptine accumulation data were converted to presence/absence and coded 1/0. QTL were detected using the nonparametric Kruskal–Wallis test and significance threshold of P = 0.0001 in MapQTL® 6 software (Van Ooijen and Kyazma 2009).
Phenotypic validation of Colorado potato beetle resistance extremes in vitro
Detached-leaf choice bioassays with adults
Host plant resistance to Colorado potato beetle adult feeding was assessed using a detached-leaf choice assay. Choice assays, where insects are confined with multiple host genotypes, offered insight into Colorado potato beetle host preferences under field conditions. Assays on the 20 phenotypic extremes (the ten-most susceptible and ten-most resistant) of the F2 generation (Table S1) were conducted using adult beetles collected from the Montcalm Research Center and undamaged leaves collected from plants in the 2018 field trial. An undamaged, fourth fully-expanded leaf was selected from three plants of each line 4 weeks after transplanting to the field. Leaves were placed in a 2.35 cm floral tube (Tezula Plants, FL) filled with distilled water. Each choice arena consisted of a 25.4 × 30.5 cm disposable foil cake pan with a fitted plastic lid (Gordon Food Service, Grand Rapids, MI), with a piece of moist cheese cloth placed in the bottom. Each arena was large enough to hold ten leaves. The single leaves from five resistant and five susceptible lines were randomly selected to be tested in each arena; this resulted in a total of six arenas (pans) to test each of the 20 lines in triplicate. Ten adult beetles were placed on the cheesecloth in the center of each arena. Pans were held at room temperature under fluorescent lights (30 umol m−2 s−1) on a 16-h photoperiod. After 48 h, defoliation was visually scored as the percent of each leaf consumed.
Detached leaf no-choice bioassays with larvae
Three plants of the parental lines and the 20 resistance extremes were also grown in the greenhouse in 2018 from in vitro plantlets (N = 66). No-choice first instar detached leaf assays were conducted using the fourth fully-expanded leaf of each plant according to methodology described for developmental profiling of parental lines at 12-wks post-transplant. Non-parametric Kruskal–Wallis tests were used for pairwise comparison of defoliation among lines (α = 0.05) in JMP® (Version Pro 13. SAS Institute Inc., Cary, NC) in both choice and no-choice bioassays. Spearman’s rank correlation coefficients (ρ) were used to compare both bioassay phenotypes to the beetle resistance phenotypes observed in the two field seasons and measured glycoalkaloids for these 20 F2 individuals.
Glycoalkaloid profiling
At the same time the no-choice bioassays were conducted, glycoalkaloids were extracted from a pooled sample of the terminal leaflet from three biological replicates of the third fully-expanded leaf for each genotype (N = 22) and quantified as described above.
Whole genome sequencing bulk segregant analysis
Sample preparation and sequencing
Tissue was harvested by hole punching a young, terminal leaf of the two parental lines and the 20 phenotypic extremes of the F2 generation (Table S1). A single hole punch from the ten susceptible and ten resistant individuals were bulked to form a susceptible sample and a resistant sample prior to DNA extraction using the Qiagen DNAeasy Plant Mini Kit. Illumina libraries were prepared using the TruSeq DNA sample prep kit and sequenced in paired-end mode (150 nt) on the Illumina Hiseq platform to 30 × depth for parental lines and 10 × depth for resistant and susceptible bulks.
Read alignment and variant calling
Raw reads were processed with Trimmomatic (v0.35) (MINLEN = 100, LEADING = 3, TRAILING = 3, SLIDINGWINDOW = 4,20) to remove adapters, primers and low-quality bases (Bolger et al. 2014). As currently only 508 Mb of the 826 Mb S. chacoense M6 assembly (Leisner et al. 2018) is anchored to the 12 chromosomes, we aligned trimmed reads from bulk samples to an alternate M6 assembly built from the more complete Solanum tuberosum DM pseudomolecules (PGSC Version 4.03) (henceforth referred to as the M6-corrected DM assembly). First, cleaned M6 reads were aligned to the DM genome using BWA-mem (v0.7.12.r1044) (Li 2013) in paired-end mode. The resulting alignments were sorted, depurated from duplicates and indexed using Picard tools (v1.113) (broadinstitute.github.io/picard) and indel realignment performed with the Genome Analysis Toolkit (GATK, v3.5.0) (DePristo et al. 2011). SNPs and indels were subsequently called using GATK HaplotypeCaller, employing a standard minimum confidence threshold for calling of 20.0. Called variants were subject to hard filtering using the GATK VariantFiltration tool with the following parameters: quality by depth < 10.0, mapping quality < 40, strand bias estimated by Fisher’s exact test > 60.0, Haplotype Score > 13.0, mapping quality rank sum test less than − 12.5, read position rank sum test less than − 8.0. Using the FastaAlternateReferenceMaker utility in GATK, bases in the DM assembly were replaced at variation sites with bases supplied by this SNP set. Alignments of resistant and susceptible bulk reads to this M6-corrected DM assembly were then processed, and variants called and filtered as described for M6, with the exception that SNPs and indels were called jointly in all samples using GATK HaplotypeCaller and GATK GenotypeGVCF.
Bulk-segregant analysis
A total of 11,070,484 SNPs resulting from alignment of bulk samples to the M6-corrected DM assembly were imported into R (v1.1.423) and further filtered by reference allele frequency to remove 5,000,179 SNPs over or under-represented in both bulks, SNPs with read depth discrepancies > 100 between bulks, and SNPs with total depth < 10 and > 200. We independently assessed the differences in allele frequency between the resistant and susceptible bulks using the G’ statistic method proposed by Magwene et al. (2011), which accounts for linkage disequilibrium and minimizes SNP calling error noise, within the R package QTL-seqr (Mansfeld and Grumet 2018). Briefly, SNPs were counted in a one Mb sliding window and a tri-cubed ΔSNP-index calculated within each window. A SNP-index is the proportion of reads with a SNP that differs from the sequence of the susceptible reference genome. A SNP-index value of 0 indicates that all read sequences in the bulk contain the reference allele. Conversely, if all reads contain the non-reference allele the SNP-index = 1. The ΔSNP-index is the difference in SNP-index between resistant and susceptible pools and is expected to be 0 in regions not associated with the trait of interest. Both p-values and genome-wide Benjamini–Hochberg FDR (Benjamini and Hochberg 1995) adjusted p-values are calculated for each SNP. We used an absolute ΔSNP-index value of 0.1 to filter outlier regions and an FDR of 0.01 to identify significant QTL associated with Colorado potato beetle resistance.
Gene expression of Colorado potato beetle resistance extremes
Sample preparation and RNA sequencing
Three strongly resistant and three susceptible F2 individuals were selected from the 20 characterized phenotypic extremes for expression profiling. The resistant individuals selected contained leptines and the susceptible individuals did not contain leptines (Table S1). Three in vitro plants of each F2 individual and the two parental lines were grown in a growth chamber (20 °C, 16-h photoperiod, 70% relative humidity) (N = 24). RNA was isolated from leaf tissue at 16-week post-transplant using the Qiagen RNeasy Plant Mini Kit. Samples were Turbo DNase (Thermo Fisher Scientific, Waltham, MA, USA) treated, and RNA concentration and quality were measured using Qubit 2.0 Fluorometer (Life Technologies, Inc., Carlsbad, CA, USA) and the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). All samples had a minimum RNA integrity number (RIN) score of 8. For each genotype, three biological replicates were prepared from equal quantities of leaflets from the fourth fully-expanded leaf. Libraries were prepared using the Illumina TruSeq Stranded mRNA Library Preparation Kit and approximately 30,000,000 50 nt single-end Illumina reads were generated for each sample on the Illumina HiSeq 4000 platform. Raw reads were processed with Trimmomatic (v0.35) (MINLEN = 36, LEADING = 20, TRAILING = 20) to remove low-quality bases, adapters, and primers (Bolger et al. 2014).
RNAseq read-mapping and differential expression analysis
Cleaned reads were aligned to the DM genome (PGSC Version 4.03) using STAR (v2.6.0) (Dobin et al. 2013). Reads aligning to annotated DM reference genes were counted using subread (v1.6.2) package featureCounts in reverse stranded mode (Liao et al. 2013). Counts were then analyzed using the R package DESeq 2 to determine normalized expression values (Love et al. 2014). Analyzed genes were required to have counts greater than 10 in at least 3 individuals. Spearman’s rank correlation coefficients (ρ) were calculated between individual candidate transcripts and measured traits.
Weighted gene co-expression network analysis
The weighted gene co-expression network analysis (WGCNA) package was used to conduct co-expression analysis in R (Langfelder and Horvath 2008), with a soft-thresholding power of 22 (Fig. S1). The varianceStabilizingTransformation function was used to transform counts for the 21,996 genes passing the count threshold (> 10 in at least 3 individuals). Modules within the signed network were identified in a single block using the BlockwiseConsensusModule function with a minimum module size of 30 and medium sensitivity (deepSplit value of 2). Module eigengenes, the first principal component of the expression matrix in the module, were correlated with defoliation (RAUDC), total leptine accumulation (mg/g DW) and the ratio of acetylated to nonacetylated glycoalkaloids measured. Average gene significance was plotted for each module and trait. Hub genes within interesting modules were required to have absolute module membership values (kME) greater than 0.9, indicative of strong intra-module connectivity, and an absolute gene significance for the trait of interest greater than 0.2. Networks of selected modules were visualized separately in Cytoscape 3.7 (Kohl et al. 2011), in the Attribute Circle Layout with log2FoldChange as the selected attribute.
Data availability
Raw whole genome sequence data is available at NCBI SRA (accession # SRR10197400; SRR10197399) and raw expression and count data is available at NCBI GEO (accession # GSE138184).
Molecular marker development and fine mapping of QTL region
Design of PCR indel markers
The .bam file containing SNPs and indels identified between 80-1 and M6 from whole genome sequence data were visualized using the Integrative Genome Viewer (IGV, v.2.4.9). Candidate indel markers flanking and within the QTL region were required to be homozygous in both parents and greater than 15 bp in length to permit adequate size separation on an electrophoresis gel. Primers were designed in house using Primer3 (Rozen and Skaletsky 2000) and validated using genomic DNA extracted from young leaf tissue of 80-1, M6 and the F1 hybrid plants. All reactions were performed in a 15 ul volume using 7.5 ul of GoTaq Green Master Mix (Promega, Madison, WI), 0.5 ul of each primer, 2 ul of DNA and 4 ul of distilled water following manufacturer protocols. Cycling conditions included an initial denaturing step of 3 min at 94 °C followed by 34 cycles of 30 s denaturing 94 °C, 30 s annealing 50 °C, 1 min elongation 72 °C and a final elongation step of 5 min at 72 °C. PCR products were run on a 2.5% agarose gel stained with SYBR Safe (Thermo Fisher Scientific, Waltham, MA, USA) at 120 V for 45 min.
Screening additional F2 progeny to identify recombinants
Two validated PCR primers within the QTL region (Table S2) were then tested on an additional 406 F2 individuals generated from the F1 hybrid and grown in greenhouse conditions under a 16-h photoperiod at 20 °C. The F2 individuals with recombination in the QTL region (n = 96), as well as the two parental lines and their F1 hybrid, were replicated by stem cuttings in the greenhouse to produce nine biological replicate plants (N = 891). Rooted stem cuttings were planted as transplants in the Colorado potato beetle nursery at the Montcalm Research Center on 21 June 2019 in a randomized complete block design of three replicates of three plants. Percent defoliation of each three-plant plot was assessed visually each week, for a total of 5 weeks and the RAUDC calculated as described previously.
Fine mapping of candidate QTL region on chromosome 2
Eleven validated PCR markers (Table S2) within the candidate QTL region were then used to genotype the 96 F2 individuals phenotyped for Colorado potato beetle resistance in the field. Linkage map construction was achieved with Joinmap® 5.1 (Van Ooijen 2006) using the F2 population type. Permutation testing to establish a LOD threshold and MQM mapping were conducted in MapQTL® 6 (Van Ooijen and Kyazma 2009) as described above to confirm the association of PCR markers with the Colorado potato beetle field resistance phenotype.
Results
S. chacoense Colorado potato beetle resistance is tissue and age-dependent
Younger leaves (1–2) of both S. chacoense parental lines 80-1 and M6 were more defoliated by Colorado potato beetle larvae than older leaves (5–6) throughout plant development (α = 0.05) (Fig. 1). In addition to leaf age, plant age was observed to impact defoliation resistance. Defoliation of 80-1 leaves (1–5) was least at 7 weeks post-transplant and increased in the subsequent sample time points (Fig. 1). This timing has practical significance because Colorado potato beetles typically emerge before or with potato plants. However, the larval mortality was higher on old leaves (3–6) than on young leaves (1–2) of young 80-1 plants (7 weeks post-transplant) (P = 0.0036).
Phenotypic evaluation of the S. chacoense F2 population
Leptine I/II was detected in 162 F2 progeny, ranging from 0.1–25.9 and 0.1–41.6 mg/g DW, respectively (Table 1). Presence of leptines in this population was determined to be not significantly different from a 3:1 ratio by Chi squared test (χ2 = 0.053). Leptine levels exceeded 8.6 mg/g DW, the concentration previously reported to reduce larval feeding (Sanford et al. 1997), in 130 F2 individuals; 40 individuals had greater leptine content than parent 80-1. All F2 progeny contained α-chaconine and α-solanine. The average foliar concentration of α-chaconine (21.8 mg/g DW) and α-solanine (28.5 mg/g DW) was higher than leptines (14.9 mg/g DW) (Table 1). In individuals containing leptines, leptine concentration was weakly and inversely correlated with α-chaconine levels, but not correlated with α-solanine (Table 2).
In field trials under natural beetle pressure, the susceptible parent M6 and cv. Atlantic were completely defoliated. In contrast, 80-1 and the F1 hybrid exhibited minimal feeding (RAUDC < 2.9). Field defoliation among F2 lines was continuously distributed, with RAUDC ranging from 0.0 (no damage) to 54.3 (Fig. 2). Increased leptine I, II and total leptine concentration were moderately correlated to decreased field defoliation while α-chaconine and α-solanine content were moderately correlated with increased field defoliation (Table 2). The ratio of acetylated compounds to nonacetylated compounds was the best predictor of field defoliation (Table 2.)
Linkage map construction
To identify QTL underlying this observed Colorado potato beetle resistance, we SNP genotyped the mapping population of 233 F2 individuals to generate a linkage map with 12 chromosomes covering 97% of the 12 assembled S. tuberosum clone DM1-3 (DM) pseudomolecules (PGSC Version 4.03) (Table 3, Table S3). The resultant map spanned a genetic distance of 1193.8 cM with an average of 63 SNP markers per chromosome distributed at an average distance of 1.6 cM between SNPs (Table 3). Comparison of the genetic location and physical position on the DM pseudomolecules of SNPs used for mapping showed good concordance (Fig. S2).
The percentage of distorted segregation in the F2 progeny was assessed at three levels of significance. Distorted segregation at the 5% and 1% conservative significance levels was detected on 56% and 40% of all mapped loci. At the 0.1% significance level, corresponding to highly distorted loci, 26% of all mapped loci exhibited distorted segregation and were located on all chromosomes except 4, 5 and 10 (Table S4). The chromosome with the greatest number of loci with distorted segregation was chromosome 12 with 90% of distorted loci at the 0.1% significance level. Plotting the maternal, paternal and heterozygous genotypic frequency at each SNP position along the 12 chromosomes showed preferential inheritance of M6 alleles on chromosomes 1, 3 and 12 (Fig. S3). Compared to other chromosomes, chromosome 8 exhibited a high preferential inheritance of heterozygous genotypes (Fig. S3).
Identification of QTL associated with Colorado potato beetle field resistance and leptine accumulation
Two QTLs were detected for Colorado potato beetle field defoliation resistance by MQM analysis of the F2 mapping population. A major QTL on chromosome 2 with partial dominance effect (the 80-1 allele contributing to lower RAUDC) explained 49.3% of the variance (Fig. 3, S4, Table 4). The 1.5 LOD interval of this QTL region is delimited by SNP markers solcap_snp_c2_4521 and PotVar0039036 which corresponds to positions 7,676,939 and 22,151,711 bp on the DM genome (PGSC 2011; Sharma et al. 2013) (Fig. 3). A second, minor QTL located on chromosome 7 explained 6.2% of phenotypic variation with overdominance effect from M6 that contributed to decreased Colorado potato beetle defoliation (Fig. S5, Table 4). Chromosome 2 also contained a major QTL with partial dominance effects that explained between 29.1 and 34.3% of variation, with 80-1 contributing to higher accumulation values for leptine I, leptine II, and total leptine while decreasing α-chaconine (Fig. 3, S4, Table 4). Loci significantly associated with leptine synthesis also colocalized to chromosome 2 and peaked at 4.1–4.5 cM which corresponds to 22.1–22.4 Mb (Kruskal–Wallis test P value < 0.0001). An overlapping minor QTL with partial dominance effects was also present on chromosome 2 and explained 38.2% variation in acetylated to non-acetylated glycoalkaloids (Fig. 3, S5, Table 4). Significant QTL were not detected for solanine or total glycoalkaloids.
The QTL regions on chromosome 2 associated with Colorado potato beetle resistance under field conditions and foliar concentration of glycoalkaloids identified by bi-parental linkage mapping. QTL are represented by solid bars (1-LOD interval) and extended lines (2-LOD interval) in unique colors for each trait. The genetic positions (cM) are shown on the left and the corresponding physical position of mapped SNPs on the PGSC Version 4.03 Doubled Monoploid pseudomolecule 2 (bp) are shown on the right of the linkage map. The significance (LOD) of SNP association to each trait is plotted against these SNP positions. Figure prepared with MapChart 2.3. (R.E. Voorrips. Plant Research International, Wageningen, The Netherlands)
Validation of the F2 Colorado potato beetle resistance phenotypic extremes
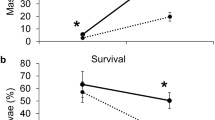
We validated the Colorado potato beetle resistance phenotype in 20 F2 individuals exhibiting high resistance or susceptibility in a second field trial as well as choice and non-choice detached leaf assays. There was high positive correlation between the phenotypes evaluated in each of these experiments (Table S5). The ten putatively resistant F2 lines demonstrated significantly less defoliation (mean RAUDC = 1.3) over the five-week field observational period than the ten putatively susceptible F2 individuals (mean RAUDC = 51.3), the susceptible parent M6 (mean RAUDC = 60.3) and susceptible check cultivar ‘Atlantic’ (mean RAUDC = 84.8) (P = 0.0002, α = 0.05) (Fig. 4a). The ten field susceptible F2 lines were also significantly more defoliated than resistant parent 80-1 and resistant F2 lines in both no-choice larval detached leaf assays (P = 0.0003, α = 0.05) and choice adult detached assays (P = 0.0002, α = 0.05). Percent larval mortality recorded after 9 days in detached leaf conditions was much more variable than percent defoliation between resistant and susceptible lines. Larval development was impeded on resistant lines evidenced by significantly more larvae progressing to third instar on susceptible lines (mean number of third instar larvae = 3.7) than resistant lines (mean number of third instar larvae = 2.7) (P = 0.0092). Furthermore, resistant line foliar mean concentration of leptine I (mean mg/g DW = 5.8, P = 0.001), leptine II (mean mg/g DW = 8.2, P = 0.0045), α-chaconine (mean mg/g DW = 17.4, P = 0.0073) but not α-solanine (mean mg/g DW = 32.6) differed significantly from susceptible lines (Fig. 4b). Resistant lines also had a significantly higher ratio of acetylated/non-acetylated glycoalkaloids (mean ratio = 0.031) than susceptible lines (mean ratio = 0.05) (P = 0.0017).
Colorado potato beetle resistance phenotypic validation of the F2 phenotypic extremes. a Percent defoliation by Colorado potato beetle under field conditions of Solanum chacoense parental lines 80-1 and M6 and F2 phenotypic extremes selected from the previous field season. Data represent means of five biological replicates for each parent and for each of the ten F2 individuals in the resistant bulk and ten F2 individuals in the susceptible bulk. b Foliar leaf concentration of leptines I/II, alpha-solanine and alpha-chaconine in the ten resistant F2 individuals and ten susceptible F2 individuals evaluated in the field
Detection of QTL associated with Colorado potato beetle resistance by BSA-seq
Whole genome sequencing parental lines and bulked resistant and susceptible F2 samples yielded 353,005,026 total reads to provide ~ 29x depth of coverage. G’ analysis identified QTL associated with Colorado potato beetle resistance on five chromosomes. The most significant QTL region contains two peaks between 7.2 and 31.7 Mb on chromosome 2 and is donated by resistant parent 80-1 (Fig. S6, Table S6). In addition, a QTL contributed by susceptible parent M6 on chromosomes 7 spanning 0.1–2.2 Mb was detected (Fig. S6, S7). Both of these intervals correspond to the physical positions of the QTL identified by biparental linkage mapping. However, QTL contributed by resistant parent 80-1 not identified by biparental mapping were also mapped to chromosomes 4, 6 and 12 using the BSA-seq approach (Fig. S7, Table S6). Two peaks between 60.7 and 62.3 Mb and 63.5–68.7 Mb reside on chromosome 4 and a single peak was located on chromosome 6 between 54.6 and 59.5 Mb (Fig. S6, Table S6). Chromosome 12 also contains two peaks on opposite chromosomal arms (Fig. S7, Table S6). The glycoalkaloid metabolism genes (GAME4, GAME12) located on chromosome 12, but not the reported glycoalkaloid genes on chromosome 7 (GAME11, GAME6, SGT1, and SGT3), fall within these minor QTL (Table S6) (Itkin et al. 2013).
Fine mapping of candidate QTL on chromosome 2
In an expanded set of 406 additional F2 progeny, we identified 96 recombinant individuals between custom PCR indel markers ch02_5451852 and ch02_29095760 spanning the candidate QTL region on chromosome 2. Linkage map construction with nine additional PCR indel markers within the QTL region and MQM mapping identified a QTL peak at marker ch02_21096852 with a dominant effect decreasing defoliation (6.76) explaining 27.5% of phenotypic variation (Fig. S8). This fine mapping narrowed the QTL region interval to a region of 7.3 Mb.
Identification of candidate genes within the QTL region on chromosome 2
A total of 351 genes, were predicted within the QTL region (15.1–22.4 Mb) on chromosome 2 identified by BSA-seq and biparental linkage mapping (PGSC 2011; Sharma et al. 2013). We investigated differential gene expression of the parental lines, three resistant and three susceptible F2 individuals to narrow this slate of candidate genes. After filtering, a total of 21,996 genes were used for expression analysis. Analysis of the mapped reads showed that 923 transcripts are differentially expressed between resistant and susceptible plants at 16-wks post-transplant and distributed across the 12 chromosomes (Table S7). A principal component analysis reveals a clear pattern of expression differentiating resistant and susceptible plants (Fig. S9). Of these, 360 transcripts are down-regulated while 563 are upregulated in the resistant F2 lines and parent 80-1 (Fig. S10). Chromosome 2 is enriched for downregulated transcripts which are distributed toward the distal end of the long arm (Fig. S10, 11). Four of the five upregulated transcripts and two of the five downregulated transcripts with the highest magnitude of log2foldchange are located on chromosome 2 (Table S7).
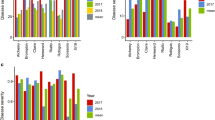
Differentially expressed genes were then grouped into 29 color-coded modules with similar patterns of expression by hierarchical average linkage clustering for network analysis (Fig. S13a). The size of modules ranged from 45 genes (skyblue module) to 8499 genes (grey module) (Table S8). We identified modules with eigengenes correlated to Colorado potato beetle defoliation, total leptine accumulation and the ratio of acetylated to non-acetylated compounds (Fig. 5). Defoliation was strongly positively (0.94, P = 8.22e−12) and leptine accumulation negatively correlated (− 0.77, P = 1.19e−05) to the midnightblue module, respectively. The darkgrey module was strongly negatively correlation with beetle resistance (− 0.74 P = 3.0e−05) and moderately correlated with gene significance for total leptines (0.53, P = 4e−06). The lightcyan module was also strongly positively correlated with leptine accumulation (0.66, P = 5.0e−04). For the ratio of acetylated to non-acetylated compounds, two related modules grey60 and red were the most highly correlated with this trait (0.91, P = 5.2e−10; 0.83, P = 6.4e−7) (Fig. 5, S13b).
Weighted gene co-expression network module associations with three traits: defoliation (RAUDC), total leptines (mg/g DW) and Ratio (the ratio of acetylated compounds to non-acetylated compounds accumulated). Each row corresponds to a module eigengene and each column to a trait. Each cell contains the Pearson correlation value and corresponding Student t test P value. The color legend indicates the scale for color-coding of the module-trait correlation values
We also examined individual gene significance within these interesting modules for the traits leptine accumulation, Colorado potato beetle defoliation resistance and the glycoalkaloid ratio. Gene significance for leptine accumulation and Colorado potato beetle defoliation were correlated with module membership in the midnightblue (0.39, P = 3.8e−09; 0.79, P = 1.0e−46) and darkgrey (0.53, P = 4.0e−06; 0.65, P = 2.6e−09) modules. Gene significance for glycoalkaloids ratio was strongly correlated with module membership in the red and grey60 modules (0.68, P = 1.5e−61; 0.68, P = 5.8e−22).
We then focused on the core hub genes of these modules, identifying 55 genes in the red module, 9 in the darkgrey module, 23 in the grey60 module and 27 in the midnightblue module (Table S9). To better understand the connectivity between hub genes within modules, the chromosome location, proximity to a QTL region, co-expression relationships, and metabolism associated were analyzed. Genes in the midnightblue module were located on chromosomes 00, 1, 2, 3, 4, 8 and 12, and all the most strongly upregulated genes in resistant lines resided on chromosome 2 (Table S9, Fig. 6). Interestingly an upregulated gene on chromosome 12 (PGSC0003DMG400004286) within our minor QTL annotated as a regulatory subunit Tap46 was found to be highly connected to six upregulated genes within our major QTL on chromosome 2 (PGSC0003DMG400015505, PGSC0003DMG400013094, PGSC0003DMG400042914, PGSC0003DMG400012650, PGSC0003DMG400017873, and PGSC0003DMG400004521) (Fig. 6). One of these genes (PGSC0003DMG400017873) is annotated as a tetratricopeptide repeat 5 oligo-binding fold domain containing protein spanning chromosome 2 positions 18,356,198 to 18,362,279. Tetratricopeptide repeats function as protein–protein interaction motifs (Blatch and Lässle 1999) and have recently been implicated in hormone signaling in plants (Schapire et al. 2006). Notably, counts of transcripts aligning to this gene were only detected in counts < 2.5 in susceptible lines and based on publicly available expression data, this transcript is expressed not at all in Solanum tuberosum DM tubers and at levels > 0.5 FPKM in other tissues (Hirsch et al. 2014).
Interaction of hub genes in the midnight blue module visualized using Cytoscape 3.7 software. The node colors are coded from white to dark blue to indicate differential gene expression. The thickness of connective lines between nodes is representative of connection weight between two nodes. Node labels provide PGSC V4.03 chromosome number (ch) followed by the PGSC numeric gene identifier
Another of these coordinately up-regulated genes (PGSC0003DMG400015505) on chromosome 2 is annotated as an anthranilate N-benzoyltransferase protein in SpudDB (Hirsch et al. 2014) and shares 100% identity with a S. tuberosum predicted uncharacterized acetyltransferase protein, At3g50280-like (LOC102606194), which is significantly and substantially up-regulated in resistant lines (Fig. 6). The 1.56 kb genic sequence spans positions 21,003,553 to 21,005,147 on chromosome 2. Expression of this gene is positively correlated with foliar leptine I (ρ = 0.7306, P < 0.0396), leptine II (ρ = 0.7319, P < 0.0396), and the ratio of acetylated/non-acetylated glycoalkaloids (ρ = 0.8571, P < 0.0065). Moreover, this gene is also not expressed in susceptible lines (Table S7) or Solanum tuberosum DM tissues (Hirsch et al. 2014). This subnetwork also contained genes involved in biotic stress response (Table S9).
The hub genes for the darkgrey module, correlated with defoliation and leptine accumulation, were located on chromosomes 3, 6, 7, 8 and 9 and were all up-regulated in resistant lines (Table S9, Fig. S14). This module contained genes associated with plant defense (Table S9). The red and grey60 modules associated with the ratio of acetylated to non-acetylated glycoalkaloids contained highly interconnected nodes (Fig. S15, S16). The red module was enriched for genes without annotated function and there were no intuitive candidate genes within the hub genes of these modules (Table S9).
We then searched for differential expression of known genes involved in the synthesis of glycoalkaloid precursors and genes previously associated with leptine accumulation. We identified a glycosyltransferase (PGSC0003DMG402004500) within the chromosome 2 QTL region downregulated in resistant lines and negatively correlated with leptine I/II content (ρ = − 0.7306, P < 0.0396; ρ = − 0.8051, P < 0.0159) and the ratio of acetylated/non-acetylated glycoalkaloids (ρ = 0.8333, P < 0.0102). We did not detect changes in expression between resistant and susceptible lines of genes 3-hydroxy-3-methylglutaryl coenzyme A reductase 2, sterol-C5(6)-desaturase, or squalene epoxidase on chromosome 2 involved in the early stages of glycoalkaloid biosynthesis. Genes GAME4 and GAME12 on chromosome 12 as well as GAME7 and GAME 11 on chromosome 7 were upregulated in resistant lines (Table S7).
Discussion
S. chacoense Colorado potato beetle resistance is tissue and age-dependent
The resistance of old leaves on young M6 plants, which do not produce leptines, in a no-choice context observed in this study provides evidence for another resistance mechanism. Colorado potato beetles can detect leaf age (De Wilde et al. 1969) and prefer young to mature foliage (Noronha and Cloutier 2006), but will feed on mature S. tuberosum leaves in a choice setting (Mitchell and Low 1994). Structural characteristics, such as leaf toughness, of mature leaves can serve as a mechanical deterrent to beetle herbivory (Larson and Csiro 1988; RAUPP 1985; Tanton 1962). Yet, that the differences in Colorado potato beetle feeding by leaf age in M6 disappear over time suggests instead a mechanism with transient properties. The production of leaf surface compounds, volatiles and cuticular waxes, is a key determinate of Colorado potato beetle feeding preference (Szafranek et al. 2006, 2008; Visser et al. 1979) and is dependent on plant developmental stage and tissue type (Agelopoulos et al. 2000; Szafranek and Synak 2006). Although the role of leaf volatile compounds in Colorado potato beetle host plant acceptance and rejection has been characterized in S. tuberosum (Bolter et al. 1997; Dickens 2002; Landolt et al. 1999; Martel et al. 2007), their effect on beetle feeding preferences in S. chacoense is incomplete (Hufnagel et al. 2017).
Phenotypic evaluation of the S. chacoense F2 population
While all F2 individuals accumulated the non-acetylated glycoalkaloids α-solanine and α-chaconine, accumulation of the acetylated leptine glycoalkaloids was detected only in a subset of the population (70%). There was strong positive correlation between accumulation of α-solanine and α-chaconine and between leptine I and leptine II. There was a modest negative correlation between accumulation of α-chaconine and individual leptines I and II as well as total leptines. Interestingly, there was no significant correlation between accumulation of α-solanine and leptine I and a slight negative correlation between accumulation of α-solanine and leptine II. This observation supports differential regulation of the accumulation of non-acetylated and acetylated glycoalkaloids, which has been previously proposed (Manrique-Carpintero et al. 2014; Sanford et al. 1996). Although previous studies using mapping populations with a S. tuberosum parent have implicated recessive genes in leptine synthesis (Hutvágner et al. 2001; Manrique-Carpintero et al. 2014; Ronning et al. 1999; Sagredo et al. 2006; Sanford et al. 1996), the presence of leptines in this S. chacoense population followed a single dominant gene model which has been previously observed by (Rangarajan et al. 2000) in a S. phureja × S. chacoense population. Production of the aglycone leptinidine and leptinines by both parental lines in the population used in this study offers the unique opportunity to examine the segregation of downstream glycoalkaloid derivatives alone (Cárdenas et al. 2019). It is possible that other recessive genes involved in leptine biosynthesis are fixed in the two largely homozygous parental lines of this population. Variability in the concentration of acetylated and non-acetylated glycoalkaloids in the lines accumulating all four compounds is indicative of previously described polygenic control of the glycoalkaloid biosynthesis pathway.
The F2 population exhibited a range of resistance to Colorado potato beetle defoliation under field conditions. We observed transgressive segregation for both foliar leptine content and Colorado potato beetle field defoliation resistance in the F2 population, which suggests the contribution of alleles controlling leptine accumulation and beetle resistance from both parental lines. However, the F2 lines with greater leptine content than resistant parent 80-1 were not significantly more beetle resistant than 80-1 and lines transgressively segregating for beetle resistance do not contain more leptines than 80-1. We also identified several F2 lines intermediately susceptible to defoliation with leptine levels not significantly different than 80-1 and resistant lines with low leptine accumulation, which casts doubt on the necessity of leptines alone for resistance (Lorenzen et al. 2001; Sagredo et al. 2009). Interestingly, the best predictor of field defoliation resistance was not leptine concentration but rather the ratio of acetylated/non-acetylated glycoalkaloids (i.e. a higher ratio of acetylated/non-acetylated glycoalkaloids is significantly correlated with lower field defoliation).
Genotyping the F2 population and distorted segregation analysis
The modest number of informative SNPs (754) generated from genotyping the F2 population with the Illumina Infinium V3 22K SNP array may be attributable to a high degree of similarity between the two S. chacoense parental lines and ascertainment bias inherent in interrogating individuals with divergent genetic landscapes relative to those used to develop the SNP array. We observed distorted segregation of mapped SNPs within the range previously reported (6–40%) in diploid potato (Bonierbale et al. 1988; Felcher et al. 2012; Gebhardt et al. 1991; Jacobs et al. 1995; Kreike and Stiekema 1997; Manrique-Carpintero et al. 2016; Rivard et al. 1996). The regions with the greatest distorted segregation were located on chromosome 1, 3 and 12 with preferential inheritance of M6 alleles, the self-compatible male parent of this population. Because of the self-compatibility selection of the F1 and F2, strong distorted segregation patterns were expected toward regions associated with self-compatibility and associated mechanisms from M6. The greatest distorted segregation observed in this population was located on the long arm of chromosome 12, where the frequency of the 80-1 genotype decreases to zero in some regions. The preferential inheritance of the paternal M6 genotype in the distal region of chromosome 12 is most likely explained by the presence of the Sli locus, associated with self-compatibility in S. chacoense (Hosaka and Hanneman 1998). Linkage of Sli to recessive lethal genes on chromosome 12 has also been proposed (Hosaka et al. 1998). Transmission of a recessive lethal allele on chromosome 12 from maternal parent 80-1 to the F1 hybrid could be responsible for the preferential inheritance of the heterozygous genotype in the pericentromeric region, where reduced recombination could prevent the purging of deleterious alleles as mentioned by Zhang et al. (2019). However, this distinct pattern of heterozygous genotype retention is indicative of an independent region of segregation distortion which could be driven by genomic interactions between the two S. chacoense parental lines (Moyle and Graham 2006).
The self-incompatibility multiallelic locus (S) on chromosome 1 is the most common source of distorted segregation in potato and contains tightly linked S-RNase and F-box (SLF/SFB) genes expressed in the style and pollen, respectively (Enciso-Rodriguez et al. 2019; Ye et al. 2018). The distorted segregation on chromosome 1 observed in this population is most likely a product of gametic selection in the self-pollinated F1 hybrid against pollen with the 80-1 S-allele, resulting in the absence of individuals with the homozygous 80-1 SLF/SFB genotype in the F2 progeny. Zhang et al. (2019) similarly reported segregation distortion resulting from gametic selection of S-locus alleles on chromosome 1 in S. stenotomum. Our observation of selection against the homozygous 80-1 genotype on chromosome 1 suggests that Sli in M6 does not completely inactivate the gametophytic incompatibility reaction. The possibility that other factors contribute to transmission of self-compatibility has important ramifications for the introduction of M6-mediated self-compatibility into diploid potato breeding programs.
The distorted segregation identified in the population did not interfere with QTL detection since major QTL were located on different chromosomes. Interestingly, the distorted segregation on chromosome 3 favoring M6 alleles points to another potential region associated with self-compatibility. Preferential inheritance of heterozygous loci on chromosome 8 could be associated with zygotic selection driven by sublethal and meiotic mutant alleles hypothesized to reside on chromosome 8 (Jacobs et al. 1995).
QTL and candidate gene identification
A major QTL was detected for Colorado potato beetle resistance and leptine accumulation on chromosome 2. The moderate negative correlation between increased leptine accumulation and lower Colorado potato beetle field defoliation reported in our study supports a single QTL for both traits. Localization of loci associated with the presence of leptines within the QTL on chromosome 2 suggests that this QTL enables leptine biosynthesis. A lack of recombination within the QTL region on chromosome 2 in Colorado potato beetle individuals with low leptine levels suggests a single gene at this locus associated with the presence of leptines. As a polygenic trait, we propose that a separate regulatory element contributes to the variation observed in leptine accumulation. We identified a putative acetyltransferase within the major QTL on chromosome 2 expressed only in resistant lines which may be responsible for leptine synthesis in this germplasm. Regulation of leptine accumulation may be then accomplished by co-expression of the regulatory elements we identified within the major QTL on chromosome 2 and minor QTL on chromosome 12 (Table S7, S8, Fig. 6). Alternatively, it is possible that glycosylation of acetyl-leptinidine to produce leptines I/II is achieved by a unique UDP-glycosyltransferase(s) that is absent in M6, the non-leptine producer parent. Three distinct UDP-glycosyltransferases are required for the conversion of solanidine to α-solanine and α-chaconine (McCue et al. 2005, 2007; Moehs et al. 1997), but enzymes involved in glycosylation of other aglycones remain undefined. We identified unique expression patterns of uncharacterized UDP-glycosyltransferases on chromosome 2 in 80-1 and resistant F2 lines that may contribute to leptine I/II production.
The complete glycoalkaloid profile of an individual plant may also contribute to the variation in Colorado potato beetle defoliation resistance. There is evidence that the Colorado potato beetle neurosensory response to leptines is modified by the presence of other glycoalkaloids. Hollister et al. (2001) demonstrated that the Colorado potato beetle neurosensory response to leptine I is reduced in the presence of α-solanine. We observed a modest positive correlation between α-chaconine accumulation and Colorado potato beetle defoliation (Table 2) and negative contribution of the 80-1 genotype in the QTL region on chromosome 2 for α-chaconine accumulation (Table 4). The ratio of acetylated to non-acetylated glycoalkaloids was also the best predictor of field defoliation in this study. Cárdenas et al. (2019) demonstrated that the product of GAME32, responsible for leptinine production in M6, can hydroxylate either the aglycone solanidine or the glycosylated α-solanine and α-chaconine. Taken together, synthesis of leptines by our candidate gene within the QTL on chromosome 2 may involve the acetylation of hydroxylated α-solanine and α-chaconine, resulting in a higher ratio of acetylated to non-acetylated compounds.
The QTL identified in this study overlaps with a large region previously associated with leptine synthesis and accumulation in a pseudo F2 population using 80-1 and S. tuberosum Grp. Phureja DH as parents (Manrique-Carpintero et al. 2014). The large size of the QTL region may be explained by the moderate population size and suppression of recombination due to the close proximity of the nucleolar organizer region on the short arm of chromosome 2 (Pijnacker and Ferwerda 1984), while the presence of two peaks within the region identified by BSA-seq is most likely explained by a potential assembly error in constructing the DM pseudomolecules. Fine mapping using F4 individuals derived from the F2 population to further delineate the region critical to Colorado potato beetle resistance will facilitate efficient introgression of this trait into cultivated backgrounds and contribute to the development of beetle resistant varieties.
References
Agelopoulos N, Chamberlain K, Pickett J (2000) Factors affecting volatile emissions of intact potato plants, Solanum tuberosum: variability of quantities and stability of ratios. J Chem Ecol 26:497–511
Alyokhin A, Baker M, Mota-Sanchez D, Dively G, Grafius E (2008) Colorado potato beetle resistance to insecticides. Am J Potato Res 85:395–413. https://doi.org/10.1007/s12230-008-9052-0
Alyokhin A, Vincent C, Giordanengo P (2012) Insect pests of potato: global perspectives on biology and management. Academic Press, New York, p 634
APRD (2019) Arthropod pesticide resistance database. https://www.pesticideresistance.org. Accessed 29 Oct 2019
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Methodol) 57:289–300
Blatch GL, Lässle M (1999) The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays 21:932–939
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Bolter CJ, Dicke M, Van Loon JJ, Visser J, Posthumus MA (1997) Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. J Chem Ecol 23:1003–1023
Boluarte-Medina T, Fogelman E, Chani E, Miller AR, Levin I, Levy D, Veilleux RE (2002) Identification of molecular markers associated with leptine in reciprocal backcross families of diploid potato. Theor Appl Genet 105:1010–1018. https://doi.org/10.1007/s00122-002-1020-3
Bonierbale MW, Plaisted RL, Tanksley SD (1988) Rflp maps based on a common set of clones reveal modes of chromosomal evolution in potato and tomato. Genetics 120:1095–1103
Cárdenas PD, Sonawane PD, Heinig U, Jozwiak A, Panda S, Abebie B, Kazachkova Y, Pliner M, Unger T, Wolf D (2019) Pathways to defense metabolites and evading fruit bitterness in genus Solanum evolved through 2-oxoglutarate-dependent dioxygenases. Nat Commun 10:1–13
Coombs JJ, Douches DS, Li W, Grafius EJ, Pett WL (2003) Field evaluation of natural, engineered, and combined resistance mechanisms in potato for control of Colorado potato beetle. J Am Soc Hortic Sci 128:219–224
Crossley MS, Schoville SD, Haagenson DM, Jansky SH (2018) Plant resistance to Colorado potato beetle (Coleoptera: Chrysomelidae) in diploid f2 families derived from crosses between cultivated and wild potato. J Econ Entomol 111:1875–1884. https://doi.org/10.1093/jee/toy120
De Wilde J, Bongers W, Schooneveld AH (1969) Effects of hostplant age on phytophagous insects. Entomol Exp Appl 12:714–720
Deahl K, Cantelo W, Sinden S, Sanford L (1991) The effect of light intensity on Colorado potato beetle resistance and foliar glycoalkaloid concentration of four Solanum chacoense clones. Am Potato J 68:659–666
Depristo MA, Banks E, Poplin RE, Garimella KV, Maguire JR, Hartl C, Philippakis AA, Del Angel G, Rivas MA, Hanna M, Mckenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ (2011) A framework for variation discovery and genotyping using next- generation DNA sequencing data. Nat Genet 43:491–498. https://doi.org/10.1038/ng.806.A
Dickens JC (2002) Behavioural responses of larvae of Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae), to host plant volatile blends attractive to adults. Agric For Entomol 4:309–314
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) Star: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21
Enciso-Rodriguez F, Manrique-Carpintero NC, Nadakuduti SS, Buell CR, Zarka D, Douches DS (2019) Overcoming self-incompatibility in diploid potato using CRISPR-Cas9. Front Plant Sci 10:376
Felcher KJ, Coombs JJ, Massa AN, Hansey CN, Hamilton JP, Veilleux RE, Buell CR, Douches DS (2012) Integration of two diploid potato linkage maps with the potato genome sequence. PLoS One 7:e36347
Friedman M, Mcdonald GM (1997) Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Crit Rev Plant Sci 16:55–132. https://doi.org/10.1080/07352689709701946
Gebhardt C, Ritter E, Barone A, Debener T, Walkemeier B, Schachtschabel U, Kaufmann H, Thompson R, Bonierbale M, Ganal M (1991) RFLP maps of potato and their alignment with the homoeologous tomato genome. Theor Appl Genet 83:49–57
Ginzberg I, Tokuhisa JG, Veilleux RE, Ginzberg I, Tokuhisa JG, Veilleux RE (2009) Potato steroidal glycoalkaloids: biosynthesis and genetic manipulation. Potato Res 52:1–15. https://doi.org/10.1007/s11540-008-9103-4
Grafius EJ, Douches DS (2008) The present and future role of insect-resistant genetically modified potato cultivars in IPM. In: Romeis J, Shelton AM, Kennedy GG (eds) Integration of insect-resistant genetically modified crops within IPM programs. Springer, Dordrecht, pp 195–221
Hamilton JP, Hansey CN, Whitty BR, Stoffel K, Massa AN, Van Deynze A, De Jong WS, Douches DS, Buell CR (2011) Single nucleotide polymorphism discovery in elite North American potato germplasm. BMC Genom 12:302. https://doi.org/10.1186/1471-2164-12-302
Hare JD (1980) Impact of defoliation by the Colorado potato beetle on potato yields. J Econ Entomol 73:369–373. https://doi.org/10.1093/jee/73.3.369
Hirsch CD, Hamilton JP, Childs KL (2014) Spud DB: a resource for mining sequences, genotypes, and phenotypes to accelerate potato breeding. The Plant Genome 7:1–12. https://doi.org/10.3835/plantgenome2013.12.0042
Hollister B, Dickens JC, Perez F, Deahl KL (2001) Differential neurosensory responses of adult Colorado potato beetle, Leptinotarsa decemlineata, to glycoalkaloids. J Chem Ecol 27:1105–1118
Hosaka K, Hanneman RE (1998) Genetics of self-compatibility in a self-incompatible wild diploid potato species Solanum chacoense. 2. Localization of an S locus inhibitor (Sli) gene on the potato genome using DNA markers. Euphytica 103:265–271
Hosaka K, Hanneman J, Robert E (1998) Genetics of self-compatibility in a self-incompatible wild diploid potato species Solanum chacoense. 1. Detection of an s locus inhibitor (sli) gene. Euphytica 99:191–197. https://doi.org/10.1023/a:1018353613431
Hufnagel M, Schilmiller AL, Ali J, Szendrei Z (2017) Choosy mothers pick challenging plants: maternal preference and larval performance of a specialist herbivore are not linked. Ecol Entomol 42:33–41
Hutvágner G, Bánfalvi Z, Milánkovics I, Silhavy D, Polgár Z, Horváth S, Wolters P, Nap JP (2001) Molecular markers associated with leptinine production are located on chromosome 1 in Solanum chacoense. Theor Appl Genet 102:1065–1071. https://doi.org/10.1007/s001220000450
Ioannidis P, Grafius E, Whalon M (1991) Patterns of insecticide resistance to azinphosmethyl, carbofuran, and permethrin in the Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 84:1417–1423
Ioannidis PM, Grafius EJ, Wierenga JM, Whalon ME, Hollingworth RM (1992) Selection, inheritance and characterization of carbofuran resistance in the Colorado potato beetle (Coleoptera: Chrysomelidae). Pestic Sci 35:215–222. https://doi.org/10.1002/ps.2780350304
Itkin M, Heinig U, Tzfadia O, Bhide AJ, Shinde B, Cardenas PD, Bocobza SE, Unger T, Malitsky S, Finkers R, Tikunov Y, Bovy A, Chikate Y, Singh P, Rogachev I, Beekwilder J, Giri AP, Aharoni A (2013) Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 341:175–179. https://doi.org/10.1126/science.1240230
Izzo VM, Chen YH, Schoville SD, Wang C, Hawthorne DJ (2018) Origin of pest lineages of the Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 111:868–878
Jacobs J, Van Eck H, Arens P, Verkerk-Bakker B, Te Lintel Hekkert B, Bastiaanssen H, El-Kharbotly A, Pereira A, Jacobsen E, Stiekema W (1995) A genetic map of potato (Solanum tuberosum) integrating molecular markers, including transposons, and classical markers. Theor Appl Genet 91:289–300
Jansky SH, Chung YS, Kittipadukal P (2014) M6: a diploid potato inbred line for use in breeding and genetics research. J Plant Regist 8:195. https://doi.org/10.3198/jpr2013.05.0024crg
Kohl M, Wiese S, Warscheid B (2011) Cytoscape: software for visualization and analysis of biological networks. Data mining in proteomics, pp 291–303
Kreike C, Stiekema W (1997) Reduced recombination and distorted segregation in a Solanum tuberosum (2 ×) × S. spegazzinii (2 ×) hybrid. Genome 40:180–187
Kumar A, Fogelman E, Weissberg M, Tanami Z, Veilleux R, Ginzberg I (2017) Lanosterol synthase-like is involved with differential accumulation of steroidal glycoalkaloids in potato. Planta 246:1189–1202
Lachman J, Hamouz K, Orsák M, Pivec V (2001) Potato glycoalkaloids and their significance in plant protection and human nutrition-review. Rostlinna Vyroba UZPI 47:181–191
Landolt PJ, Tumlinson J, Alborn D (1999) Attraction of Colorado potato beetle (Coleoptera: Chrysomelidae) to damaged and chemically induced potato plants. Environ Entomol 28:973–978
Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinform 9:559
Larson S, Csiro C (1988) Leaf age and larval performance of the leaf beetle Paropsis atomaria. Ecol Entomol 13:19–24
Lawson DR, Veilleux RE, Miller AR (1993) Biochemistry and genetics of Solanum chacoense steroidal alkaloids: natural resistance factors to the Colorado potato beetle. Curr Top Bot Res 1(33335):33352
Leisner CP, Hamilton JP, Crisovan E, Manrique-Carpintero NC, Marand AP, Newton L, Pham GM, Jiang J, Douches DS, Jansky SH, Buell CR (2018) Genome sequence of M6, a diploid inbred clone of the high-glycoalkaloid-producing tuber-bearing potato species solanum chacoense, reveals residual heterozygosity. Plant J 94:562–570. https://doi.org/10.1111/tpj.13857
Li H (2013) Aligning sequence reads, clone sequences and assembly contigs with BWA-mem. arXiv 00:1-3. arXiv:1303.3997 [q-bio.GN]
Liao Y, Smyth GK, Shi W (2013) Featurecounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930
Lorenzen JH, Balbyshev NF, Lafta AM, Casper H, Tian X, Sagredo B (2001) Resistant potato, selections contain leptine and inhibit development of the Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 94:1260–1267. https://doi.org/10.1603/0022-0493-94.5.1260
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
Magwene PM, Willis JH, Kelly JK (2011) The statistics of bulk segregant analysis using next generation sequencing. PLoS Comput Biol 7:e1002255
Manrique-Carpintero NC, Tokuhisa JG, Ginzberg I, Veilleux RE (2014) Allelic variation in genes contributing to glycoalkaloid biosynthesis in a diploid interspecific population of potato. Theor Appl Genet 127:391–405
Manrique-Carpintero NC, Coombs JJ, Veilleux RE, Buell CR, Douches DS (2016) Comparative analysis of regions with distorted segregation in three diploid populations of potato. G3: genes. Genom Genet 6:2617–2628
Mansfeld BN, Grumet R (2018) Qtlseqr: an R package for bulk segregant analysis with next-generation sequencing. bioRxiv:208140. https://doi.org/10.1101/208140
Martel J, Alford A, Dickens J (2007) Evaluation of a novel host plant volatile-based attracticide for management of Colorado potato beetle, Leptinotarsa decemlineata (Say). Crop Prot 26:822–827
Mccue KF, Shepherd LVT, Allen PV, Maccree MM, Rockhold DR, Corsini DL, Davies HV, Belknap WR (2005) Metabolic compensation of steroidal glycoalkaloid biosynthesis in transgenic potato tubers: using reverse genetics to confirm the in vivo enzyme function of a steroidal alkaloid galactosyltransferase. Plant Sci 168:267–273. https://doi.org/10.1016/j.plantsci.2004.08.006
Mccue KF, Allen PV, Shepherd LVT, Blake A, Malendia Maccree M, Rockhold DR, Novy RG, Stewart D, Davies HV, Belknap WR (2007) Potato glycosterol rhamnosyltransferase, the terminal step in triose side-chain biosynthesis. Phytochemistry 68:327–334. https://doi.org/10.1016/j.phytochem.2006.10.025
Mitchell B, Low R (1994) The structure of feeding behavior in the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). J Insect Behav 7:707
Moehs CP, Allen PV, Friedman M, Belknap WR (1997) Cloning and expression of solanidine UDP-glucose glucosyltransferase from potato. Plant J 11:227–236
Mondy NI, Gosselin B (1988) Effect of peeling on total phenols, total glycoalkaloids, discoloration and flavor of cooked potatoes. J Food Sci 53:756–759
Mondy NI, Munshi CB (1990) Effect of nitrogen fertilization on glycoalkaloid and nitrate content of potatoes. J Agric Food Chem 38:565–567
Mondy NI, Leja M, Gosselin B (1987) Changes in total phenolic, total glycoalkaloid, and ascorbic acid content of potatoes as a result of bruising. J Food Sci 52:631–634
Morris SC, Petermann JB (1985) Genetic and environmental effects on levels of glycoalkaloids in cultivars of potato (Solanum tuberosum L.). Food Chem 18:271–282
Mota-Sanchez D, Hollingworth RM, Grafius EJ, Moyer DD (2006) Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Pest Manag Sci 62:30–37. https://doi.org/10.1002/ps.1120
Moyle LC, Graham E (2006) Genome-wide associations between hybrid sterility QTL and marker transmission ratio distortion. Mol Biol Evol 23:973–980
Mweetwa AM, Hunter D, Poe R, Harich KC, Ginzberg I, Veilleux RE, Tokuhisa JG (2012) Steroidal glycoalkaloids in Solanum chacoense. Phytochemistry 75:32–40. https://doi.org/10.1016/j.phytochem.2011.12.003
Noronha C, Cloutier C (2006) Effects of potato foliage age and temperature regime on prediapause Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Environ Entomol 35:590–599
Osman S, Sinden SL, Deahl K, Moreau R (1987) The metabolism of solanidine by microsomal fractions from Solanum chacoense. Phytochemistry 26:3163–3165. https://doi.org/10.1016/S0031-9422(00)82462-0
PGSC (2011) Genome sequence and analysis of the tuber crop potato. Nature 475:189–195. https://doi.org/10.1038/nature10158
Pijnacker L, Ferwerda M (1984) Giemsa C-banding of potato chromosomes. Can J Genet Cytol 26:415–419
Rangarajan A, Miller AR, Veilleux RE (2000) Leptine glycoalkaloids reduce feeding by Colorado potato beetle in diploid Solanum sp. hybrids. J Am Soc Hortic Sci 125:689–693
Raupp MJ (1985) Effects of leaf toughness on mandibular wear of the leaf beetle, Plagiodera versicolora. Ecol Entomol 10:73–79
Riley CV (1871) Third annual report on the noxious, beneficial and other insects, of the state of missouri. Public Printer, Jefferson City
Rivard SR, Cappadocia M, Landry BS (1996) A comparison of RFLP maps based on anther culture derived, selfed, and hybrid progenies of Solanum chacoense. Genome 39:611–621
Ronning CM, Sanford LL, Kobayashi RS, Kowalsld SP (1998) Foliar leptine production in segregating F1, inter-F1, and backcross families of Solanum chacoense Bitter. Am J Potato Res 75:137–143
Ronning CM, Stommel JR, Kowalski SP, Sanford LL, Kobayashi RS, Pineada O (1999) Identification of molecular markers associated with leptine production in a population of Solanum chacoense Bitter. Theor Appl Genet 98:39–46. https://doi.org/10.1007/s001220051037
Rozen S, Skaletsky H (2000) Primer3 on the www for general users and for biologist programmers. Bioinformatics methods and protocols. Springer, pp 365–386
Sagredo B, Lafta A, Casper H, Lorenzen J (2006) Mapping of genes associated with leptine content of tetraploid potato. Theor Appl Genet 114:131–142. https://doi.org/10.1007/s00122-006-0416-x
Sagredo B, Balbyshev N, Lafta A, Casper H, Lorenzen J (2009) A QTL that confers resistance to Colorado potato beetle (Leptinotarsa decemlineata [Say]) in tetraploid potato populations segregating for leptine. Theor Appl Genet 119:1171–1181. https://doi.org/10.1007/s00122-009-1118-y
Sanford LL, Kobayashi RS, Deahl KL, Sinden SL (1996) Segregation of leptines and other glycoalkaloids in Solanum tuberosum (4 ×) × S. chacoense (4 ×) crosses. Am Potato J 73:21
Sanford LL, Kobayashi RS, Deahl KL, Sinden SL (1997) Diploid and tetraploid Solanum chacoense genotypes that synthesize leptine glycoalkaloids and deter feeding by Colorado potato beetle. Am Potato J 74:15–21
Schapire AL, Valpuesta V, Botella MA (2006) Tpr proteins in plant hormone signaling. Plant Signal Behav 1:229–230
Shaner G, Finney R (1977) The effect of nitrogen fertilization on the expression of slow-mildewing resistance in knox wheat. Phytopathology 67:1051–1056
Sharma SK, Bolser D, De Boer J, Sønderkær M, Amoros W, Carboni MF, D’ambrosio JM, De La Cruz G, Di Genova A, Douches DS (2013) Construction of reference chromosome-scale pseudomolecules for potato: integrating the potato genome with genetic and physical maps. G3: Genes. Genom Genet 3:2031–2047
Silhavy D, Szentesi A, Bánfalvi Z (1996) Solanum chacoense lines with different alkaloid contents-a potential source of genes involved in leptine synthesis. Acta Agron Hung 44:113–120
Sinden SL, Webb RE (1972) Effect of variety and location on the glycoalkaloid content of potatoes. Am Potato J 49:334–338
Sinden SL, Sanford LL, Osman SF (1980) Glycoalkaloids and resistance to the Colorado potato beetle in Solanum chacoense Bitter. Am Potato J 57:331–343. https://doi.org/10.1007/BF02854028
Sinden SL, Sanford LL, Webb RE (1984) Genetic and environmental control of potato glycoalkaloids. Am Potato J 61:141–156
Sinden SL, Sanford LL, Cantelo WW, Deahl KL (1986) Leptine glycoalkaloids and resistance to the Colorado potato beetle (Coleoptera: Chrysomelidae) in Solanum chacoense. Environ Entomol 15:1057–1062
Slanina P (1990) Solanine (glycoalkaloids) in potatoes: toxicological evaluation. Food Chem Toxicol 28:759–761
Stürekow B, Löw I (1961) Die wirkung einger Solanum alkaloidglykoside auf den kartoffelkafer Leptinotarsa decemlineata. Entomol Exp Appl 4:133–142
Szafranek BM, Synak EE (2006) Cuticular waxes from potato (Solanum tuberosum) leaves. Phytochemistry 67:80–90
Szafranek B, Chrapkowska K, Waligóra D, Palavinskas R, Banach A, Szafranek J (2006) Leaf surface sesquiterpene alcohols of the potato (Solanum tuberosum) and their influence on Colorado beetle (Leptinotarsa decemlineata Say) feeding. J Agric Food Chem 54:7729–7734
Szafranek B, Synak E, Waligóra D, Szafranek J, Nawrot J (2008) Leaf surface compounds of the potato (Solanum tuberosum) and their influence on Colorado potato beetle (Leptinotarsa decemlineata) feeding. Chemoecology 18:205–216
Szendrei Z (2014) Colorado potato beetle management in potatoes. Michigan State University Extension. https://www.canr.msu.edu/news/colorado_potato_beetle_management_in_potatoes. Accessed 1 July 2020
Tanton M (1962) The effect of leaf “toughness” on the feeding of larvae of the mustard beetle Phaedon cochleariae Fab. Entomol Exp Appl 5:74–78
Tingey WM (1984) Glycoalkaloids as pest resistance factors. Am Potato J 61:157–167. https://doi.org/10.1007/BF02854036
Van Gelder WM, Dellaert LMW (1988) Alkaloids in potatoes. Prophyta 42:236–238
Van Ooijen J (2006) Joinmap 4. Software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen, Netherlands
Van Ooijen J., Kyazma B. (2009) Mapqtl 6. Software for the mapping of quantitative trait loci in experimental populations of diploid species. Kyazma BV, Wageningen, Netherlands
Veilleux RE, Miller AR (1998) Hybrid breakdown in the F1 between Solanum chacoense and S. phureja and gene transfer for leptine biosynthesis. J Am Soc Hortic Sci 123:854–858
Visser J, Van Straten S, Maarse H (1979) Isolation and identification of volatiles in the foliage of potato, Solanum tuberosum, a host plant of the Colorado beetle, Leptinotarsa decemlineata. J Chem Ecol 5:13–25
Voorrips RE (2002) Mapchart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78. https://doi.org/10.1093/jhered/93.1.77
Vos PG, Uitdewilligen JGAML, Voorrips RE, Visser RGF, Van Eck HJ (2015) Development and analysis of a 20 k SNP array for potato (Solanum tuberosum): an insight into the breeding history. Theor Appl Genet. https://doi.org/10.1007/s00122-015-2593-y
Vreugdenhil D, Bradshaw J, Gehardt C, Govers F, Mackerron DKL, Taylor M, Ross H (2007) Potato biology and biotechnology: advances and perspectives 91–111
Whalon ME, Mota-Sanchez D, Hollingworth RM (2008) Analysis of global pesticide resistance in arthropods. Global pesticide resistance in arthropods. CABI, Wallingford, pp 5–31
Ye M, Peng Z, Tang D, Yang Z, Li D, Xu Y, Zhang C, Huang S (2018) Generation of self-compatible diploid potato by knockout of S-RNase. Nat Plants 4:651–654
Yencho GC, Kowalski SP, Kennedy GG, Sanford LL (2000) Segregation of leptine glycoalkaloids and resistance to Colorado potato beetle (Leptinotarsa decemlineata (Say)) in F2 Solanum tuberosum (4 ×) × S. chacoense (4 ×) potato progenies. Am J Potato Res 77:167–178
Zhang C, Wang P, Tang D, Yang Z, Lu F, Qi J, Tawari NR, Shang Y, Li C, Huang S (2019) The genetic basis of inbreeding depression in potato. Nat Genet 51:374–378
Acknowledgements
The authors are grateful to Robin Buell and Gina Pham for advice on sequencing and bioinformatic analyses. We would also like to thank Anthony Schilmiller, Assistant Core Manager of the Michigan State University Mass Spectrometry and Metabolomics Core facility, for guidance on methods and Grant Billings for his technical assistance conducting Colorado potato beetle bioassays, extracting metabolite and DNA samples, and providing plant maintenance in the greenhouse.
Funding
This project was supported by Michigan State University AgBioResearch Project GREEEN (Generating Research and Extension to meet Economic and Environmental Needs) (Grant No. GR17-014) funding.
Author information
Authors and Affiliations
Contributions
NRK and DD conceived of this study and contributed to funding acquisition. NRK, CD, DD, NCMC, and JC designed the research. NRK executed the experiments. NRK and NCMC defined linkage mapping protocols. NRK, CD. and JC defined insect assay experimental design and protocols. NRK defined genomic analysis pipelines. NRK analyzed the data with input from all authors. NRK and NCMC wrote the manuscript with contributions from all coauthors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Jeffrey Endelman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaiser, N., Manrique-Carpintero, N.C., DiFonzo, C. et al. Mapping Solanum chacoense mediated Colorado potato beetle (Leptinotarsa decemlineata) resistance in a self-compatible F2 diploid population. Theor Appl Genet 133, 2583–2603 (2020). https://doi.org/10.1007/s00122-020-03619-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-020-03619-8