Abstract
Key message
A new gene for adult plant leaf rust resistance in wheat was mapped to chromosome 3BL. This gene was designated as Lr77.
Abstract
‘Santa Fe’ is a hard red winter cultivar that has had long-lasting resistance to the leaf rust fungus, Puccinia triticina. The objective of this study was to determine the chromosome location of the adult plant leaf rust resistance in Santa Fe wheat. A partial backcross line of ‘Thatcher’ (Tc) wheat with adult plant leaf rust resistance derived from Santa Fe was crossed with Thatcher to develop a Thatcher//Tc*2/Santa Fe F6 recombinant inbred line (RIL) population. The RIL population and parental lines were evaluated for segregation of leaf rust resistance in three field plot tests and in an adult plant greenhouse test. A genetic map of the RIL population was constructed using 90,000 single-nucleotide polymorphism (SNP) markers with the Illumina Infinium iSelect 90K wheat bead array. A significant quantitative trait locus for reduction of leaf rust severity in all four tests was found on chromosome 3BL that segregated as a single adult plant resistance gene. The RILs with the allele from the resistant parent for SNP marker IWB10344 had lower leaf rust severity and a moderately resistant to moderately susceptible response compared to the susceptible RILs and Thatcher. The gene derived from Santa Fe on chromosome 3BL was designated as Lr77. Kompetitive allele-specific polymerase chain reaction assay markers linked to Lr77 on 3BL should be useful for selection of wheat germplasm with this gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leaf rust, caused by Puccinia triticina Erikss., is an important disease of wheat (Triticum aestivum L) in many regions throughout the world (Roelfs et al. 1992; Saari and Prescott 1985; Samborski 1985). The disease is usually most common and destructive in non-arid areas where wheat matures in warm to hot weather or in coastal regions. In the Great Plains of North America, leaf rust occurs regularly and can cause significant yield losses. In 2007, a heavy and widespread epidemic occurred throughout the Great Plains region, causing an estimated 14% loss in Kansas (Kolmer et al. 2009). In research plots and farm fields, many of the commonly grown hard red winter wheat cultivars are completely susceptible to leaf rust. The resistance genes Lr3a, Lr14a, Lr21, Lr24, Lr26, Lr37, and Lr39 (Kolmer, unpublished data) are present in the hard red winter wheat cultivars; however, races of P. triticina with virulence to these race-specific genes are present in high frequency, which has diminished the effectiveness of the genes. Wheat cultivars with combinations of the genes Lr34, Lr46, Lr67, and Lr68 that are most effective in the adult plant stage to all current leaf rust races have had long-lasting resistance compared to cultivars that carry only the race-specific genes that are effective in seedling plants (Lagudah 2011). Only a few current hard red winter wheat cultivars carry Lr34; however, the presence of other effective adult plant resistance genes in these wheat cultivars is not known, since robust gene-specific diagnostic markers for most of these genes have not been developed.
The hard red winter wheat cultivar ‘Santa Fe’ was highly resistant in the 2007 leaf rust epidemic and in the immediate following years when it was still widely grown in the southern Great Plains. Genetic analysis determined that the genes Lr3a and Lr37 were present in Santa Fe (Kolmer 2017). Since there are many races with virulence to both Lr3a and Lr37, the effective resistance must have been due to other resistance gene(s). A homozygous line of Tc*2/Santa Fe, derived from a Tc*2/Santa Fe F2 family that lacked Lr3a and Lr37, had a low infection type (IT) of flecks with small to moderate size uredinia surrounded by chlorosis (IT; 2−) in the adult plant stage in a greenhouse test, but had high IT of large uredinia without necrosis or chlorosis in seedling plants (IT 4). In inoculated field plots with a mixture of races, the Tc*2/Santa Fe line had an intermediate level of leaf rust resistance that was easily distinguished from the susceptible Thatcher. This line did not carry the adult plant resistance genes Lr34, Lr37 or Lr46 based on the absence of markers tightly linked to these genes. The objective of this study was to determine the chromosome location and further characterize the additional adult plant leaf rust resistance derived from Santa Fe.
Materials and methods
Population development
The Tc*2/Santa Fe 8-1 F4 line with adult plant leaf rust resistance derived from Santa Fe (Kolmer 2017) was used as the resistant parent for the recombinant inbred line (RIL) population in this study. The Tc*2/Santa Fe 8-1 line had 20–30% leaf rust severity with a moderately resistant to moderately susceptible response of small to moderate uredinia surrounded by necrosis and chlorosis in a field plot test at St. Paul, MN, 2011. To evaluate the RIL population in a spring wheat background that was not segregating for winter wheat type or heading date, the Tc*2/Santa Fe 8-1 line was crossed with Thatcher, and the Tc//Tc*2/Santa Fe 8-1 RILs were advanced to the F6 generation by single seed descent in a greenhouse. The RILs were increased one time in the greenhouse.
Leaf rust phenotyping
One hundred and thirteen RILS and the two parental lines were evaluated for field resistance at St. Paul MN from 2014 to 2016. In the field plots, 50–60 seeds of the parents and each RIL were planted in 2-m rows spaced 30 cm apart perpendicular to spreader rows of the wheat cultivars Tc, ‘Morocco’, ‘Max’, and ‘Little Club,’ that are all highly susceptible to leaf rust. A mixture of P. triticina races with designations, MHDSB, MFPSB, MLDSD, TBBGJ, TFBJQ, TFBGQ, and TBBGS in 2014, and MHDSB, MFPSB, MLDSD, TFBGQ, TBBGS, and MJBJG in 2015 and 2016, were inoculated to the spreader rows by atomizing a Soltrol 170 oil (Chevron-Phillips Petroleum, Bartlesville, OK)—urediniospore mixture on the plants, approximately 30 days after planting, when the plants were at the jointing stage. The five letter race designations were adapted from the hexadecimal nomenclature system (Long and Kolmer 1989). These P. triticina races had virulence to the seedling resistance genes and race-specific adult plant resistance genes Lr12, Lr13, and Lr37 that are present in U.S. wheat cultivars. The parents and RILs were evaluated once for leaf rust severity approximately 60 days after planting using the modified Cobb scale (Peterson et al. 1948). The RILs and parents were also evaluated at the same time for leaf rust resistance response: R = necrosis surrounding small uredinia; MR = necrosis surrounding moderate size uredinia; MS = distinct chlorosis surrounding moderate to large uredinia; S = large uredinia lacking necrosis or chlorosis. The plots were rated when the susceptible parent Tc had leaf rust severity of 50–80% with a susceptible response. Five flag leaves collected from different parts of the row were assessed for severity and response. The field plots in 2014 and 2015 had sufficient rainfall and dew periods after inoculation to allow the rust epidemic to develop normally. In 2016, hot dry weather set in immediately after inoculation of the plots in early June and persisted through the first week of July when the plots were evaluated. The lack of rainfall slowed the leaf rust spread in the plots and also significantly stressed the plants.
The RILs and parents were also tested as adult plants in a greenhouse test. Four seeds of the parents and each RIL were planted in 15-cm pots filled with steamed topsoil. The plants were grown in a greenhouse set at 20 °C with a 16 h light period. The RIL population was inoculated at anthesis with the mixture of P. triticina races MHDSB, MFPSB, TBBGJ, TFBJQ, TFBGQ. The flag leaves were inoculated individually with a mixture of Soltrol 170 oil and urediniospores. Eight to ten flag leaves of each RIL and parent were inoculated with an atomized mixture of 5 mg of rust spores and 750 μl of Soltrol oil. After inoculation, the plants were dried for at least 1 h and then placed in a closed chamber with a humidifier filled with distilled water running for 60 s every 5 min. The adult plants were removed the following morning and placed on a greenhouse bench. The RILs and parents were evaluated for IT using the standard 0–4 scale (Long and Kolmer 1989) 14 days after inoculation. Infection type scores were converted to a 0–100 score for quantitative trait analysis. Infection types and equivalent severity scores are given in Supplemental Table 1. Leaf rust severity in the greenhouse test was also recorded; however, there were little differences in severity among the RILs; therefore, only the converted infection type scores were used for subsequent analysis. The leaf rust severity (percentage of leaf area covered) of the RILs in the three field tests in St. Paul and the converted infection type in the greenhouse test were tested for correlation using Pearson’s coefficient with PROC CORR in Statistical Analysis Software (SAS Institute 2012).
Genotyping, mapping and QTL analysis
High-quality DNA of the parents and RILs was isolated using the cetyltrimethylammonium bromide (CTAB) method (Akbari et al. 2006). The RILs and parental lines were genotyped using the Infinium iSelect 90K wheat bead chip array described in Wang et al. (2014). Since the resistant parent was derived from Thatcher, it would be expected that many of the SNPs on the 90K wheat chip would not be polymorphic between the two parents, resulting in an incomplete genetic map. However, since the Tc*2/Santa Fe parent was selected for resistance relative to Thatcher, it would be expected to find polymorphic SNP markers in regions associated with leaf rust resistance.
Single-nucleotide polymorphism (SNP) genotype calling for the RILs and parental lines was done using GenomeStudio v.2011.1 (Illumina 2011). Redundant SNP markers showing complete linkage (100%), and markers with missing values equal to or more than 10%, were removed using the BIN function in QTL ICI Mapping (Meng et al. 2015). Linkage groups of SNP marker loci were assembled with Mapmaker v2.0 for MacIntosh (Lander et al. 1987) with the Kosambi map function and logarithm of odds (LOD) of 10 and r of 0.3. Map order was confirmed using R/qtl (Broman et al. 2003). Location of SNP markers was confirmed using the Chinese Spring NRGene-IWGSC v1.0 genome assembly (http://www.wheatgenome.org/News/Latest-news/RefSeq-v1.0-URGI). Composite interval mapping (CIM) was implemented using QGENE (Nelson 1997). The marker closest to the LOD peak was selected by QGENE as a cofactor. The coefficient of determination (R2) and LOD scores for each marker interval were determined with a significance level of α = 0.05 with 1000 permutations of the dataset. Analysis of variance of markers and disease severity was conducted with PROC GLM in SAS (SAS Institute 2012).
Based on the genotypic data from the 90K SNP chip, seven 90K SNP markers on chromosome 3BL that showed polymorphism between two contrasting genotype groups were selected for conversion of Kompetitive Allele-Specific PCR (KASP) markers (Supplemental Table 2). The KASP markers were assayed on the parents and 80 of the RILs in a 5-μl volume reaction including 2.5 μl 2× KASP master mix, 0.07 μl KASP primer mix and 2.5 μl DNA (~ 20 ng/μl) following the manufacturer’s instructions (LGC Genomics, http://www.lgcgenomics.com) using an ABI 7900HT fast real-time PCR system (Applied Biosystems).
Results
Inheritance of leaf rust resistance
In 2014 and 2015, the Tc*2/Santa Fe 8-1 parent had leaf rust severity of 10–20% and response of MR-MS. In 2016, the Tc*2/Santa Fe 8-1 parent had leaf rust severity of 30% with an MR–MS response. In all 3 years of field tests, the susceptible parent Thatcher had leaf rust severity of 50–60% with a susceptible response. In the greenhouse adult plant test, Thatcher had an IT of 3+–4 (large uredinia without chlorosis or necrosis) and the Tc*2/Santa Fe 8-1 parent had an IT of ;2–2+ (flecks with small to larger size uredinia surrounded by distinct chlorosis) (Fig. 1). Correlation for leaf rust severity in the RIL population between the different tests varied from 0.87 for the field tests in 2014 and 2015, and 0.32 for the greenhouse test with the field test in 2016 (Table 1). The average severity in all tests varied from 32.1 to 37.6%, with a range of 5–70% (Table 2).
Genetic mapping and QTL analysis
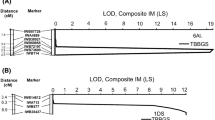
A total of 4163 polymorphic SNP markers were scored for the population. After removing completely linked (100%) markers, 460 markers remained, which were grouped into 30 linkage groups that covered 1571 centimorgans (cM). Single marker regression and composite interval mapping identified a region on chromosome 3BL, designated as QLr.cdl-3BL, which was associated with lower leaf rust severity. SNP marker IWB10344 mapped at the LOD peak at 40.9 cM on chromosome 3BL (Fig. 2). The marker IWB32805 was 3.5 cM proximal to the LOD peak, and IWB73555 was 0.9 cM distal to the peak. Sixty-four of the RILs had the ‘T’ allele from the resistant parent and 49 had the ‘G’ allele from Thatcher, which fit a single gene segregation of 1:1 with a χ2 of 1.99 (p = 0.16). The marker interval from IWB32805 to IWB73555 spanned 4.4 cM that is 10.9 million bases in the Chinese Spring NRGene v1.0 genome assembly and 7.02 cM in the 92K SNP wheat consensus map (Supplemental Table 3). LOD scores for QLr.cdl-3BL were greater than 6.0 in all tests, with values over 35 in the field tests in 2014 and 2015 (Table 3). The corresponding R2 values were all over 0.23, with values greater than 0.75 in the 2014 and 2015 field tests. In the ANOVA of leaf rust severity over all the tests, there was significant genotype × environment interaction (p < 0.001) that did not allow the overall means and R2 values to be used. The RILs homozygous for the IWB10344 ‘T’ allele associated with resistance derived from Tc*2/Santa Fe 8-1 had an average leaf rust severity less than 20.0% in the 2014 and 2015 field tests, and a severity of 32.7% in the 2016 field test (Table 2). In the greenhouse adult plant test, the resistant RILs had an average severity of 22.9% based on the converted IT scores. The RILs with the susceptibility ‘G’ allele derived from Thatcher had over 50% leaf rust severity in the 2014 and 2015 field tests: 43.3% severity in the 2016 field test, and 46.3% severity based on converted IT scores in the adult plant greenhouse test (Table 2). In the 2014 and 2015 field tests (Fig. 3a, b), the distribution of RILs based on alleles at IWB10344 had little overlap, as only three RILs in 2014 with the IWB10344 Thatcher allele had leaf rust severity less than 30%; and one RIL with the resistance allele had severity of 50%. In 2015, only five RILs with the IWB10344 resistance allele had leaf rust severity greater than 40%. In the 2016 field test (Fig. 3c), there was more overlap, as nine RILS with the Thatcher type allele had 30% or less severity, and nine RILs with the resistance allele had severity greater than 50%. Similarly in the greenhouse test (Fig. 3d), eight RILs with the resistance allele had converted IT severity of 60% or greater, while 20 RILs with the Thatcher type allele had 20% or less severity.
Frequency distributions of Thatcher//Thatcher*2/Santa Fe 8-1 recombinant inbred line (RIL) population for leaf rust severity based on segregation at IWB10344. RILs are segregating for the ‘T’ allele associated with resistance from Thatcher*2/Santa Fe and the ‘G’ allele associated with susceptibility from Thatcher. a 2014 field plot test, b 2015 field plot test, c 2016 field plot test, d greenhouse adult plant test
Among seven SNPs tightly linked to Lr77 from the 90K chip, six (IWB9095, IWB73555, IWB12260, IWB32805, IWB76509, IWB23680) were successfully converted into KASP markers and used to genotype the parental lines and 80 of the RILs (Supplemental Table 2). KASP marker 12260 had the same allele calls as the original SNP marker for all 80 RILs. The other five KASP markers also had high rates of agreement between the KASP and original SNP markers. Seven of the fourteen total SNP–KASP discrepancies were for individual RIL that were heterozygous based on the KASP assay but had the resistant parent allele in the original SNP genotyping. Five RIL marker combinations had different allele calls in the KASP assay and the original SNP genotyping and two combinations were heterozygous in the original SNP genotyping and were homozygous in the KASP assay. For SNP IWB10344, the KASP assay failed because the designed primers did not amplify the expected PCR product.
Discussion
A major QTL derived from the winter wheat cultivar Santa Fe for adult plant leaf rust resistance that segregated as single gene was mapped to chromosome 3BL. This gene expressed adult plant resistance in three field plot tests and in an adult plant greenhouse test. In 2014, two of the RILs with the Thatcher allele at IWB10344 had severity of 30%, but with a susceptible response. The lower leaf rust severity may have resulted from lower inoculum levels in that part of the plot. Both these RILs had 50–60% severity with a susceptible response in 2015. One of the RILs with the Thatcher allele had a 20 MR–MS response in 2014, but had 50% severity with a susceptible response in 2015. One RIL with the resistant allele had a 50% severity with a susceptible response in both 2014 and 2015. This RIL may be a recombinant between IWB10344 and the 3BL gene, since IWB32805 that is immediately proximal to IWB10344 could not be scored in the 90K chip genotyping, and the KASP assay for IW32805 indicated that both alleles were present. In 2015, three RILs with the resistant allele had 40% severity with a susceptible response. These RILs had 20–30% severity in 2014, with an MR–MS response. An additional RIL with the resistance allele had 50% severity in 2015; however, this RIL was not scored for leaf rust in 2014.
Previous to this research, no other Lr genes on chromosome 3BL had been designated; therefore, the gene on 3BL was designated as Lr77 (McIntosh et al. 2017). Subsequent to the designation of Lr77, a seedling leaf rust resistance gene derived from durum wheat was mapped to chromosome 3BL (Qureshi et al. 2018). Based on common markers, Lr79 was located approximately 9.2 million bases distal to Lr77.
The Tc*2/Santa Fe 8-1 parent with Lr77 had more effective resistance manifested by distinct necrosis and chlorosis compared to Thatcher lines with the adult plant resistance genes Lr34, Lr46, and Lr67 in field plot tests in St. Paul in 2014 and 2015 (Kolmer, unpublished data). The parental line and RILs with Lr77 had a very similar severity and response compared to lines with the adult plant resistance gene Lr78, derived from the cultivar Toropi (Kolmer et al. 2017).
Expression of adult plant resistance (Drijepondt and Pretorius 1989; Dyck 1987; Singh 1992; Kolmer et al. 2015; Singh et al. 1998) is dependent on temperature and moisture conditions in the field plot tests. In 2014 and 2015, sufficient rainfall occurred prior to and following inoculation of the field plots to allow leaf rust to spread relatively evenly throughout the plots. In 2016 as a result of the drought-stressed plants, it was difficult to distinguish susceptible from resistant RILs. This accounted for the much lower LOD score and R2 value associated with IWB10344 for 2016 compared to 2014 and 2015. Resistance conditioned by Lr77 in the parental line could also be distinguished in the adult plant greenhouse test; however, the LOD score and R2 value were also much lower compared to the 2014 and 2015 field plot tests. Evaluation of germplasm with Lr77 would best be conducted in field plot tests. The race specificity of Lr77 is not known at this time; however, the consistent expression of resistance in field plots with different mixtures of races suggests a non-race specific type of resistance.
Long-lasting resistance to P. triticina has been especially challenging to obtain in the hard red winter wheat cultivars grown in the southern Great Plains of the United States. Cultivars with seedling effective genes Lr21, Lr24, Lr26, and Lr39 have had leaf rust resistance eroded by the rapid increase of races with virulence to these genes. Santa Fe also has Lr3a and Lr37 (Kolmer 2017); however, many races have virulence to both genes, rendering them completely ineffective for resistance. The effective resistance of Santa Fe to leaf rust must be due to Lr77 and possibly other genes. Since only a single Tc*2/Santa Fe F4 derived plant was selected for crossing to Thatcher and development of the RIL population, it is possible that other effective adult plant resistance genes are also present in Santa Fe. The most well-known adult plant leaf rust resistance gene, Lr34, is also present in hard red winter wheat germplasm, although at relatively low frequency (Kolmer et al. 2008). In the 2017 Southern Regional Performance Nursery (SRPN), only 6 of 50 hard red winter wheat breeding lines had the marker associated with Lr34 (Bai, unpublished data). In contrast, 22 of the same breeding lines had the ineffective gene Lr37. Lr77 may already be widely present in the hard red winter wheat germplasm. In the same 2017 SRPN test, 18 of the entries had the same allele for the KASP marker for IWB12260 as the Tc*2/Santa Fe parental line with Lr77. However, since IWB12260 mapped 6.2 cM distal to IWB10344, this KASP marker may not be optimal for detection of Lr77. A specifically designed marker in the IWB10344–IWB73555 region would be more suitable for marker-assisted selection of germplasm with Lr77.
In conclusion, a highly effective adult plant leaf rust resistance gene derived from the winter wheat cultivar Santa Fe was mapped to chromosome 3BL and was designated as Lr77. Cultivars with this gene can be crossed with cultivars that have other effective adult plant leaf rust resistance genes such as Lr34 and Lr46 to develop germplasm with improved leaf rust resistance. The soft red winter wheat cultivars Clark (Li et al. 2017) and Caldwell (Kolmer et al. 2018) both carry effective adult plant leaf rust resistance that mapped to chromosome 3BS, and may be the same gene as Lr74. The resistance in Santa Fe could also be combined with the 3BS resistance to develop winter wheat cultivars with two sources of adult plant leaf rust resistance that should remain effective over a long period of time.
Author contribution statement
JAK designed the study, developed the mapping population, phenotyped the population, mapped the population, did the quantitative trait analysis, and prepared the manuscript. ZS, AB and GB designed the KASP markers and conducted the KASP assays on the population. GB revised the manuscript. SC genotyped the population with the 90K Illumina wheat bead chip.
References
Akbari M, Wenzl P, Caig V, Carling J, Yang S, Uszynski G, Mohler V, Lehmensiek A, Kuchel H, Hayden MJ, Howes N, Sharp P, Vaughn P, Rathmell B, Huttner E, Kilian A (2006) Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor Appl Genet 113:1409–1420
Broman KW, Wu H, Sen S, Churchill GA (2003) QTL mapping in experimental crosses. Bioinformatics 19:889–890
Drijepondt SC, Pretorius ZA (1989) Greenhouse evaluation of adult-plant resistance conferred by the gene Lr34 to leaf rust of wheat. Plant Dis 73:669–671
Dyck PL (1987) The association of a gene for leaf rust resistance with the chromosome 7D suppressor of stem rust resistance in common wheat. Genome 29:467–469
Illumina (2011) GenomeStudio genotyping module software: release 1.0. Illumina, San Diego
Institute SAS (2012) The SAS system for Windows, release 9.4. SAS Institute, Cary
Kolmer JA (2017) Genetics of leaf rust resistance in the hard red winter wheat cultivars Santa Fe and Duster. Crop Sci 57:2500–2505
Kolmer JA, Singh RP, Garvin DF, Viccars L, William HM, Huerta-Espino JH, Obonnaya FC, Raman H, Orford S, Bariana HS, Lagudah ES (2008) Analysis of the Lr34/Yr18 rust resistance region in wheat germplasm. Crop Sci 48:1841–1852
Kolmer JA, Long DL, Hughes ME (2009) Physiologic specialization of Puccinia triticina on wheat in the United States in 2007. Plant Dis 93:538–544
Kolmer JA, Lagudah ES, Lillemo M, Lin M, Bai G (2015) The Lr46 gene conditions partial adult-plant resistance to stripe rust, stem rust, and powdery mildew in Thatcher wheat. Crop Sci 55:2557–2565
Kolmer JA, Bernardo A, Bai G, Hayden MJ, Chao S (2017) Adult plant resistance derived from Toropi wheat is conditioned by Lr78 and three minor QTL. Phytopathology 108:246–253
Kolmer JA, Chao S, Brown-Guedira G, Bansal U, Bariana H (2018) Adult plant leaf rust resistance derived from the soft red winter wheat cultivar ‘Caldwell’ maps to chromosome 3BS. Crop Sci 58:152–158
Lagudah ES (2011) Molecular genetics of race non-specific resistance in wheat. Euphytica 179:81–91
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Li C, Wang Z, Li C, Bowden R, Bai G, Li C, Li C, Su Z, Carver BF (2017) Mapping of quantitative trait loci for leaf rust resistance in the wheat population Ning7840 × Clark. Plant Dis 101:1974–1979
Long DL, Kolmer JA (1989) A North American system of nomenclature for Puccinia recondita f.sp. tritici. Phytopathology 79:525–529
McIntosh RA, Dubcovsky J, Rogers J, Morris C, Xia XC (2017) Catalogue of gene symbols for wheat. 2017 supplement. Komugi Wheat Genetic Resources Database, Yokohama
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3:269–283
Nelson JC (1997) QGENE: software for marker-based genomic analysis and breeding. Mol Breed 3:239–245
Peterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can J Res 26:496–500
Qureshi N, Bariana H, Kumran VV, Muruga S, Forrest KL, Hayden MJ, Bansal U (2018) A new leaf rust resistance gene Lr79 mapped in chromosome 3BL from the durum wheat landrace Aus26582. Theor Appl Genet. https://doi.org/10.1007/s00122-08-3060-3
Roelfs AP, Singh RP, Saari EE (1992) Rust diseases of wheat: concepts and methods of disease management. CIMMYT, Mexico
Saari EE, Prescott JM (1985) World distribution in relation to economic losses. In: Roelfs AP, Bushnell WR (eds) The cereal rusts, vol 2. Academic Press, Orlando, pp 259–298
Samborski DJ (1985) Wheat leaf rust. In: Roelfs AP, Bushnell WR (eds) The cereal rusts, vol 2. Academic Press, Orlando, pp 39–59
Singh RP (1992) Expression of wheat leaf rust resistance gene Lr34 in seedlings and adult plants. Plant Dis 76:489–491
Singh RP, Mujeebkazi A, Huerta-Espino J (1998) Lr46—a gene conferring slow rusting resistance to leaf rust in wheat. Phytopathology 88:890–894
Wang S, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L, Mastrangelo AM, Whan A, Stephen S, Barker G, Wieseke R, Plieske J, International Wheat Genome Sequencing Consortium, Lillemo M, Mather D, Appels R, Dolferus R, Brown-Guedira G, Korol A, Akhunova AR, Feuillet C, Salse J, Pozniak C, Luo M-C, Dvorak J, Morell M, Dubcovsky J, Ganal M, Tuberosa R, Lawley C, Mikoulitch I, Cavanagh C, Edwards KJ, Hayden MJ, Akhunov E (2014) Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol 12:787–796
Acknowledgements
We thank K. Xiao for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Communicated by Evans Lagudah.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kolmer, J.A., Su, Z., Bernardo, A. et al. Mapping and characterization of the new adult plant leaf rust resistance gene Lr77 derived from Santa Fe winter wheat. Theor Appl Genet 131, 1553–1560 (2018). https://doi.org/10.1007/s00122-018-3097-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-018-3097-3