Abstract
Key message
Frost tolerance in durum wheat is mainly controlled by copy number variation of CBF - A14 at the Fr - A2 locus.
Abstract
Frost tolerance is a key trait for successful breeding of winter durum wheat (Triticum durum) which can increase the yield performance in regions favoring autumn-sown winter cereals. The aim of this study was to investigate the genetic architecture of frost tolerance in order to provide molecular support for the breeding of winter durum wheat. To this end, a diverse panel of 170 winter and 14 spring durum wheat genotypes of worldwide origin was evaluated for frost tolerance in the field, as well as in a semi-controlled test. A total of 30,611 polymorphic genome-wide markers obtained by a genotyping-by-sequencing approach and markers for candidate loci were used to assess marker-trait associations. One major QTL was detected on chromosome 5A, likely corresponding to Frost Resistance-A2 (Fr-A2). Further analyses strongly support the conclusion that copy number variation of CBF-A14 at the Fr-A2 locus is the causal polymorphism underlying this major QTL. It explains 91.6 % of the genotypic variance and a haploblock of two strongly associated markers in the QTL region also allowed to capture the variance of this QTL. In addition to this major QTL, a much smaller contribution of 4.2 % was observed for Fr-B2. We further investigated this major QTL and found that the copy number of CBF-A14 and the frequency of the frost tolerant haplotype mirrored the climatic conditions in the genotypes’ country of origin, suggesting selection through breeding. Two functional KASP markers were developed which facilitate a high-throughput screening of the haploblock and thus a marker-based breeding of frost tolerance in winter durum wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Durum wheat (Triticum durum) is predominantly produced in the Mediterranean area (South Europe, West Asia and North Africa) due to the prevailing climatic conditions. Using an autumn-sown spring type, Mediterranean farmers are able to take advantage of the humidity in the mild winters as well as in spring, and avoid heat and drought stresses in summer with a harvest in early June. In general, spring types in cereals are lacking in frost tolerance, photoperiodic sensitivity, and the need for vernalization, and consequently their survival during harsh winters with recurring frost periods as prevalent in Central Europe or North America, is dramatically reduced. Due to the low availability of frost tolerance in high grain quality winter type durum wheat, the production of durum in cold climate areas is restricted to spring-sown spring type. An autumn-sown winter type, however, would prolong the growing time, extend the vegetative phase, and result in higher yield (Kling et al. 2006; Lafferty 2010). Furthermore, it would allow to extend the range of growing regions, thereby supporting the production of regional products, preventing soil erosion due to constant soil coverage, and suppressing weeds by growth advantage. Breeding efforts of the last decade showed that it is possible to combine frost tolerance with high quality in winter durum varieties (Longin et al. 2013). The understanding of the genetic control of frost tolerance is therefore a key element towards a knowledge-based selection for frost tolerance in winter durum wheat breeding programs.
Well known frost tolerance loci are Frost Resistance-1 (Fr-1) (Sutka and Snape 1989) and Fr-2 each with homoeologs on group 5 chromosomes (Toth et al. 2003; Vágújfalvi et al. 2003). These two loci have been mapped within a distance of approximately 30 cM (Vágújfalvi et al. 2003). However, as Fr-1 maps close to the vernalization locus Vernalization-1 (Vrn-1), it is assumed that Fr-1 is actually Vrn-1 exhibiting a pleiotropic effect (Dhillon et al. 2010; Sutka and Snape 1989). Vrn-1 encodes for a MADS box protein (Trevaskis et al. 2003) and controls the transition from vegetative to generative growth. Furthermore, it was recently shown that an increased copy number of Vrn-A1 resulted in an increased requirement for vernalization (Díaz et al. 2012). The Fr-2 locus consists of a cluster of eleven C-Repeat Binding Factor (CBF) genes which are activated during vernalization and in turn activate Cold Regulated (COR) genes required for an increased winter survival (Knox et al. 2008; Miller et al. 2006; Vágújfalvi et al. 2005). For Fr-A2, Zhu et al. (2014) identified a number of completely linked polymorphisms in the CBF-A12 and CBF-A15 genes, which distinguish a frost susceptible Fr-A2-S and tolerant Fr-A2-T haplotype. The two haplotypes were also associated with different copy numbers of CBF-A14 and showed an interaction with Vrn-A1 copy number variation. For Fr-B2 a nine-gene deletion in this cluster of eleven CBF genes, encompassing all except the two most proximal genes, was found to be characteristic for tetraploid wheat and results in reduced frost tolerance (Pearce et al. 2013). However, little is known on the effects of these loci and in general on the genetic control of frost tolerance in durum wheat.
The aim of this study was therefore, to phenotype and genotype a diverse set of 184 durum wheat lines in order to investigate the genetic control of frost tolerance. In particular, the objectives were to (1) determine the phenotypic variation of frost tolerance in durum wheat under field and semi-controlled conditions, (2) perform genome-wide association mapping to detect frost tolerance QTL and investigate markers for candidate loci, (3) analyze their effects and the proportion of explained genotypic variance in durum wheat as well as their geographic distribution, and (4) draw conclusions on the prospects of marker-assisted breeding for frost tolerance in winter durum wheat.
Materials and methods
Plant materials and field trials
In this study a diverse set of 184 durum wheat lines (T. durum) was used that has been described by Sieber et al. (2015). In brief, the set included 14 spring types, while the remaining 170 lines were winter types, comprising old as well as important modern varieties and breeding lines. The genotypes were grouped based on country of origin into six groups (Sieber et al. 2015): Genotypes from Austria and Germany (Group 1), France (Group 2), Canada and USA (Group 3), Italy and Spain (Group 4), Hungary (Group 5), and Bulgaria, Romania, Russia, Slovakia, Turkey, and Ukraine (Group 6).
Field trials were located at Hohenheim (Germany, Hoh) in 2012/13 and 2013/14, Probstdorf (Austria, Prob) in 2012/13 and 2013/14, and Seligenstadt (Germany, Selig) in 2013/14. The genotypes were sown in an α-lattice design with two replications as observation plots of two rows and 1.25 m length. In addition, the genotypes were sown in a semi-controlled test (SCT), the ‘Weihenstephaner Auswinterungsanlage’ (Hoeser 1954; Sieber et al. 2014), located at the field station Oberer Lindenhof on the Swabian Alb (Germany, Oli) in 2012/13, 2013/14 and 2014/15. The genotypes in the semi-controlled test were sown by hand in an α-lattice design with two replications in micro-plots of two rows and 0.2 m length (Fig. S1). The semi-controlled test is described in detail by Sieber et al. (2014). Temperature profiles of all locations are available in the supporting information (Fig. S2).
Evaluations followed the protocol described by Sieber et al. (2014). Emergence of the plants was noted before winter in the field as well as in the semi-controlled test. Frost tolerance in the field was determined after winter by scoring the plots for visually healthy and green plants on a scale from 1 to 9 (FTField: 1 = all plants healthy and green, 9 = all plants brown or no plant survival). Frost tolerance in the semi-controlled test (FTSCT in %) was evaluated as leaf damage caused by frost in percentage of discolored leaf area, 10 days after a frost period (Fig. S1, Fig. S2).
Phenotypic data analysis
To analyze the phenotypic data, a one-step procedure was applied, using the following model: y ijko = μ + g i + envj + (g × env)ij + repjk + b jko + e ijko, where y ijko denotes the phenotypic observation of the ith genotype tested at the jth environment in the oth incomplete block of the kth replication, μ is the conditional mean, g i the genetic effect of the ith genotype, envj the effect of the jth environment, (g × env)ij the genotype-by-environment interaction, repjk the effect of the kth replication at the jth environment, b jko the effect of the oth incomplete block of the kth replication at the jth environment and e ijko the residual. All effects were considered as random, except g i which was modelled as fixed effect to obtain the best linear unbiased estimates (BLUEs). Error variances were assumed to be heterogeneous among locations. The environment was defined as the combination of location and year. Variance components were determined by the restricted maximum-likelihood (REML) method assuming a random model (cf. Cochran and Cox 1957) and model comparison with likelihood ratio tests were used to test the significance of the variance component estimates (Stram and Lee 1994).
Heritability (h 2) was estimated as broad-sense heritability following the approach suggested by Piepho and Möhring (2007): h 2 = σ 2G / (σ 2G + (\(\overline{vd}\))/2), where σ 2G is the genotypic variance and \(\overline{vd}\) the mean variance of a difference of two adjusted genotype means (BLUEs).
Molecular data analysis and candidate genes
DNA was extracted from young leaves following standard procedures. Diversity Arrays Technology (DArT) Pty Ltd (Yarralumla, Australia) conducted genotyping-by-sequencing (GBS) with the DArTseq assay yielding silico-DArTs and single nucleotide polymorphism (SNP) markers. In the following the marker Clone IDs are given a ‘D’ or ‘S’ prefix for DArT and SNP markers, respectively. Markers that were monomorphic, had more than 20 % missing values or a minor allele frequency (MAF) of <5 % were not considered for further analyses. This resulted in 30,611 markers, for 13,431 of which a map position was known. More detailed information about the markers, procedures and relationships between the genotypes is given in Sieber et al. (2015).
In addition to the genome-wide markers, a candidate gene approach was employed. For the Fr-A2 locus the approach described by Zhu et al. (2014) was used. PCR products of CBF-A15 were digested by SalI and fragments of 403/605 bp identified the frost susceptible haplotype (Fr-A2-S) and the undigested fragment of 1017 bp the frost tolerant haplotype (Fr-A2-T). Genotyping for the deletion at the Fr-B2 locus was done following Pearce et al. (2013) using primers CBF-B9-pair 1, CBF-B4, CBF-B10, and CBF-B12-pair 2. CBF-B9 is located in the proximal gene cluster and was used as positive control as it is not part of the nine CBF genes that were found to be deleted in frost-susceptible durum lines. CBF-B4 is also located in the proximal cluster, CBF-B12 in the central cluster and CBF-B10 is the most distal gene in the distal cluster.
The protocol of Chen et al. (2009) was used to identify the allele variants vrn-A1a (350 bp, winter type with shorter vernalization requiremnet) and vrn-A1b (375 bp, winter type with longer vernalization requiremnet) at the Vrn-A1 locus. Additionally, all genotypes were analyzed for the Vrn-A1 alleles: Vrn-A1a (965 bp + 876 bp, spring type), Vrn-A1b (714 bp, spring type), Vrn-A1c (714 bp, spring type) and vrn-A1 (714 bp, winter type) (714 bp) (Yan et al. 2004; Zhang et al. 2008). Beside those known PCR fragments, one new fragment of approximately 680 bp was detected in 2 genotypes. Furthermore, plants were screened for polymorphisms at Vrn-B1 and Vrn-B3 (Chu et al. 2011; Yan et al. 2006), as well as for copy number variation (CNV) at Ppd-B1 (Beales et al. 2007; Díaz et al. 2012) and Vrn-A1 (Díaz et al. 2012; Zhu et al. 2014). CNV at CBF-A14 (Zhu et al. 2014) was scored but the signal ratio between target gene and control did not allow a clear grouping into distinct copy number classes and consequently the signal ratio itself was used for the subsequent analyses. The protocols for the CNV at Ppd-B1, Vrn-A1, and CBF-A14 were adapted from the protocol described by Langer et al. (2014).
Association mapping
To analyze the marker-trait associations, a genome-wide association scan (GWAS) and a candidate gene based scan were performed. A mixed linear model approach with a kinship matrix as variance–covariance matrix for the random genotypic effect was employed (Yu et al. 2006). Due to a high genome-wide inflation (λ) the model was fitted by the maximum likelihood implementation in the function polygenic of GenABEL (Aulchenko et al. 2007). P values obtained from the one-degree-of-freedom score test were corrected for possible inflation (Aulchenko et al. 2007). To account for multiple testing, a Bonferroni-corrected significance level of P < 0.05 was chosen. For the calculation of the proportion of genotypic variance explained by the detected QTL (ρ G ), the segregating candidate genes and all significant QTL were simultaneously fitted in a linear model in the order of the strength of their association. The ρ G values of the candidate genes and the significant QTL were then derived from the sums of squares of the QTL (SSMarker) in this linear model (Utz et al. 2000; Würschum et al. 2015b). Cross-validation was done as described previously (Würschum and Kraft 2014; Würschum et al. 2015b).
All calculations were done with the open-source programming language and statistical software R (R Core Team 2013) and the statistical software package ASReml-R 3.0 (Gilmour et al. 2009).
Development of functional markers
Two SNP markers from the genome-wide assay, Clone IDs S2269949 and S1077313, were converted into KASP-markers (http://www.lgcgroup.com/kasp/), which are codominant, competitive allele-specific PCR-based markers. The KASP assay for marker S2269949, comprised of the FAM-tailed primer (5′-FAM-tail-ACTGCAGCGAGGATTCCCA-3′) for the frost susceptible allele, the HEX tailed primer (5′-HEX-tail- CTACTGCAGCGAGGATTCCCG-3′) for the frost tolerant allele and the common primer (5′-AGCCACCTCTGAGATGCCGCTT-3′), was run as a standard KASP touchdown PCR program (http://www.lgcgroup.com/products/kasp-genotyping-chemistry/kasp-technical-resources/) with 68–62 °C as annealing temperatures decreasing by 0.6 °C per cycle to reach the final annealing temperature of 62 °C after 10 cycles. The assay for marker S1077313, comprised of the FAM-tailed primer (5′-FAM-tail-ATATGTCTCCCTCACCATGGCG-3′) for the frost susceptible allele, the HEX tailed primer (5′-HEX-tail-CATATGTCTCCCTCACCATGGCA-3′) for the frost tolerant allele and the common primer (5′-GTCAACTGCTTTGTACTGATTCGTGATAT-3′), was run as a standard KASP touchdown PCR program with 61–55 °C as annealing temperatures decreasing by 0.6 °C per cycle to reach the final annealing temperature of 55 °C after 10 cycles.
Results
Phenotypic variation for frost tolerance
The panel of 184 durum wheat genotypes, including 170 winter and 14 spring types, was evaluated for frost tolerance in the field (FTField) as well as in the semi-controlled test (FTSCT). For frost tolerance evaluated in the field only the ratings from the location Hohenheim in the seasons 2012/13 and 2013/14 could be used due to the lack of sufficiently cold temperatures and frosts at the other environments (Fig. S2). The genotypic variance σ 2G was significant (P < 0.05) for FTField and highly significant (P < 0.001) for FTSCT (Table 1). Estimates for the genotype-by-environment interaction variance σ 2G×E were highly significant and for FTSCT smaller than the genotypic variance. Heritability was medium for FTField with 0.56 and high for FTSCT with 0.79. The adjusted entry means for FTField were in a rather narrow range from 4.6 to 5.6 while for FTSCT the observed values ranged from 34 to 66 % leaf damage, thereby approximating a bimodal distribution (Fig. S1; Table 1). The small phenotypic variation observed in the field trials, resulting in a small genotypic variance and the medium heritability, substantiates the utility of the semi-controlled test, which not only yielded results in each year, but also a better differentiation of the genotypes and a higher heritability. Consequently, the following analyses were only performed based on the results obtained with the semi-controlled test.
The spring type group of this data set had a lower frost tolerance in the semi-controlled test with 60.4 % leaf damage, as compared to the winter type group with only 47.9 % leaf damage. Differences were also obtained between the different origins: Group 1 (Austria and Germany) had a mean of 49.3 % leaf damage, Group 2 (France) 60.4 %, Group 3 (Canada and USA) 51.8 %, Group 4 (Italy and Spain) 60.7 %, Group 5 (Hungary) 46.5 %, and Group 6 (Bulgaria, Romania, Russia, Slovakia, Turkey, and Ukraine) had a mean value of 45 % leaf damage.
Genome-wide association mapping and candidate loci analysis
After quality checks 30,611 markers remained which were used for the genome-wide association mapping and for 13,431 of these markers a map position was known. The genome-wide scan for marker-trait associations identified a single peak with 37 significantly associated markers (Table S1), located in the middle of chromosome 5A (Fig. 1).
With regard to markers for known polymorphisms in candidate genes, we found that Vrn-B1 and Vrn-B3 were monomorphic in this panel of durum lines. For the Vrn-A1 locus only the vrn-A1 variants segregated, and we observed the vrn-A1a spring allele (350 bp) in 104 genotypes and the vrn-A1b winter allele (375 bp) in 68 genotypes, while 12 genotypes could not be scored. For the Fr-A2 locus the CBF-A15 marker described by Zhu et al. (2014) that differentiates two haplotypes (Fr-A2-S and Fr-A2-T) was used, but of the 183 reliably scored lines only five carried the frost susceptible haplotype while the remaining 178 lines all carried the frost tolerant haplotype. For the Fr-B2 locus, we found that 171 lines had no deletion at this locus, 12 lines showed the nine-gene deletion reported for tetraploid wheat, and one genotype was identified that showed a deletion of the entire CBF cluster, as reported for bread wheat.
To assess copy number variation (CNV) of CBF-A14 at the Fr-A2 locus, the CNV assay was repeated four times and we observed only little variation among replications, indicating the technical robustness of the obtained signal ratios. However, the signal ratio between the CBF-A14 target gene and the TaCO2 control did not allow a clear-cut grouping into distinct copy number classes (Fig. 2). Nevertheless, the distribution of the signal ratio was not continuous but showed two larger groups of genotypes with a signal ratio of ~0.3 and ~0.7. Two lines had a slightly lower signal ratio and one a substantially higher one of 0.94. In addition, several genotypes showed a signal ratio in between the two major groups, with a continuous transition from the lower to the higher signal ratio. Copy number variation at the Vrn-A1 locus was also scored in this panel of durum lines, and 103, 12, 6, and 63 lines were found to carry the one, two, three or four copy number variant, respectively. In the group of spring types only the one-copy allele was found, except for one Italian variety (’Simeto’) having two copies. Of the candidate loci, CBF-A14 copy number variation at Fr-A2 was highly significantly associated with frost tolerance, with the association being even stronger than that observed for the most significantly associated marker, while Fr-B2 was weakly (P < 0.1) associated with frost tolerance.
As expected from the close proximity of the markers revealed in the Manhattan plot (Fig. 1), only two of these highly collinear markers explained more than 2 % of the genotypic variance. Marker S2269949 explained 82.2 % and marker S1077313 explained 8.5 % of the genotypic variance (Table 2). To investigate the genotypic variance explained by the candidate loci and the markers significantly associated with frost tolerance, both were simultaneously fitted in a linear model in the order of the strength of their association. This revealed that CBF-A14 copy number variation and Fr-B2 explained 91.6 % and 4.2 % of the genotypic variance, respectively (Fig. 2). Fitting the two markers after CBF-A14 in the model, reduced their proportion of explained genotypic variance to only 0.1 and 3.2 % for markers S2269949 and S1077313, respectively. These estimates for the proportion of explained genotypic variance were observed irrespective of whether all 184 lines or only the winter durum wheats were used.
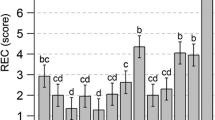
Consistent with the estimated α-effects, the homozygous classes of marker S2269949 and S1077313 showed a clear difference in frost damage (Fig. 3). As both markers explained a substantial proportion of the genotypic variance, we next formed a haploblock consisting of these two markers and observed four different haplotypes. The haplotype ‘AT’ consisting of the two frost tolerant alleles, ‘A’ for S2269949 and ‘T’ for S1077313, carried by 49.5 % of the lines (n = 91), had a substantially lower leaf damage compared to the other haplotypes. This haploblock was able to explain 93.2 % of the genotypic variance of frost tolerance (Table 2). To obtain a more unbiased estimate for this parameter, we performed a fivefold cross-validation and found that cross-validated, the haploblock still explained 90.4 % of the genotypic variance.
Frost tolerance (FTSCT, leaf damage in %) in the semi-controlled test (SCT) for genotypes carrying different candidate gene alleles, copy numbers and detected QTL alleles. Numbers underneath the box plots indicate the number of genotypes in each group. The mean value for the traits in each group is given by mean FTSCT. Non-overlapping notches indicate statistically significant differences between the medians
We next assessed the frequency of copy number variation at CBF-A14 and Vrn-A1, the nine-gene deletion at Fr-B2, and the alleles at the two markers, as well as the frost tolerant haplotype, dependent on the geographic origin of the genotypes, employing the groups defined above. For only one of the 184 durum wheat genotypes the origin was unknown. The origin was defined as the country of registration or in case of the breeding or unregistered lines, the country of the breeder (Fig. 4). For CBF-A14 copy number variation, the average signal ratio of the groups was lowest in Groups 2 and 4, intermediate in Groups 1, 3, and 5, and highest in Group 6. The frequency of the nine-gene deletion at Fr-B2 also varied between groups, with the highest frequency in Group 1 and the lowest in Group 5. For copy number variation at Vrn-A1, we observed a general trend for increasing copy numbers moving from south to north within Europe. The frost tolerant haplotype has a frequency of 0.49 in the entire population and within the six groups the frequency varied from 0.00 in Group 2 and 4 to 0.68 in Group 6. Both markers of the haploblock are in strong LD (r 2 = 0.79) and therefore showed a similar pattern and the frequencies of the frost tolerant alleles at both markers resembled those of the haplotype.
Establishment of functional markers
The two markers that were used to form the haploblock, enabling a very high predictive power for the proportion of genotypic variance, are both SNP markers. In order to make this haploblock useable for applied marker-assisted breeding, we converted these two markers obtained from a genome-wide assay, into single marker assays. To enable a cheap, fast and high-throughput screening, we chose to convert them into competitive allele-specific PCR based (KASP) markers. For both S2269949 and S1077313 a KASP assay could be established that allows to separate the three genotypic classes (Fig. 5). We genotyped the entire durum panel and verified the functionality of both markers by obtaining the same high predictive power of the haploblock.
Discussion
Frost tolerance is an important trait in cereals and other crops, particularly in growing regions with harsh winters and regularly occurring frost periods. In durum wheat the improvement of frost tolerance is essential for the establishment of winter durum and the aim of this study was therefore, to investigate the genetic control of frost tolerance towards a marker-based breeding.
Phenotypic evaluation of frost tolerance in durum wheat
The ability of a breeder to select for frost tolerance strongly depends on the occurrence and the intensity of this abiotic stress at a particular location and year. Absence of sufficiently cold temperatures or protection of the plants in the field by an isolating snow coverage can prohibit selection. In our case, only two of the field trials conducted at five environments could be scored for frost tolerance and the obtained phenotypic data showed only a small variation. Additionally, high variance for genotype-by-environment interaction resulted in a relatively low heritability (h 2 = 0.56) compared to other results in durum wheat (h 2 = 0.89; Sieber et al. 2014), bread wheat (h 2 = 0.89; Zhao et al. 2013), or triticale (h 2 = 0.87 and 0.91; Liu et al. 2014) and the field data were therefore not investigated further. By contrast, the semi-controlled test produced highly heritable data (h 2 = 0.79), confirming results from durum wheat of other years (h 2 = 0.83–0.88; Longin et al. 2013; Sieber et al. 2014). Thus, the semi-controlled test (Fig. S1) employed at a location with recurring frosts is a valuable tool that enables a reliable selection for this trait in breeding programs.
We observed differences in frost tolerance, depending on the country of origin of the durum lines. Generally, lines from the more northern countries and especially those from the more eastern, continental countries showed a higher frost tolerance. This indicates different requirements for frost tolerance and consequently selection intensities in the different growing regions. It is assumed that the origin of winter hardiness including frost tolerance in durum wheat lies in Russia, the Ukraine, and a few other countries surrounding the Black Sea, which were then used to create the winter durum lines of Central Europe (Akar et al. 2009; Palamarchuk 2005; Szucs et al. 2003). Durum wheat from Central Europe on the other hand is known for its excellent quality (Longin et al. 2013) and consequently, targeted crosses with genotypes from group 1 and group 5 or 6, are promising to further enhance traits related to winter hardiness in European winter durum germplasm, while maintaining the high quality.
The observed variation between groups but also within the Central European winter wheat shows that the trait is genetically not fixed yet, indicating the presence of different QTL alleles. Although high quality phenotypic selection can be conducted using the semi-controlled test, identification of frost tolerance QTL and their utilization in marker-assisted breeding would help to improve the efficiency of winter durum breeding programs.
Genetic architecture of frost tolerance in winter durum wheat
The distribution of the phenotypic values obtained in the semi-controlled test approximated a bimodal distribution with peaks at about 40 and 60 % leaf damage (Fig. S1), indicating the contribution of a major QTL to the expression of this trait. In agreement with the bimodal distribution, the genome-wide scan identified only a single peak on chromosome 5A (Fig. 1). This chromosome harbors both Vrn-A1 and Fr-A2 and is therefore well known to be involved in the response to vernalization requirement and frost tolerance (Sutka and Snape 1989; Vágújfalvi et al. 2003). Vrn-A1 is the more distally located of the two loci and as the identified QTL (S2269949) mapped to the middle of chromosome 5A at 111.66 cM, we concluded, that the QTL is either a novel frost tolerance locus or corresponds to Fr-A2. We found that another marker (S2277317, association P value 1.3e-08) mapped also at 111.66 cM and is located in the center of the Fr-A2 locus, 21.9 kb proximal of CBF-A15, as its sequence was found in the Triticum monococcum BAC 60J11, which harbors the Fr-A m 2 locus. Together with the well-established importance of Fr-A2 in frost tolerance this strongly supported the conclusion that the identified QTL is indeed Fr-A2. The three adjacent genes, CBF-A12, CBF-A14, and CBF-A15, are located in the central cluster of the eleven-gene Fr-A2 locus and are assumed to play a critical role in frost tolerance (Knox et al. 2008; Pearce et al. 2013; Zhu et al. 2014). We first evaluated the candidate gene marker reported by Zhu et al. (2014) that distinguishes a frost tolerant and susceptible haplotype, each with a number of characteristic polymorphisms in CBF-A12 and CBF-A15. However, in contrast to the frequency of the identified major QTL with 55 % in this population, only five individuals carried the frost susceptible haplotype for the candidate gene marker. This suggested another causal polymorphism in Fr-A2 underlying the identified major QTL.
Copy number variation (CNV) is another type of DNA polymorphism that results in multiple copies of a gene being present at a locus. CNV are well researched in humans (Redon et al. 2006), but the relevance of CNV in plants and especially in crops is just beginning to be understood (Żmieńko et al. 2014). All three genes of the central cluster have recently been shown to be present in different copy numbers and particularly CBF-A14 copy number variation has been shown to be associated with frost tolerance in cereals (Knox et al. 2010; Zhu et al. 2014). The CBF-A14 to control gene signal ratios indicated two major groups of copy number variants but also revealed a number of genotypes with a signal ratio somewhere in between the two major groups. These were very consistently observed across 4 replications and thus appear to be technically robust. As it is unlikely that all these ratios in between correspond to different copy number variants, this may be caused by residual heterozygosity in some of the lines or may indicate a certain level of cross-hybridization signal from other chromosomal regions in some genotypes, thereby obscuring the true signal ratio. It was therefore not possible to unambiguously assign the genotypes to copy number classes illustrating that the detailed characterization of this locus requires further work on the molecular level. Nevertheless, using the signal ratio for the analyses, we found that CBF-A14 copy number variation was most strongly associated with frost tolerance and explained 91.6 % of the genotypic variance. While it cannot be ruled out that the causal polymorphism is copy number variation at one of the closely linked CBF genes at the Fr-A2 locus, CBF-A14 appears the most likely candidate as previous work indicated a primary role of this gene in frost tolerance (Zhu et al. 2014).
The high number of polymorphic markers in the QTL region resulted in 37 significantly associated markers. Correcting for collinearity by simultaneously fitting them in a linear model, resulted in two markers explaining 82.2 and 8.5 % of the genotypic variance (Table 2). However, in a model with CBF-A14 copy number variation, the two markers could not explain any genotypic variance any more, suggesting that they were identified due to their association with CBF-A14 CNV. We reasoned that the two markers which have the same map position, likely flank the causal polymorphism but in light of the causal polymorphism being copy number variation, it appears likely that neither of the two biallelic markers with their two states is able to fully capture the variation of CBF-A14 CNV which is probably caused by more than two variants (Fig. 3a). We therefore combined both markers into a haploblock which explained 93.2 % of the genotypic variance. This is slightly more than explained by CBF-A14 CNV itself which might corroborate the conclusion of an altered signal ratio in some genotypes.
Taken together, our results illustrate that the genetic architecture of frost tolerance in durum wheat is controlled by a single major QTL, likely being copy number variation of CBF-A14 at the Fr-A2 locus, with small contributions from Fr-B2 and some other unidentified small effect QTL.
Geographic patterns of frost tolerant alleles
The different frost tolerance in the five groups of durum defined previously (Sieber et al. 2015) suggested a differential use of the major frost tolerance QTL. While the frost tolerant haplotype of the major QTL was not present in Group 2 (France), it segregated in all other groups (Fig. 4). There was, however, a trend for higher frequencies of this haplotype in regions with colder winters and more regularly occurring frosts, as the lowest frequency was found for Group 4 (Italy and Spain) and the highest frequency for Group 6 (continental Europe). While the absence of clear-cut copy number classes did not allow the same analysis with CBF-A14 copy number variation, the average signal ratio mirrored the geographic pattern observed for the haplotype. This indicates that the Fr-A2 locus is already the target of selection and has likely been used in breeding programs to increase frost tolerance in the respective germplasm. A similar trend was observed for Fr-B2 for which the frost tolerant allele is almost fixed in the regions with harsher winters.
Díaz et al. (2012) found that Vrn-A1 can be present at one to three copies in wheat and that an increased copy number results in an increased vernalization requirement. Furthermore, Würschum et al. (2015a) reported a four copy variant and showed a clear geographic trend of the Vrn-A1 copy number variations, suggesting a role in adaptation. We observed a similar South to North trend in durum wheat, with the higher copy number variants becoming increasingly present when moving from South to North (Fig. 4). As this pattern is unlikely to have arisen by chance, this indicates that although Vrn-A1 copy number variation did not appear to be involved in the regulation of frost tolerance, it is likely involved in the adaptation of durum wheat to regions where an extended vernalization requirement increases winter survival.
Conclusions for frost tolerance breeding in durum wheat
Our analyses revealed that frost tolerance in winter durum can almost be considered as monogenic, with the major QTL Fr-A2 explaining about 90 % of the genotypic variance while the second identified locus Fr-B2 explains a comparably negligible proportion of around 4 %. This presents an ideal situation for the implementation of marker-assisted selection, which works best with a genetic architecture of one or few major QTL as opposed to many small effect QTL. In order to make the two identified markers useable for applied durum breeding, we converted them into functional KASP markers, which together constitute the haploblock described above. This haploblock provides a very high predictive power and cross-validated still explains 90.4 % of the genotypic variance of frost tolerance. Notably, this haploblock only selects for frost tolerance while other traits also contributing to winter hardiness must be improved in parallel. Interestingly, the frost tolerant allele of the identified major QTL is not fixed yet in Central European durum wheat or in any of the six durum groups and consequently, the established markers should provide a valuable tool for a marker-based breeding of frost tolerance in durum wheat.
Author contribution statement
CFHL and TW designed the study. ANS collected phenotypic data and performed the analyses. ANS, CFHL, WLL and TW wrote the paper.
References
Akar T, Mert Z, Yazar S, Sanal T, Avci M (2009) Sustainable use of winter durum wheat landraces under Mediterranean conditions. Afr J Biotechnol 8:4108–4116
Aulchenko YS, Ripke S, Isaacs A, van Duijn CM (2007) GenABEL: an R library for genome-wide association analysis. Bioinformatics 23:1294–1296. doi:10.1093/bioinformatics/btm108
Beales J, Turner A, Griffiths S, Snape JW, Laurie DA (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet 115:721–733. doi:10.1007/s00122-007-0603-4
Chen Y, Carver BF, Wang S, Zhang F, Yan L (2009) Genetic loci associated with stem elongation and winter dormancy release in wheat. Theor Appl Genet 118:881–889. doi:10.1007/s00122-008-0946-5
Chu C, Tan CT, Yu G, Zhong S, Xu SS, Yan L (2011) A novel retrotransposon inserted in the dominant Vrn-B1 allele confers spring growth habit in tetraploid wheat (Triticum turgidum L.). G3 (Bethesda) 1:637–645. doi: 10.1534/g3.111.001131
Cochran WG, Cox GM (1957) Experimental designs. Wiley, New York
Dhillon T, Pearce SP, Stockinger EJ, Distelfeld A, Li C, Knox AK, Vashegyi I, Vágújfalvi A, Galiba G, Dubcovsky J (2010) Regulation of freezing tolerance and flowering intemperate cereals: the VRN-1 connection. Plant Physiol 153:1846–1858. doi:10.1104/pp.110.159079
Díaz A, Zikhali M, Turner AS, Isaac P, Laurie DA (2012) Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS One 7:e33234. doi:10.1371/journal.pone.0033234
Gilmour AR, Gogel BJ, Cullis BR, Thompson R (2009) ASReml user guide release 3.0. International Ltd, Hemel Hempstead
Hoeser K (1954) Testing winter wheat for winter kill [Über die Prüfung von Winterweizen auf Winterfestigkeit in Auswinterungskästen]. Züchter 24:353–357
Kling CI, Utz HF, Münzing K (2006) Autumn sowing of durum wheat-effects on quality and yield [Herbstanbau von Durumweizen–Auswirkungen auf Qualität und Ertrag]. Getreidetechnologie 60:141–147
Knox AK, Li C, Vágújfalvi A, Galiba G, Stockinger EJ, Dubcovsky J (2008) Identification of candidate CBF genes for the frost tolerance locus Fr-Am 2 in Triticum monococcum. Plant Mol Biol 67:257–270. doi:10.1007/s11103-008-9316-6
Knox AK, Dhillon T, Cheng H, Tondelli A, Pecchioni N, Stockinger EJ (2010) CBF gene copy number variation at Frost Resistance-2 is associated with levels of freezing tolerance in temperate-climate cereals. Theor Appl Genet 121:21–35. doi:10.1007/s00122-010-1288-7
Lafferty J (2010) Durum-between yield and quality? In: Gesellschaft für Pflanzenzüchtung e.V. (ed), Tagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs. Lehr- und Forschungszentrum für Landwirtschaft, Gumpenstein, pp 41–44
Langer SM, Longin Friedrich CFH, Würschum T (2014) Flowering time control in European winter wheat. Front Plant Sci 5:537. doi:10.3389/fpls.2014.00537
Liu W, Maurer HP, Li G, Tucker MR, Gowda M, Weissmann EA, Hahn V, Würschum T (2014) Genetic architecture of winter hardiness and frost tolerance in triticale. PLoS One 9(6):e99848. doi:10.1371/journal.pone.0099848
Longin CFH, Sieber A-N, Reif JC (2013) Combining frost tolerance, high grain yield and good pasta quality in durum wheat. Plant Breed 132:353–358. doi:10.1111/pbr.12064
Miller A, Galiba G, Dubcovsky J (2006) A cluster of 11 CBF transcription factors is located at the frost tolerance locus Fr-Am 2 in Triticum monococcum. Mol Gen Genomics 275:193–203. doi:10.1007/s00438-005-0076-6
Palamarchuk A (2005) Selection strategies for traits relevant for winter and facultative durum wheat. In: Royo C, Nachit M, Di Fonzo N, Araus J (eds) Durum wheat breeding: Current approaches and future strategies, vol 2. The Haworth Press Inc, New York, pp 599–644
Pearce S, Zhu J, Boldizsár Á, Vágújfalvi A, Burke A, Garland-Campbell K, Galiba G, Dubcovsky J (2013) Large deletions in the CBF gene cluster at the Fr-B2 locus are associated with reduced frost tolerance in wheat. Theor Appl Genet 126:2683–2697. doi:10.1007/s00122-013-2165-y
Piepho HP, Möhring J (2007) Computing heritability and selection response from unbalanced plant breeding trials. Genetics 77:1881–1888. doi:10.1534/genetics.107.074229
R Core Team (2013) R a language and environment for statistical computing. R foundation for statistical computing, Vienna
Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews T Daniel, Fiegler H, Shapero MH, Carson AR, Chen W, Cho EK, Dallaire S, Freeman JL, Gonzalez JR, Gratacos M, Huang J, Kalaitzopoulos D, Komura D, MacDonald JR, Marshall CR, Mei R, Montgomery L, Nishimura K, Okamura K, Shen F, Somerville MJ, Tchinda J, Valsesia A, Woodwark C, Yang F, Zhang J, Zerjal T, Zhang J, Armengol L, Conrad DF, Estivill X, Tyler-Smith C, Carter NP, Aburatani H, Lee C, Jones KW, Scherer SW, Hurles ME (2006) Global variation in copy number in the human genome. Nature 444:444–454. doi:10.1038/nature05329
Sieber A-N, Würschum T, Longin CFH (2014) Evaluation of a semi-controlled test as a selection tool for frost tolerance in durum wheat (Triticum durum). Plant Breed 133:465–469. doi:10.1111/pbr.12181
Sieber A-N, Longin CFH, Würschum T (2015) Molecular characterization of winter durum wheat (Triticum durum) based on a genotyping-by-sequencing approach. Plant Genetic Resour. doi:10.1017/S1479262115000349
Stram DO, Lee JW (1994) Variance components testing in the longitudinal mixed effects model. Biometrics 50:1171–1177. doi:10.2307/2533455
Sutka J, Snape JW (1989) Location of a gene for frost resistance on chromosome 5A of wheat. Euphytica 42:41–44
Szucs P, Veisz O, Vida G, Bedo Z (2003) Winter hardiness of durum wheat in Hungary. Acta Agronomica Hung 51:389–396. doi:10.1556/AAgr.51.2003.4.3
Tóth B, Galiba G, Fehér E, Sutka J, Snape JW (2003) Mapping genes affecting flowering time and frost resistance on chromosome 5B of wheat. Theor Appl Genet 107:509–514. doi:10.1007/s00122-003-1275-3
Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES (2003) MADS box genes control vernalization-induced flowering in cereals. PNAS 100:13099–13104. doi:10.1073/pnas.1635053100
Utz HF, Melchinger AE, Schön CC (2000) Bias and sampling error of the estimated proportion of genotypic variance explained by quantitative trait loci determined from experimental data in maize using cross validation and validation with independent samples. Genetics 154:1839–1849
Vágújfalvi A, Galiba G, Cattivelli L, Dubcovsky J (2003) The cold-regulated transcriptional activator Cbf3 is linked to the frost-tolerance locus Fr-A2 on wheat chromosome 5A. Mol Gen Genomics 269(1):60–67. doi:10.1007/s00438-003-0806-6
Vágújfalvi A, Aprile A, Miller A, Dubcovsky J, Delugu G, Galiba G, Cattivelli L (2005) The expression of several Cbf genes at the Fr-A2 locus is linked to frost resistance in wheat. Mol Gen Genomics 274(5):506–514. doi:10.1007/s00438-005-0047-y
Würschum T, Kraft T (2014) Cross-validation in association mapping and its relevance for the estimation of QTL parameters of complex traits. Heredity 112(4):463–468. doi:10.1038/hdy.2013.126
Würschum T, Boeven PH, Langer SM, Longin CFH, Leiser WL (2015a) Multiply to conquer: copy number variations at Ppd-B1 and Vrn-A1 facilitate global adaptation in wheat. BMC Genet 16:96. doi:10.1186/s12863-015-0258-0
Würschum T, Langer SM, Longin CFH (2015b) Genetic control of plant height in European winter wheat cultivars. Theor Appl Genet 128:865–874. doi:10.1007/s00122-015-2476-2
Yan L, Helguera M, Kato K, Fukuyama S, Sherman J, Dubcovsky J (2004) Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor Appl Genet 109:1677–1686. doi:10.1007/s00122-004-1796-4
Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. PNAS 103:19581–19586. doi:10.1073/pnas.0607142103
Yu J, Pressoir G, Briggs WH, Vroh Bi I, Yamasaki M, Doebley JF, McMullen MD, Gaut BS, Nielsen DM, Holland JB, Kresovich S, Buckler ES (2006) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nature Genet 38:203–208. doi:10.1038/ng1702
Zhang XK, Xiao YG, Zhang Y, Xia XC, Dubcovsky J, He ZH (2008) Allelic variation at the vernalization genes, and in Chinese wheat cultivars and their association with growth habit. Crop Sci 48(2):458. doi:10.2135/cropsci2007.06.0355
Zhao Y, Gowda M, Würschum T, Longin C, Friedrich H, Korzun V, Kollers S, Schachschneider R, Zeng J, Fernando R, Dubcovsky J, Reif JC (2013) Dissecting the genetic architecture of frost tolerance in Central European winter wheat. J Exp Bot 64:4453–4460. doi:10.1093/jxb/ert259
Zhu J, Pearce S, Burke A, See DR, Skinner DZ, Dubcovsky J, Garland-Campbell K (2014) Copy number and haplotype variation at the VRN-A1 and central FR-A2 loci are associated with frost tolerance in hexaploid wheat. Theor Appl Genet 127:1183–1197. doi:10.1007/s00122-014-2290-2
Żmieńko A, Samelak A, Kozłowski P, Figlerowicz M (2014) Copy number polymorphism in plant genomes. Theor Appl Genet 127:1–18. doi:10.1007/s00122-013-2177-7
Acknowledgments
This research was funded by the Deutsche Forschungsgemeinschaft (Grant ID: LO 1816/2-1). For conductance of field trials, we thank Dr. Julia Lafferty from Saatzucht Donau GesmbH & CoKG, Otto Kram from KWS LOCHOW GMBH, and Helmut Bimek from the University of Hohenheim. Furthermore we thank Angela Harmsen and Barbara Renz for excellent technical assistance in the laboratory and Bernd Habeck, Sabit Recaj, Bianca Yildirim, Verena Till, Sabine Frey-Tippelt, Ortwin Schildmann, Marcel Mosdorf, and Martina Schnieder for outstanding work in the field. Many thanks to Anna Tyler from Jackson Laboratory for the helpful advices in running cape.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The authors declare that the experiments comply with the current laws of Germany.
Additional information
Communicated by X. Xia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sieber, AN., Longin, C.F.H., Leiser, W.L. et al. Copy number variation of CBF-A14 at the Fr-A2 locus determines frost tolerance in winter durum wheat. Theor Appl Genet 129, 1087–1097 (2016). https://doi.org/10.1007/s00122-016-2685-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-016-2685-3