Abstract
Key message
The Rpp6 locus of PI 567102B was mapped from 5,953,237 to 5,998,461 bp (chromosome 18); and a novel allele at the Rpp6 locus or tightly linked gene Rpp[PI567068A] of PI 567068A was mapped from 5,998,461 to 6,160,481 bp.
Abstract
Soybean rust (SBR), caused by the obligate, fungal pathogen Phakopsora pachyrhizi is an economic threat to soybean production, especially in the Americas. Host plant resistance is an important management strategy for SBR. The most recently described resistance to P. pachyrhizi (Rpp) gene is Rpp6 contributed by PI 567102B. Rpp6 was previously mapped to an interval of over four million base pairs on chromosome 18. PI 567068A was recently demonstrated to possess a resistance gene near the Rpp6 locus, yet PI 567068A gave a differential isolate reaction to several international isolates of P. pachyrhizi. The goals of this research were to fine map the Rpp6 locus of PI 567102B and PI 567068A and determine whether or not PI 567068A harbors a novel Rpp6 allele or another allele at a tightly linked resistance locus. Linkage mapping in this study mapped Rpp6 from 5,953,237 to 5,998,461 bp (LOD score of 58.3) and the resistance from PI 567068A from 5,998,461 to 6,160,481 bp (LOD score of 4.4) (Wm82.a1 genome sequence). QTL peaks were 139,033 bp apart from one another as determined by the most significant SNPs in QTL mapping. The results of haplotype analysis demonstrated that PI 567102B and PI 567068A share the same haplotype in the resistance locus containing both Rpp alleles, which was designated as the Rpp6/Rpp[PI567068A] haplotype. The Rpp6/Rpp[PI567068A] haplotype identified in this study can be used as a tool to rapidly screen other genotypes that possess a Rpp gene(s) and detect resistance at the Rpp6 locus in diverse germplasm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soybean rust (SBR), caused by the obligate, basidiomycete pathogen Phakopsora pachyrhizi Syd., is a detrimental disease to soybean (Glycine max L. Merill) production. P. pachyrhizi has a broad host range and is capable of infecting over 50 genera of plants (Lynch et al. 2006a, b; Ono et al. 1992; Slaminko et al. 2008a, b). Susceptible soybean genotypes typically develop lesions on the abaxial side of their leaves that form uredinia and subsequently urediniospores that are primarily disseminated to other plants by wind (Goellner et al. 2010). The susceptible lesion type of soybean is referred to as TAN; due to the often tan-colored lesion type that is associated with uredinia and high levels of sporulation. Resistant genotypes are defined by a reddish-brown (RB) lesion that can be sporulating or non-sporulating; or immunity (IM), where plants have no visible lesion to the naked eye after being challenged with P. pachyrhizi (Miles et al. 2006; Walker et al. 2014a, b; Harris et al. 2015).
Soybean rust was first reported in the continental USA in 2004 in Louisiana, and may have been transported by hurricane Ivan (Isard et al. 2005; Schneider et al. 2005). P. pachyrhizi has low to no infectivity after freezing temperatures; therefore, SBR is more prevalent in the Southern USA (Jurick et al. 2008). In the USA, it was estimated that Alabama, Arkansas, Georgia, Louisiana, North Carolina, Oklahoma, South Carolina, and Texas had 53.65 million metric tons of yield losses from 2005 to 2007 due to SBR (Wrather and Koenning 2009).
Developing cultivars with host plant resistance is the preferred means of managing SBR, allowing for minimal reliance on fungicides and fossil fuels (Hartman et al. 2005, 2011). Six resistance loci to P. pachyrhizi (Rpp), Rpp1 to Rpp6 have been reported that harbor at least 10 described resistance alleles, named in order of discovery: Rpp1, Rpp1-b, Rpp2, rpp2, Rpp3, Rpp?(Hyuuga), Rpp4, Rpp5, rpp5, and Rpp6 (Bromfield and Hartwig 1980; Chakraborty et al. 2009; Garcia et al. 2008; Hartwig 1986; Hartwig and Bromfield 1983; Li et al. 2012; McLean and Byth 1980; Monteros et al. 2007).
Mapping work of the Rpp1 (PI 200492) and Rpp3 (PI 462312) loci was performed using the P. pachyrhizi India 1973 (IN73-1) isolate (Hyten et al. 2007, 2009). Rpp2 (PI 230970) was fine mapped using the Georgia 2008 bulk isolate (GA08) (Yu et al. 2015). The rpp2 (PI 224270), Rpp5 alleles (sources are PI 200487, PI 200526, and PI 471904), and rpp5 (PI 200456) were mapped using a Cambé, Brazil 2004 isolate (BZ04) (Garcia et al. 2008). Rpp?(Hyuuga) (PI 506764) was mapped using the Georgia 2005 bulk isolate (GA05) (Monteros et al. 2007). Rpp4 (PI 459025B) was fine mapped using a Brazilian isolate; the area it was collected from within Brazil was not disclosed (Meyer et al. 2009). Rpp6 (PI 567102B) was mapped using the Louisiana 2004 (LA04-1) and Mississippi 2006 (MS06-1) isolates (Li et al. 2012).
In the Southeastern USA, Rpp1, Rpp2, rpp2, Rpp3, Rpp?(Hyuuga), Rpp4, and Rpp6 have been shown to condition varying levels of resistance to SBR (Walker et al. 2014a, b). The source of Rpp1-b does not provide effective resistance against field populations of P. pachyrhizi in the Southeastern USA (Walker et al. 2014a). PI 200487 and PI 471904 are believed to contain the Rpp resistance alleles at the Rpp3 and Rpp5 loci and have effective resistance against soybean rust in the southeastern USA (Kendrick et al. 2011; Walker et al. 2014a, b). PI 200526, which only has a known Rpp gene at the Rpp5 locus, is susceptible to P. pachyrhizi in the southeastern USA (Kendrick et al. 2011; Walker et al. 2014b).
Breeding efforts to introgress Rpp genes into elite germplasm have been successful. Diers et al. (2013) developed eight elite near isogenic lines (NILs) with Rpp1 (PI 200492), Rpp1-b (PI 594538A), Rpp?(Hyuuga) (PI 506764), Rpp5 (PI 200456), or Rpp5 PI 471904 alleles. In each NIL, the Rpp gene of interest was integrated via marker-assisted backcrossing into the elite lines, LD01-7323 and LD00-3309 in maturity group (MG) II and IV, respectively. The NILs with the various Rpp genes yielded as well as their recurrent parents (Diers et al. 2013). G01-PR16 (PI 659503) is another example of an elite germplasm line that contains an Rpp gene. G01-PR16 was developed as an MG VI germplasm line with the Rpp?(Hyuuga) allele contributed by PI 506764, and demonstrated 90 % of the yield of its elite parent ‘Dillon’ (PI 592756) (Boerma et al. 2011). These examples illustrate that marker-assisted breeding can be used successfully to develop useful germplasm for the areas at risk for SBR epidemics. Additionally, NILs possessing different Rpp genes could allow breeders to pyramid-specific Rpp genes in the same genetic background.
Rpp6 from PI 567102B was previously mapped to chromosome (Chr) 18 (Li et al. 2012). Rpp6 is a single, dominant gene that was mapped using two independent populations, whereby each population was phenotyped with the P. pachyrhizi isolates MS06-1, or LA04-1. Linkage mapping in each population placed Rpp6 on Chr 18 between the simple sequence repeat (SSR) markers Satt324 and Satt394, with an interval of over 4 Mb (Wm82.a1 genome sequence). Resistant progeny from either population developed an IM or RB lesion phenotype when challenged with the MS06-1 or the LA04-1 isolate (Li et al. 2012).
When Rpp genes map to the same locus, new potential alleles or tightly linked Rpp genes may be differentiated from one another using a panel of diverse P. pachyrhizi isolates, or a single P. pachyrhizi isolate that is informative. For example, PI 200492, the source of Rpp1 is susceptible to the Zimbabwe 2001 (ZM01-1) isolate, while PI 594538A, the source of Rpp1-b is resistant to the ZM01-1 isolate, which was used to map the Rpp1-b allele (Chakraborty et al. 2009). Hyuuga was originally believed to have a novel allele at the Rpp3 locus (Monteros et al. 2007). A combination of recombinant inbred line (RIL) mapping and a panel of eight geographically diverse P. pachyrhizi isolates was used to identify that the cultivar ‘Hyuuga’ (PI 506764) harbors two Rpp genes (Rpp3 and Rpp5) (Kendrick et al. 2011). Therefore, differential isolates can differentiate between resistance alleles and identify multiple Rpp genes that a single isolate may not be able to identify.
P. pachyrhizi isolates Columbia 2004 (CO04-2), Hawaii 1998 (HW98-1), India 1973 (IN73-1), Louisiana 2004-1 (LA04-1), South Africa 2001 (SA01-1), Taiwan 1972 (TW72-1), Louisiana 2004-3 (LA04-3), Zimbabwe 2001 (ZM01-1), and Australia 1979 (AU79-1) are regularly used by the USDA-ARS Foreign Disease-Weed Science Research Unit (Ft. Detrick, MD) singularly or in multiple tests to differentiate types of resistance or to assist mapping Rpp genes and have been described in detail (Chakraborty et al. 2009; Harris et al. 2015; Hyten et al. 2007, 2009; Kendrick et al. 2011; Pham et al. 2009). When Harris et al. (2015) challenged PI 567102B with this panel of isolates, it reacted with RB-resistant lesions for all isolates except TW72-1, to which PI 567102B reacted with a mixed reaction of plants that had TAN or RB lesions. Results of bulked segregant analysis (BSA) indicated that the resistance of PI 567068A was located within 5 cM of the Rpp6 locus, and PI 567068A had RB-resistant reactions to HW98-1, LA04-1, and LA04-3; however, it reacted with TAN lesions when challenged with the isolates ZM01-1, AU79-1, SA01-1, and TW72-1. Additionally, PI 567068A did not have haplotype allele matches for the Rpp1 or Rpp4 loci defined by Harris et al. (2015) that are also on Chr 18. These data supported that PI 567068A may harbor another allele at the Rpp6 locus, or may possess a new gene that is linked to the Rpp6 locus of PI 567102B (Harris et al. 2015).
Recently, a SoySNP50K iSelect SNP BeadChip was developed with Illumina and used to genotype G. max and G. soja in the USDA Soybean Germplasm Collection (Song et al. 2013; http://soybase.org/dlpages/index.php#snp50k). This has provided a wealth of genomic information, as the 50 K SNPs span primarily euchromatic regions and cover all 20 chromosomes of the G. max and G. soja genomes. Polymorphisms are now easily located by accessing Soybase data (soybase.org) for mapping regions of interest of the soybean genome. Additionally, Kompetitive Allele Specific PCR (KASP) marker assays can be developed for reliable and cost-efficient genotyping and QTL mapping using the SNP data and sequence surrounding the SNP (Pham et al. 2013). Regions of the soybean genome associated with Rpp genes can be translated to the SoySNP50K data. Harris et al. (2015) used SoySNP50K data, in combination with BSA, and diverse panels of P. pachyrhizi isolates as tools to rapidly screen PIs with known Rpp gene resistance. They were able to identify PIs that likely harbor the same Rpp genes in different PI sources, or to identify PIs with putatively novel resistance. This approach allowed Harris et al. (2015) to define haplotype windows using the SoySNP50K data for Rpp1, Rpp3, and Rpp4. Yu et al. (2015) recently fine mapped the Rpp2 locus and defined the unique haplotype window of this locus. One of the PIs identified by Harris et al. (2015) that putatively contained a novel mode of Rpp resistance near the Rpp6 locus was PI 567068A. The objectives of this study were to: map the Rpp gene from PI 567068A and saturate the resistance gene locus from PI 567102B with SNP markers to determine if the resistance allele from PI 567102B is allelic to the Rpp6 allele from PI 567068A.
Materials and methods
Plant material and population development
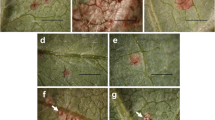
PI 567068A was selected for mapping because BSA data and differential P. pachyrhizi isolate data compared to PI 567102B (Rpp6) supported that PI 567068A possessed a putatively novel resistance allele within 5 cM of the Rpp6 locus (Harris et al. 2015; Li et al. 2012). Genetic mapping populations were created by crossing a susceptible, elite cultivar or breeding line to a plant introduction (PI) with known soybean rust (SBR) resistance. The cross of ‘Prichard’ (PI 612157) × PI 567068A was made in the summer of 2011 in Athens, GA. The F1 plants were grown in the winter (2011–2012) in the University of Georgia (UGA) greenhouse located in Athens, GA. F2 plants were grown in the summer of 2012 and threshed individually to form the F2:3 families. Prichard, a maturity group (MG) VIII cultivar released from UGA with white flowers, gray pubescence, and tan pod walls (Boerma et al. 2001), is susceptible to the Georgia 2012 bulk P. pachyrhizi isolate (GA12) (Fig. 1; Walker et al. 2014b; Harris et al. 2015).
The reactions of mapping population parents to the Georgia 2012 (GA12) P. pachyrhizi bulk isolate: a PI 567102B (Rpp6), b G00-3213, c PI 567068A, and d Prichard. G00-3213 and Prichard had TAN, highly sporulating lesions (b, d). PI 567102B (Rpp6) and PI 567068A had faint reddish-brown resistant lesions that did not sporulate (a, c). The presence of urediniospores is indicated by the white arrows. Bar 1 mm
The cross of G00-3213 × PI 567102B (Rpp6) was created to fine map the Rpp6 locus that was described by Li et al. (2012). G00-3213 is an elite MG VII soybean breeding line developed at UGA, and was derived from a cross of ‘N7001’ (Carter et al. 2003) × ‘Boggs’ (Boerma et al. 2000). G00-3213 has white flowers, tawny pubescence, tan pod walls, black hila, and is susceptible to the GA12 isolate of P. pachyrhizi (Fig. 1). The G00-3213 × PI 567102B cross was made in the 2011–2012 winter greenhouse at UGA located in Athens, GA. The F1 seeds from the cross were grown in the summer in the UGA greenhouse in 2012. The F2 seeds were planted in the summer of 2013 at Athens, GA and were advanced using a single-seed descent method. The F3 and F4 generations were advanced at the USDA-ARS station in Isabella, Puerto Rico in winter of 2013–2014 by single-seed descent. The F5 seed were grown in the summer of 2014 at the UGA Plant Science Farm and at harvest, 184 single plants were pulled and threshed to establish the F5:6 RIL population.
Greenhouse phenotyping assay and phenotypic classification
The Prichard × PI 567068A F2 population previously described by Harris et al. (2015) was advanced to an F2:3 population. The experimental design, including planting, P. pachyrhizi inoculation, growing conditions, and disease rating was the same as that described by Harris et al. (2015). Twelve plants were rated for SBR reaction per family. The Prichard × PI 567068A population was rated for SBR reaction using the GA12 bulk isolate. The GA12 isolate has been used in previous studies and was collected from P. pachyrhizi-infected field-grown kudzu and soybean in 2012 throughout the state of Georgia; therefore, it is referred to as a bulk isolate (Harris et al. 2015; Walker et al. 2014b). The Prichard x PI 567068A F2:3 population was phenotyped for SBR reaction in May 2014.
For the G00-3213 × PI 567102B population, 184 F5:6 RILs were rated for SBR reaction in the same manner as the above population, whereby each RIL was planted into half of a plastic tray (2 seeds per pot and 12 plants per RIL) and the parents were placed in the experiment four times each throughout the experiment in the same manner. Each plastic tray contained 15 spots for pots. Plastic pots were 10-cm × 10-cm Kord Presto sheet pots (Griffin Greenhouse Supplies, Inc., Tewksbury, MA). Plants were grown in Fafard® 3B blend potting soil (Sun Gro Horticulture, Agawam, MA). The outside 12 positions of the tray were used for planting and the three spots in each tray were left open to allow for light penetration and to reduce crowding of the seedling. The G00-3213 × PI 567102B population was rated for SBR reaction in January 2015.
All phenotyping work was done at the UGA greenhouse located at the Griffin Campus in Griffin, GA. The GA12 bulk isolate used to inoculate and rate the populations was maintained and propagated on susceptible ‘Cobb’ plants (Hartwig and Jamison 1975; Harris et al. 2015). Plants were inoculated approximately 14 days after planting and were rated approximately 14 days after inoculation, when disease symptoms were readily visible. Harris et al. (2015) has described this process in detail and a representative lesion reaction for each mapping population parent is shown in Fig. 1.
Due to variable seed germination, 10–12 plants from each of F2:3 family from Prichard × PI 567068A population and each of RILs derived from G00-3213 × PI 567102B RILs were rated. The following guidelines were developed to classify each F2:3 family or RIL as resistant or susceptible, which is similar to the method that was previously used to by Li et al. (2012) to map the Rpp6 locus. TAN lesions are a susceptible reaction classified by the presence of uredinia and profuse sporulation; RB (reddish-brown) lesions are classified as a resistance reaction and typically non-sporulating. A single family or RIL was considered homozygous susceptible if over 66 % of the plants were rated as TAN (susceptible). If 100 % of the plants were RB or IM, the family or RIL was classified as homozygous resistant. All other families or RILs were considered heterozygous or heterogeneous.
Evaluation of plant introductions with different P. pachyrhizi isolates
It was previously shown that PI 567102B (Rpp6) and PI 567068A produced different isolate × genotype patterns of resistance when challenged with a diverse panel of P. pachyrhizi isolates that were collected from South Africa in 2001 (SA01-1); Taiwan in 1972 (TW72-1); Zimbabwe in 2001 (ZM01-1); and Australia in 1979 (AU79-1) (Harris et al. 2015). We wished to test these several of these isolates again to confirm the result.
Isolate reaction experiments were conducted with P. pachyrhizi isolates SA01-1, ZM01-1, and AU79-1 at the USDA-ARS Foreign Disease-Weed Science Research Unit located at Ft. Detrick, MD. The experimental design was the same as that reported by Harris et al. (2015). Briefly, four replications were tested per isolate. A replication consisted of three plants of a given genotype in a single pot tested with a specific isolate. All pots inoculated with the same isolate were randomly arranged in trays. After planting, seedlings were allowed to grow for 3 weeks and were then transferred to a Biological Safety Level-3 Plant pathogen containment facility for inoculation. Approximately 14 days post-inoculation, seedlings were rated for their response to the given P. pachyrhizi isolate. Each replicate consisted of five lines: PI 518671 (‘Williams 82’), G00-3213, PI 612157 (Prichard), PI 567102B (Rpp6), and PI 567068A (Table 1). Williams 82 was used as a susceptible control, as it is known to be universally susceptible (TAN lesions) to SBR (Harris et al. 2015; Hyten et al. 2009; Kendrick et al. 2011). The lesion reaction types of the seedlings were scored qualitatively as TAN, RB, or INT in April of 2015.
Fingerprinting and super bulked segregant analysis
For each family or RIL, a minimum of 10 of the 12 plants were sampled, and a newly expanded trifoliolate leaf was collected from each plant. The leaf samples were combined to form a bulk for that respective family or RIL. The tissue sample from each bulk was lyophilized for 36 h and ground into a fine powder using a GenoGrinder (SPEX US). DNA extractions were performed as per Keim et al. (1988) using the CTAB (hexadecyltrimethylammonium bromide) method. DNA samples were diluted in water to obtain a final concentration ranging from 10 to 50 ng µL−1.
A modified BSA (Michelmore et al. 1991) method was used to identify the specific region on Chr 18 that harbors the Rpp resistance locus contributed by PI 567068A. This technique is referred to as “super bulked segregant analysis” (SBSA), as it includes an informative resistant or susceptible bulk of individuals not previously used in F2:3 BSA mapping (Hyten et al. 2009). Briefly, of the 140 families phenotyped, 28 families were 100 % homozygous susceptible; and 36 families were 100 % homozygous resistant, showing no segregation in any of the families. From the Prichard x PI 567068A population, an equal tissue contribution of leaf powder was taken from each of the 28 susceptible families to create the susceptible super bulk. The resistant bulk was created in the same manner using the 36 resistant families. The powdered leaf tissue in each bulk was homogenized and used for DNA extraction as described above. DNA was then diluted to a concentration of 75 ng µl−1. The resistant and susceptible DNA bulks from the Prichard × PI 567068A population (one of each) were fingerprinted with the SoySNP50K iSelect SNP BeadChips (Song et al. 2013) at the Soybean Genetics Lab at Michigan State University. Genotypes were called using the program GenomeStudio V2011.1 (Illumina, San Diego, USA). PI 567068A and Prichard were not included in the fingerprinting because SoySNP50K data for both lines are available on Soybase (Song et al. 2013). A putative resistance region from SBSA was determined when the genotypic alleles of PI 567068A matched the alleles of a resistant super bulk (e.g., both TT) and were different from the susceptible parent Prichard, the susceptible super bulk (e.g., both CC), which were also homozygous.
SNP assay design and genotyping
The parents of the mapping populations, Prichard and PI 567068A, and G00-3213 and PI 567102B, were compared to identify the polymorphic SNPs surrounding the BSA-identified genomic regions using the SoySNP50K data (Song et al. 2013) or in our laboratory database. Fifteen KASP (LGC Genomics, Middlesex, UK) assays were then developed from these SNP markers which were used for linkage and QTL mapping for both populations (Table 3). To further saturate the genomic region, additional SNPs from the region that are not included in the SoySNP50K Infinium Chips were screened. KASP assays were designed using the criteria established by the KASP User Guide and Manual available online (http://www.lgcgroup.com). Genotyping of the mapping population(s) using KASP assays was conducted using the protocol reported by Pham et al. (2013) for the master mix preparation and thermocycling conditions. The endpoint reading was determined using either a Tecan M1000 Pro Infinite Reader (Tecan Group Ltd., Männedorf, Switzerland) or a Roche LightCycler 480 II with LightCycler® Software (Roche Diagnostics Corporation Indianapolis, IN). When the Tecan Reader was used, allele calls were determined with KlusterCaller software. The allele calls that were ambiguous (did not distinctly cluster) were designated as missing for both populations. Some markers behaved as dominant with the KASP system, even though it was expected they would be co-dominant (Table 3). For the Prichard × PI 567068A F2:3 population, both homozygous and heterozygous genotypes were used to construct the linkage map and perform QTL analysis. However, heterozygous calls for the G00-3213 × PI 567102B RIL population were excluded (Yan et al. 2009).
Linkage and QTL mapping
The comparative linkage maps of the resistance loci of PI 567102B and PI 567068A were created using Kosambi’s regression model function with JoinMap 4.1 software (Van Ooijen 2006). Linkage was established using an LOD score of 3.0 (Figs. 2, 3, 4). JoinMap was used to calculate Chi-square values for both populations (Table 2). Composite interval mapping for the G00-3213 × PI 567102B RIL and Prichard × PI 567068A F2:3 populations was accomplished using Windows QTL Cartographer 2.5 (Basten et al. 2002), using the “All Marker Control Model” parameters with a 1-cM or 2-cM walking window, 2000 permutations, and a 0.001 level of significance.
Linkage map constructed with SNP markers: a recombinant inbred line of G00-3213 × PI 567102B (Rpp6) and b F2:3 population of Prichard × PI 567068A. The left side of the linkage map displays distance in centiMorgans and the right side shows the KASP SNP assay ID (Table 4). Note Rpp6 and Rpp[PI567068A] map to different intervals. Solid lines highlight shared SNP markers used to assay both mapping populations
Physical interval where Rpp6 and Rpp[PI567068A] mapped to Chr 18. The physical locations of GSM markers correspond to SNP positions in the Wm82.a1 genome sequence. The positions of Rpp6 and Rpp[PI567068A] were assigned based on linkage maps generated in this study, and therefore represent estimated positions (dagger). The solid gray lines represent the physical intervals that harbor Rpp6 (45,224 bp) and Rpp[PI567068A] (162,020 bp) determined by SNP markers. The interval that harbors both Rpp6 and Rpp[PI567068A], shown with the dotted gray line is 139,033 bp
The linkage map and QTL likelihood plot for a RILs of G00-3213 × PI 567102B (Rpp6) and b F2:3 population of Prichard × PI 567068A. Displayed on the left side of the linkage map is genetic distance in centiMorgans. The right side displays the KASP SNP assay ID (Table 4). Linkage maps were created with JoinMap 4.1 and QTL plots were generated with Windows QTL Cartographer. The black dotted line highlights where the QTL peak was determined to be on the physical map in relation to the SNP markers tested. The KASP assay IDs are on the x-axis of the QTL map
A diagram was created using Flapjack software (Milne et al. 2010), showed that the physical interval where Rpp6 and the resistance from PI 567068A, designated as Rpp[PI567068A], mapped to Chr 18. The estimated positions of Rpp6 and Rpp[PI567068A] were determined using linkage mapping. All the physical locations of SNPs correspond to the Wm82.a1 genome sequence.
Haplotype analysis and comparisons at the Rpp6 locus
After defining the interval containing Rpp6 and Rpp[PI567068A] the haplotypes of PI 567102B and PI 567068A were compared. Haplotype analysis was performed using a panel of genotypes that included 32 soybean ancestors representing 95 % of the allelic diversity of North American cultivars from 1947 to 1988; a panel of known PIs harboring resistance alleles at the Rpp1, Rpp2, Rpp3, Rpp4, Rpp5 and Rpp6 loci (Gizlice et al. 1994; Monteros et al. 2010; Yu et al. 2015), several elite U.S. cultivars (Williams 82, Prichard, 5601T, and Boggs), and PIs that possessed SBR resistance genes located at the Rpp6 locus by Harris et al. (2015). Additionally, Flapjack software (Milne et al. 2010) was used to compare all genotypes listed in Table 4 by accessing the SoySNP50K data available for all these genotypes (Song et al. 2013) with the exception of G00-3213, which is in our internal laboratory database. The SoySNP50K data were used in their entirety, except for unanchored scaffold sequences that were removed from the analysis. FlapJack software was used to create a comparative matrix and dendrogram for all lines listed in Table 4, which uses a function to create a hierarchical cluster analysis of dissimilarities across all the SoySNP50K SNPs being analyzed. Missing data was not counted as a dissimilarity, and heterozygous locus data are treated as a 50 % match to homozygous allele calls.
Results
Phenotypes of the populations and parental controls
The inoculations of the G00-3213 × PI 567102B RIL and Prichard × PI 567068A F2:3 populations and parents with the GA12 bulk isolate were as expected. In each case the susceptible parent controls G00-3213 or Prichard had TAN, susceptible lesion reactions that produced uredinia and were profusely sporulating. The resistant parents PI 567102B (Rpp6) and PI 567068A each produced faint RB lesions that were never observed to produce uredinia (Fig. 1). No segregation was observed in any of the parental controls.
The parameters for classifying families and RILs here were considered to be realistic based on analysis of data compared to expectations of segregation for each population being a 1:2:1 ratio (resistant:segregating:susceptible) or 1:1 ratio (resistant:susceptible) for the Prichard × PI 567068A and G00-3213 × PI 567102B populations, respectively (Table 2). The Rpp gene from PI 567068A behaved as a single dominant gene in this study and as did the resistance gene from PI 567102B in previous studies (Harris et al. 2015; Li et al. 2012). The segregation ratios of the Prichard × PI 567068A F2:3 and G00-3213 × PI 567102B RIL populations were as expected demonstrating that the resistance conferred by PI 567068A and PI 567102B both behaved as a single gene.
Super bulked segregant analysis, linkage, and QTL mapping
For the Prichard × PI 567068A population, 35 positive SBSA hits fell on Chr 18 between ss715630656 (4,614,748 bp) and ss715629019 (14,689,691 bp); of these 35 positive hits, 34 were from ss715630656 (4,614,748 bp) to ss715632778 (8,403,159 bp) (data not shown; Wm82.a1 genome sequence; http://www.soybase.org/dlpages/index.php#snp50k). Of the positive SBSA hits, KASP assays ss715632549 (GSM0357), ss715632566 (GSM0358), ss715632179 (GSM0374), and ss715631635 (GSM0442) were developed. GSM0374 is a flanking marker for both Rpp6 of PI 567102B and Rpp[PI567068A] of PI 567068A (Table 3). Therefore, SBSA using the Prichard × PI 567068A F2:3 families was able to detect an SNP (GSM0374) that mapped approximately 100 kb away from Rpp[PI567068A]. Additional KASP marker assays in the SBSA region were created, including some assays slightly outside of the interval, to ensure saturation of the region containing Rpp[PI567068A].
The Rpp6 gene contributed by PI 567102B and Rpp[PI567068A] contributed by PI 567068A were mapped using a RIL and an F2:3 family population, respectively. Once Rpp6 from the G00-3213 × PI 567102B population was found to be flanked by SNP markers GSM0373 and GSM0374, this region was saturated by markers GSM0435, GSM0438, and GSM0442 to further narrow the Rpp6 locus of PI 567102B (Table 3; Fig. 2).
All SNP marker coordinates and physical positions, as well as estimations of Rpp gene locations, were defined using the Wm82.a1 sequence (soybase.org). The information on the location of the SNPs used for mapping is reported in Table 3. For both populations, none of the markers showed significant segregation distortion from what was expected (data not shown, p > 0.05). The Rpp6 resistance genes contributed by PI 567102B and Rpp[PI567068A] from PI 567068A were both mapped to Chr 18. Composite interval mapping was performed on both populations using the “All Marker Control” parameters that controls for genetic background.
Linkage mapping narrowed the Rpp6 interval of PI 567102B to a 42,224 bp region that is 1.8 cM long, flanked by KASP markers GSM0374 and GSM0427 (Figs. 2, 3; Table 3). Rpp6 contributed by PI 567102B mapped from 5,953,237 to 5,998,461 bp (Figs. 2, 3; Table 3). Rpp6 had a peak LOD score of 58.3 over SNP GSM0438 (5,930,715 bp; Fig. 4). Information on the genomic configuration of individual RILs from the G00-3213 × PI 567102B RIL population that were homozygous susceptible, homozygous resistant, and had a recombination in the marker interval shown below, or a recombination on either side of the Rpp6 locus. Of the 184 RILs, two had recombinations flanking each side of the Rpp6 locus.
Rpp[PI567068A] was fine mapped from 5,998,461 to 6,160,481 bp, spanning a 161,158 bp interval (3.9-cM) flanked by KASP markers GSM0427 and GSM0374 (Figs. 2, 3; Table 3). Rpp[PI567068A] had an LOD score of 4.4 over SNP GSM0374 (5,998,461 bp). Rpp6 and Rpp[PI567068A] are both flanked by the SNP marker GSM0374 (Fig. 3; Table 3).
A diagram showing the physical interval where Rpp6 and Rpp[PI567068A] are located on Chr 18 was created to show the tight linkage between Rpp6 and Rpp[PI567068A] (Fig. 3). Based on the SoySNP50K Infinium Chip (Song et al. 2013), PI 567102B and PI 567068A have identical haplotypes from 5,961,788 (ss715632113) to 6,406,710 bp (ss715632525) of the Wm82.a1 sequence. This haplotype is defined by 16 SoySNP50K markers: ss715632113, ss715632123, ss715632129, ss715632179, ss715632196, ss715632280, ss715632362, ss715632369, ss715632399, ss715632451, ss715632467, ss715632499, ss715632517, ss715632521, ss715632523, and ss715632525. Since the haplotype is identical between PI 567102B and PI 567068A, it is referred to as the Rpp6/Rpp[PI567068A] haplotype.
Haplotype analysis at the Rpp6 locus and rust phenotypes of PIs and cultivars to inoculation with P. pachyrhizi isolates
The P. pachyrhizi isolates SA01, ZM01-1, AU79-1, and GA12 were used to challenge PI 518671 (Williams 82), G00-3213, PI 612157 (Prichard), PI 567102B (Rpp6), and PI 567068A (Table 1). Williams 82, which was used as the susceptible control, was susceptible to all isolates tested, and PI 567102B produced RB lesions when challenged with these isolates. PI 567068A produced TAN reactions to SA01 and ZM01-1; a mixture of INT and RB reactions to AU79-1; and an RB reaction to GA12. The mapping population parents G00-3213 and Prichard were susceptible to the GA12 isolate (Fig. 1; Table 1).
Data were compiled from Harris et al. (2015) using PIs with genes that mapped to the Rpp6 locus and from the current research on PI 567102B (Rpp6) and PI 567068A (Table 4). Phenotypically, when tested with a unique panel of P. pachyrhizi isolates, PI 476905A showed a unique isolate panel reaction pattern; PI 567076 and PI 567090 were similar to PI 567068A; PI 567129 was not tested with an isolate panel; and PI 567104B reacted as if it had the Rpp4 and Rpp6 loci of PI 459025B (Rpp4) and PI 567102B Rpp6 (Table 4; Harris et al. 2015).
PI 567102B and PI 567068A have an identical haplotype allele in the interval of ss715630691 (4,734,471 bp) to ss715632534 (6,591,476 bp); a region spanning over 1.85 Mbp. Within that interval, PI 476905A, PI 567068A, PI 567076, PI 567090, PI 567129, PI 567102B, and PI 567104B shared an identical haplotype from SNP markers ss715632113 to ss715632525 (5,961,788–6,406,710 bp), spanning 444,922 bp. Interestingly, the 16 SNPs that define the Rpp6/Rpp[PI567068A] haplotype window are identical amongst all the PIs that had BSA data or that mapped to the Rpp6, locus including PI 476905A, PI 567068A (Rpp[PI567068A]), PI 567076, PI 567104B, PI 567129, and PI 567102B (Rpp6) (Table 4). Other than PI 476905A, which was collected from an unknown province in China in 1983, PI 566956, PI 566984, PI 567068A, PI 567076, PI 567090, PI 567104B, PI 567123A PI 567129, and PI 567102B all were collected from East Java, Indonesia in 1993, which further suggests these genotypes may be closely related (Table 3).
Within the haplotype window defined by 16 SNPs that is shared by PI 567102B and PI 567068A, three unique SNPs were identified that create a CAG haplotype (ss715632362, ss715632523, and ss715632525) (Tables 3, 4). PI 476905A, PI 567068A (Rpp[PI567068A]), PI 567076, and PI 567102B (Rpp6), PI 567104B, and PI 567129 all possess the Rpp6/Rpp[PI567068A] haplotype and have data that support they possess an Rpp gene near the Rpp6Rpp[PI567068A] locus (Table 4). None of the 32 North American soybean ancestors, SBR susceptible soybean cultivars (Prichard, Boggs, 5601T, and Williams 82), or any other known sources of Rpp genes at loci Rpp1 to Rpp5 possess this CAG haplotype (Table 4), indicating that the CAG haplotype is unique. The haplotype window identified here to detect an Rpp gene at the Rpp6/Rpp[PI567068A] locus had three SNPs. The three SNPs would theoretically allow for eight possible haplotypes. Excluding PI 506764 (Hyuuga), which had a heterozygous haplotype at ss715632523, the panel of PIs examined in Table 4 had six of the eight possible haplotypes.
The SoySNP50K data with the exception for the SNPs from unanchored scaffold sequences were used to create a comparative matrix and dendrogram for all lines listed in Table 4 using FlapJack software (Milne et al. 2010). The dendrogram showed that all genotypes that possessed the Rpp6/Rpp[PI567068A] haplotype (PI 567068A, PI 567076, PI 567090, PI 567102B, PI 567104B, and PI 567129) that were collected from East Java, Indonesia in 1993 clustered tightly together; however, PI 476905A (collected from China) did not cluster with the other Rpp6/Rpp[PI567068A] haplotype lines (Fig. 5; Table 4).
A dendrogram describing the relationship of all lines listed in Table 4 based on the SoySNP50K SNPs
PI 567102B and PI 567068A clustered together and were 77.4 % similar (Fig. 5; data not shown). PI 476905A, which was collected from an unknown location in China in 1983 and which also that possessed the Rpp6/Rpp[PI567068A] haplotype, distinctly clustered with PI 240664 (collected from the Philippines), PI 548461 (China), PI 548485 (Jiangsu, China), and PI 594538A (Fujian, China). It is not surprising that PI 476905A clustered with other genotypes from China and the Philippines (Table 4; Fig. 5).
Discussion
The resistance gene Rpp6 contributed by PI 567102B and Rpp[PI567068A] from PI 567068A were both mapped using a relatively high density pannel of SNP markers, and each Rpp gene is flanked by the GSM0374 SNP identified in this study (Fig. 3; Table 3). Through linkage mapping, Rpp6 was mapped from 5,953,237 to 5,998,461 bp and Rpp[PI567068A] was mapped from 5,998,461 to 6,160,481 bp (Fig. 3; Table 3). Even though the Rpp6 interval of PI 567102B is less than 50 kb, recombinations on either side of the Rpp6 locus were observed in two of the 184 RILs, indicating that recombinations are possible in close proximity to the Rpp6 locus and that none of the SNPs identified in the mapping of Rpp6 are causative (data not shown). QTL peaks for Rpp6 (LOD score of 58.3), and Rpp[PI567068A] (LOD score of 4.4) were 139,033 bp apart (Fig. 2; Table 3). This suggests that Rpp6 and Rpp[PI567068A] are either tightly linked or possibly allelic. An allelism test or further fine mapping may help resolve between these two possibilities.
Harris et al. (2015) challenged numerous PIs with a panel of diverse P. pachyrhizi isolates. PI 567102B and PI 567068A had differential reactions when challenged with isolates SA01-1, TW72-1, ZM01-1, and AU79-1. Specifically, PI 567068A had a TAN lesion type when challenged by SA01-1, TW72-1, ZM01-1, and AU79-1; and PI 567102B had an RB lesion type to all these isolates with the exception of TW72-1, to which PI 567102B reacted with a mixture plants with RB or TAN lesions.
The P. pachyrhizi isolates SA01, ZM01-1, and AU79-1 that gave clean differential reactions for PI 567102B (Rpp6) and PI 567068A from Harris et al. (2015) were used in the present study with similar results. Additionally, the susceptible control PI 518671 (Williams 82) was included and was susceptible (TAN) to SA01, ZM01-1, AU79-1, as well as to the GA12 bulk isolate used to map the traits in this study (Table 1). The mapping population parents G00-3213 and Prichard were also susceptible (TAN) to the GA12 bulk isolate, as expected (Fig. 1; Table 1). PI 567102B and PI 567068A were both resistant to the GA12 bulk isolate and produced faint, relatively small RB lesions measuring approximately 1 mm in diameter. The RB lesions of PI 567102B and PI 567068A were never observed to produce uredinia when challenged with GA12 after 14 days (Fig. 1; Table 1). When PI 567102B and PI 567068A were challenged with SA01-1, and ZM01-1, and AU79-1 again in this study, PI 567102B reacted with RB lesions; PI 567068A reacted with TAN reactions to SA01-1, and ZM01-1; and a mixture of INT and RB reactions to AU79-1. The only discrepancy between the Harris et al. (2015) study and our results is when PI 567068A was challenged with the ZM01-1. Harris et al. (2015) observed a TAN reaction and we observed a mixture of INT and RB lesions on the plants. This could potentially be due to small variations in the growth conditions between experiments that may have resulted in more or less uredinia production. Additionally, the reaction of PI 567068A to the ZM01-1 isolate was difficult to score. The differential isolate reactions presented here and in Harris et al. (2015) support that PI 567068A Rpp[PI567068A] has a different source of Rpp resistance from PI 567102B (Rpp6).
PI 476905A has the Rpp6/Rpp[PI567068A] haplotype, yet has a unique P. pachyrhizi isolate pattern from PI 567102B (Rpp6) and PI 567068A (Rpp[PI567068A]) (Table 4; Harris et al. 2015). This indicates that PI 476905A may harbor a novel resistance allele at the Rpp6/Rpp[PI567068A] locus, or a tightly linked, novel Rpp gene. PI 476905A also stands out as the only PI with an Rpp gene that mapped to the Rpp6 locus, but was not collected from East Java, Indonesia (Table 4). When a panel of diverse genotypes were compared using the SoySNP50K data, all genotypes from East Java, Indonesia clustered together distinctly from all other genotypes, and PI 476905A clustered with PI 240664, PI 548461, PI 548485, and PI 594538A, all of which were collected from China, other than PI 240664 which was collected from the Philippines (Table 4; Fig. 5).
Several PIs have a natural Rpp gene pyramid based on haplotype data. It is estimated as many as 15 % of rust-resistant PIs harbor more than one Rpp gene (Harris et al. 2015; Kendrick et al. 2011). Interestingly, PI 567104B has the Rpp4 haplotype of PI 459025B and the Rpp6/Rpp[PI567068A] haplotype. The resistance of PI 567104B also maps to the Rpp4 and Rpp6 loci, and reacted like the PI 567102B (Rpp6) and PI 459025B (Rpp4) genotypes to the panel of P. pachyrhizi isolates used in this study, providing evidence that this PI may contain an Rpp gene at both the Rpp4 and Rpp6 locus (Table 4; Harris et al. 2015).
In field screens in 2008 in Quincy, Florida, PI 567104B had lower field rust severity scores than either PI 567102B (Rpp6) and PI 459025B (Rpp4), and PI 567068A was not tested (Walker et al. 2014a). Additionally, PI 567104B had a lower lesion density than PI 567102B (Rpp6), PI 567068A (Rpp[PI567068A]), and PI 459025B (Rpp4) when challenged with the GA 2008 (GA08) bulk P. pachyrhizi isolate in a greenhouse assay in 2011 (Walker et al. 2014b). These results may indicate the higher resistance of PI 567104B is caused by an additive resistance effect of the Rpp4 and Rpp6 loci.
The current study has mapped the Rpp6 and Rpp[PI567068A] SBR resistance genes. Further research is needed to resolve whether or not Rpp[PI567068A] is allelic to Rpp6 or a tightly linked resistance gene. These findings can be used to incorporate the Rpp6 or the Rpp[PI567068A] resistance allele into elite germplasm. The Rpp6/Rpp[PI567068A] haplotype provides soybean researchers with additional genomic resources to identify new, unique sources of SBR resistance.
Author contribution statement
Zachary King designed SNP markers, phenotyped populations, completed mapping experiments, generated tables and figures, and wrote the manuscript. Donna Harris and James Buck phenotyped populations and reviewed the manuscript. Kerry Pedley tested lines with a panel of Phakopsora pachyrhizi isolates and edited the manuscript. Qijian Song provided the sequences of SNPs described here. Dechun Wang and Zixiang Wen ran the SoySNP50K Infinium Chips. Zenglu Li and Roger Boerma interpreted the results, provided oversight for experiments, and edited the manuscript.
References
Basten CJ, Weir BS, Zeng Z-B (2002) QTL cartographer, version 1.16. Department of Statistics, North Carolina State University, Raleigh
Boerma HR, Hussey RS, Phillips DV, Wood ED, Rowan GB, Finnerty SL (2000) Registration of ‘Boggs’ soybean. Crop Sci 40:294–295
Boerma HR, Hussey RS, Phillips DV, Wood ED, Rowan GB, Finnerty SL, Griner JT (2001) Registration of ‘Prichard’ Soybean. Crop Sci 41:920–921
Boerma HR, Monteros MJ, Ha B-K, Wood ED, Phillips DV, Walker DR, Missaoui AM (2011) Registration of Asian soybean rust–resistant soybean germplasm G01-PR16. J Plant Regist 5:118–122. doi:10.3198/jpr2009.12.0732crg
Bromfield KR, Hartwig EE (1980) Resistance to soybean rust and mode of inheritance. Crop Sci 20:254–255
Carter TE Jr, Burton JW, Bowman DT, Cui Z, Zhou X, Villagarcia MR, Fountain MO, Niewoehner AS (2003) Registration of ‘N7001’ soybean. Crop Sci 43:1126–1127
Chakraborty N, Curley J, Frederick RD, Hyten DL, Nelson RL, Hartman GL, Diers BW (2009) Mapping and confirmation of a new allele at Rpp1 from soybean PI 594538A conferring RB lesion-type resistance to soybean rust. Crop Sci 49:783–790
Diers BW, Kim K-S, Frederick RD, Hartman GL, Unfried J, Schultz S, Cary T (2013) Registration of eight soybean germplasm lines resistant to soybean rust. J Plant Regist 8:96–101. doi:10.3198/jpr2012.11.0052crg
Garcia A, Calvo ES, Kiihl RAdS, Harada A, Hiromoto DM, Vieira LGE (2008) Molecular mapping of soybean rust (Phakopsora pachyrhizi) resistance genes: Discovery of a novel locus and alleles. Theor Appl Genet 117:545–553
Gizlice Z, Carter TE Jr, Burton JW (1994) Genetic base for North American public soybean cultivars released between 1947 and 1988. Crop Sci 34:1143–1151
Goellner K, Loehrer M, Langenbach C, Conrath U, Koch E, Schaffrath U (2010) Phakopsora pachyrhizi, the causal agent of Asian soybean rust. Mol Plant Pathol 11:169–177
Harris DK, Kendrick MD, King ZR, Pedley KF, Walker DR, Cregan PB, Buck JW, Phillips DV, Li Z, Boerma HR (2015) Identification of unique genetic sources of soybean rust resistance from the USDA germplasm collection. Crop Sci 55:2161–2176
Hartman GL, Miles MR, Frederick RD (2005) Breeding for resistance to soybean rust. Plant Dis 89:664–666
Hartman GL, West ED, Herman TK (2011) Crops that feed the world 2. Soybean—worldwide production, use, and constraints caused by pathogens and pests. Food Security 3:5–17
Hartwig EE (1986) Identification of a fourth major gene conferring resistance to soybean rust. Crop Sci 26:1135–1136
Hartwig EE, Bromfield KR (1983) Relationships among three genes conferring specific resistance to rust in soybeans. Crop Sci 23:237–239
Hartwig EE, Jamison KW (1975) The uniform soybean tests—Southern states. USDA-ARS, Stoneville
Hyten DL, Hartman GL, Nelson RL, Frederick RD, Concibido VC, Narvel JM, Cregan PB (2007) Map location of the Rpp1 locus that confers resistance to soybean rust in soybean. Crop Sci 47:837–840
Hyten DL, Smith JR, Frederick RD, Tucker ML, Song Q, Cregan PB (2009) Bulked segregant analysis using the GoldenGate assay to locate the Rpp3 locus that confers resistance to soybean rust in soybean. Crop Sci 49:265–271. doi:10.2135/cropsci2008.08.0511
Isard SA, Gage SH, Comtois P, Russo JM (2005) Principles of the atmospheric pathway for invasive species applied to soybean rust. Bioscience 55:851–861
Jurick WM, Narvaez DF, Brennan MM, Harmon CL, Marois JJ, Wright DL, Harmon PF (2008) Winter survival of the soybean rust pathogen, Phakopsora pachyrhizi, in Florida. Plant Dis 92:1551–1558
Keim P, Olson TC, Shoemaker RC (1988) A rapid protocol for isolating soybean DNA. Soybean Genet Newsl. 15:150–152
Kendrick MD, Harris DK, Ha B, Hyten DL, Cregan PB, Frederick RD, Boerma HR, Pedley KF (2011) Identification of a second Asian soybean rust resistance gene in Hyuuga soybean. Phytopathology 101:535–543
Li S, Smith JR, Ray JD, Frederick RD (2012) Identification of a new soybean rust resistance gene in PI 567102B. Theor Appl Genet 125:133–142. doi:10.1007/s00122-012-1821-y
Lynch TN, Marois JJ, Wright DL, Harmon PF, Harmon CL, Miles MR, Hartman GL (2006a) First report of soybean rust caused by Phakopsora pachyrhizi on Phaseolus spp. in the United States. Plant Dis 90:970
Lynch TN, Miles MR, Frederick RD, Bonde MR, Hartman GL (2006b) Alternative hosts to Phakopsora pachyrhizi, the causal agent of soybean rust. In: 2nd national Soybean rust symposium, American Phytopathology Society, St. Louis. Accessed August 2014
McLean RJ, Byth DE (1980) Inheritance of resistance to rust (Phakopsora pachyrhizi) in soybeans. Aust J Agric Res 31:951–956
Meyer JDF, Silva DCG, Yang C, Pedley KF, Zhang C, van de Mortel M, Hill J, Shoemaker RC, Abdelnoor RV, Whitham SA, Graham MA (2009) Identification and analyses of candidate genes for Rpp4-mediated resistance to Asian soybean rust in soybean. Plant Physiol 150:295–307
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci 88:9828–9832
Miles MR, Frederick RD, Hartman GL (2006) Evaluation of soybean germplasm for resistance to Phakopsora pachyrhizi. Plant Health Prog. doi:10.1094/php-2006-0104-01-rs (online)
Milne I, Shaw P, Stephen G, Bayer M, Cardle L, Thomas WTB, Flavell AJ, Marshall D (2010) Flapjack—graphical genotype visualization. Bioinformatics 26:3133–3134
Monteros MJ, Missaoui AM, Phillips DV, Walker DR, Boerma HR (2007) Mapping and confirmation of the ‘Hyuuga’ red-brown lesion resistance gene for Asian soybean rust. Crop Sci 47:829–834
Monteros MJ, Ha B-K, Phillips DV, Boerma HR (2010) SNP assay to detect the ‘Hyuuga’ red-brown lesion resistance gene for Asian soybean rust. Theor Appl Genet 121:1023–1032
Ono Y, Buriticá P, Hennen JF (1992) Delimitation of Phakopsora, Physopella and Cerotelium and their species on Leguminosae. Mycol Res 96:825–850
Pham TA, Miles MR, Frederick RD, Hill RD, Hill CB, Hartman G (2009) Differential responses of resistant soybean entries to isolates of Phakopsora pachyrhizi. Plant Dis 93:224–228
Pham A, McNally K, Abdel-Haleem H, Boerma HR, Li Z (2013) Fine mapping and identification of candidate genes controlling the resistance to Southern root-knot nematode in PI 96354. Theor Appl Genet 126:1825–1838
Schneider RW, Hollier CA, Whitam HK, Palm ME, McKemy JM, Hernandez JR, Levy L, DeVries-Paterson R (2005) First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Dis 89:774
Slaminko TL, Miles MR, Frederick RD, Bonde MR, Hartman GL (2008a) New legume hosts of Phakopsora pachyrhizi based on greenhouse evaluations. Plant Dis 92:767–771
Slaminko TL, Miles MR, Marios JJ, Wright DL, Hartman GL (2008b) Hosts of Phakopsora pachyrhizi identified in field evaluations in Florida. Plant Health Prog. doi:10.1094/php-2008-1103-01-rs (online)
Song QJ, Hyten DL, Quigley CV, Jia GF, Fickus EW, Cregan PB (2013) Development and Evaluation of a high-density Infinium beadchip SoySNP50K. PLoS One 8:e54985. doi:10.1371/journal.pone.0054985
Van Ooijen JW (2006) JoinMap® 4, Software for the calculation of genetic linkage maps in experimental populations. Kyazma B.V., Wageningen
Walker DR, Harris DK, King ZR, Li Z, Boerma HR, Buckley JB, Weaver DB, Sikora EJ, Shipe ER, Mueller JD, Buck JW, Schneider RW, Marois JJ, Wright DL, Nelson RL (2014a) Evaluation of soybean germplasm accessions for resistance to Phakopsora pachyrhizi populations in the southeastern United States, 2009–2012. Crop Sci 54:1673–1689. doi:10.2135/cropsci2013.08.0513
Walker DR, Harris DK, King ZR, Li Z, Phillips DV, Buck JW, Nelson RL, Boerma HR (2014b) Soybean germplasm accession seedlings to soybean rust (Phakopsora pachyrhizi) isolates from Georgia. Crop Sci 54:1433–1447. doi:10.2135/cropsci2013.09.0654
Wrather JA, Koenning SR (2009) Effects of diseases on soybean yields in the United States 1996 to 2007. Plant Health Prog. doi:10.1094/php-2009-0401-01-rs (online)
Yan J, Yang X, Shah T, Sánchez-Villeda H, Li J, Warburton M, Zhou Y, Crouch JH, Xu Y (2009) High-throughput SNP genotyping with the GoldenGate assay in maize. Mol Breed 25:441–451. doi:10.1007/s11032-009-9343-2
Yu N, Kim M, King ZR, Harris DK, Buck JW, Li Z, Diers BW (2015) Fine mapping of the Asian soybean rust resistance gene Rpp2 from soybean PI 230970. Theor Appl Genet 128:387–396
Acknowledgments
We would like to acknowledge David Spradlin, Brian Vermeer, Dale Wood, Gina Bishop, Earl Baxter, Tatyana Nienow, and Colleen Wu at the University of Georgia for providing technical support in greenhouse assays in Griffin Georgia or genotyping support in the Soybean Molecular Breeding Laboratory. We would also like to acknowledge Amy Ruck for providing technical assistance in phenotyping plant introductions with a panel of Phakopsora pachyrhizi isolates described here at the USDA-ARS Foreign Disease-Weed Science Research Unit at Ft. Detrick, Maryland. This work was funded by the United Soybean Board (USB 1420-532-5635) and the United Soybean Board Fellowship to Zachary King.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by D. A. Lightfoot.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
King, Z.R., Harris, D.K., Pedley, K.F. et al. A novel Phakopsora pachyrhizi resistance allele (Rpp) contributed by PI 567068A. Theor Appl Genet 129, 517–534 (2016). https://doi.org/10.1007/s00122-015-2645-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-015-2645-3