Abstract
Key message
Understanding the correlations of seven minerals for concentration, content and yield in maize grain, and exploring their genetic basis will help breeders to develop high grain quality maize.
Abstract
Biofortification by enhanced mineral accumulation in grain through genetic improvement is an efficient way to solve global nutrient malnutrition, in which one key step is to detect the underlying quantitative trait loci (QTL). Herein, a maize recombinant inbred population (RIL) was field grown to maturity across four environments (two locations × two years). Phenotypic data for grain mineral concentration, content and yield were determined for copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), magnesium (Mg), potassium (K) and phosphorus (P). Significant effects of genotype, location and year were observed for all investigated traits. The strongest location effects were found for Zn accumulation traits probably due to distinct soil Zn availabilities across locations. Heritability (H 2) of different traits varied with higher H 2 (72–85 %) for mineral concentration and content, and lower (48–63 %) for mineral yield. Significant positive correlations for grain concentration were revealed between several minerals. QTL analysis revealed 28, 25, and 12 QTL for mineral concentration, content and yield, respectively; and identified 8 stable QTL across at least two environments. All these QTL were assigned into 12 distinct QTL clusters. A cluster at chromosome Bin 6.07/6.08 contained 6 QTL for kernel weight, mineral concentration (Mg) and content (Zn, K, Mg, P). Another cluster at Bin 4.05/4.06 contained a stable QTL for Mn concentration, which were previously identified in other maize and rice RIL populations. These results highlighted the phenotypic and genetic performance of grain mineral accumulation, and revealed two promising chromosomal regions for genetic improvement of grain biofortification in maize.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The human body requires more than 22 mineral elements that can be supplied by an appropriate diet (Philip and Martin 2005). However, diets of human populations subsisting largely on cereals often lack adequate minerals [e.g., copper (Cu), iron (Fe), manganese (Mn) and zinc (Zn)], which are known to play essential roles in physiological processes of the human body. Over three billion people in the world are affected by micronutrient malnutrition resulting in poor health and higher rates of mortality (Cakmak 2002). Many of those afflicted are dependent upon staple crops for their sustenance (Pfeiffer and McClaferty 2007). Among the staple crops, maize (Zea mays L.) is one of the most important crops, accounting for 40 percent of the world’s cereal food production (Bouis and Welch 2010). Together with the fact that maize has the highest average yield per hectare, even a small increase in the nutritive value of maize would be important for human nutrition. Moreover, mineral concentrations (e.g., Cu, Fe, Mn, and Zn) in maize grain are relatively low when compared to animal food products (Wang et al. 2003). The concentration even decreased in the past decades due to breeders selecting exclusively for grain yield but not grain quality (Fan et al. 2008; Šimic et al. 2009; Anandan et al. 2011). Thus, since the concentration of these dietary minerals in maize grain is not sufficient to meet the dietary requirement of humans’ daily intake when these foods are consumed in typical amounts, improving the mineral concentration in maize grain is of great interest.
Traditional efforts on improvement of grain mineral accumulation in crops has been done majorly in three ways: (1) selection of germplasm with greater accumulation of essential minerals (White and Broadley 2009), (2) breeding of mineral efficient crops that accumulate more minerals (Gregorio et al. 2000), and (3) enhancing bio-available minerals in edible portions through genetic engineering (Goto et al. 1999). However, such approaches have not always been successful, mostly because of economic and technical difficulties (e.g., the large amount of time and labor required for breeding new crop varieties with high grain minerals; Mayer et al. 2008). Quantitative trait locus (QTL) mapping is a powerful approach to study and manipulate complex traits important in agriculture (Abiola et al. 2003). A wide range of genetic variation for grain mineral accumulation has been revealed among maize accessions, suggesting that traits of mineral accumulation are complex quantitative traits (Šimic et al. 2012; Qin et al. 2012; Baxter et al. 2013). Therefore, QTL analysis could greatly accelerate genetic modification of mineral amount in maize grain by marker-assisted selection (MAS) and even discovery of underlying candidate genes.
Several QTL studies have been conducted in maize for grain mineral concentration (Garcia-Oliveira et al. 2009; Zhou et al. 2010; Lung’aho et al. 2011; Qin et al. 2012; Šimic et al. 2012; Baxter et al. 2013, 2014). Stable or consistent QTL across different environments could only be identified in some of these above studies. For example, Baxter et al. (2013) identified 27 QTL for 9 mineral concentrations with most expressed in at least two of the six investigated environments. For micro-minerals, Lung’aho et al. (2011) identified 3 QTL for Fe concentration across three environments and Qin et al. (2012) identified 3 QTL for Fe and Zn concentrations across two environments. For macro-minerals, Šimic et al. (2012) identified a major QTL for phosphorus (P) concentration across four environments. However, none of common QTL located in the same genomic region was detected across populations, suggesting that QTL for maize grain mineral concentration were mainly determined by the specific genetic background. Therefore, analysis of QTL using different genetic resources is necessary to enrich number of QTL for controlling mineral accumulation in maize grain.
QTL for mineral accumulation in maize grain are strongly influenced by environment (Šimic et al. 2012; Baxter and Dilkes 2012; Baxter et al. 2013). Soil conditions (location) and climatic factors (year) are considered two important factors for crop production (Pacini et al. 2003). The impact of soil conditions on mineral accumulation has been investigated in Arabidopsis by correlating changes in elemental concentrations to different soil types (Buescher et al. 2010; Baxter et al. 2012; McDowell et al. 2013). Climate across different seasons could affect plant growth and grain yield, which might directly or indirectly affect mineral accumulation in grain. Thus, it is worthy to evaluate the environmental effects as location × years on maize mineral accumulation. Concentration was widely applied for evaluating mineral accumulation in crop grain. Content per kernel (g/kernel; calculated as concentration × kernel weight) was also considered to be important for evaluating mineral amount in a kernel. Despite few studies, analysis of phenotypic and genetic relationship between both concentration and content in a population allows to clarify a dilution effect due to the size of kernel (Cakmak et al. 2010; Imtiaz et al. 2003). Moreover, mineral yield [mineral harvest yield per hectare (g/ha): calculated as concentration × grain yield] was important for evaluating the economic value for global micronutrient malnutrition.
In the present study, a recombinant inbred line (RIL) population was used to map main-effect QTL for the accumulation traits (concentration, content, and yield) of seven minerals [Cu, Fe, Mn, Zn, potassium (K), magnesium (Mg) and P] in maize grain based on field measurements. The objectives were to (1) evaluate mineral concentration, content and yield in the RIL population; (2) analyze their consistency across environments using two locations across two years; and (3) map QTL for mineral concentration, content, and yield in this population.
Materials and methods
Plant materials and field design
A population of 218 F8RILs derived from the cross Ye478 × Wu312 was used in this study (Liu et al. 2011). RILs and their parents were grown in two locations Beijing (BJ, 40°06′N latitude, 116°11′E longitude) and Gansu (GS, 38°37′N latitude, 102°40′E) for 2 years 2009 and 2010 (Supplementary Table S1). Before planting, six soil samples were taken from 0 to 30 cm soil layer for each environment, mixed, air-dried, and sieved to remove undecomposed plant material, and used to measure the chemical properties (Supplementary Table S1). Organic matter was determined by K2Cr2O7 method (Walkley 1947), and total nitrogen (N) by Kjeldahl procedure (Bremner 1996). Available P (Olsen-P) was extracted by NaHCO3 and determined using a spectrophotometer (Olsen et al. 1954). Potassium (K) was extracted by NH4OAc and determined at flame photometer (Van Reeuwijk 1992). Micro-minerals (Cu, Fe, Mn and Zn) were extracted with DTPA (diethylenetriaminepentaacetic acid) solution (Lindsay and Norvell 1978) and served for concentration measurement by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, Perkin-Elmer, USA) (Xue et al. 2014).
The field was arranged as split-plot design. RILs and parents were planted in one-row plots with three replications. The rows were 4 m long containing 13 plants, with a plant density of 60,000 per hectare. The space between rows was 0.5 m. Seeds were sown in April and May in BJ and GS, respectively, and harvested in September for both locations (Supplementary Table S1). All fertilizers were applied before sowing, except N was supplied with two applications: 50 % at sowing and 50 % at the V6 (6 leave) stage. Rainfall during the growing season in BJ ranged from 400 to 600 mm (Supplementary Table S2), which was sufficient to avoid water deficit stress. Annual precipitation in GS was only 100–300 mm, which was insufficient for maize growth. So, 500 mm irrigation water was additionally supplied. Turf machinery and weeding were applied before sowing to ensure seedling establishment. Pest management was applied at V6 (six expanded leaves) and V8 stages in BJ and GS, respectively.
Phenotyping for quality traits
The analysis methods used are almost identical to those described by Xue et al. (2014). At harvest, five plants were chosen from the middle of each row for trait evaluation. All ears were harvested for evaluation of grain yield (GY), which was expressed as grain weight per hectare. Then, 20 kernels were collected from the middle of each ear, rapidly washed with deionized water and oven-dried at 70 °C to determine kernel weight (KW), which was expressed as dry weight per kernel. For mineral analysis, collected kernels from each row were mixed and ground with a stainless steel grinder GENO-2000 (Spex, Pittsburg, CA, USA) and 0.5 g powder was digested with HNO3–H2O2 in a microwave accelerated reaction system (CEM, Matthews, NC, USA). Mineral concentrations were determined by ICP–OES.
Data analysis
Least square mean (LSMEAN) of replicates was used for QTL analysis of measured parameters from each environment. Then, these data were analyzed by analysis of variance (ANOVA) using the SAS statistics system. For each phenotypic value, the following linear model was used:
where observation Y ijkl is the plot-based phenotype as sum of the mean (μ), the genetic effect (G) of the Ith line, the effect of the Jth location (L), the effect of the Kth Year (Y), and their respective interactions G × L ij + G × Y ik + L × Y jk + G × L × Y ijk and the error e ijkr . The PROC MIXED procedure (SAS) was used to estimate LSMEAN values, which were used to analyze trait Pearson correlations (Pillen et al. 2003).
Heritability for each trait was calculated from an ANOVA fitting effect of genotype (G), environment (E; two locations × two years were combined into 4 environments), and G × E interactions, as
where H 2 is broad sense heritability, σ 2 G is genotypic variance, σ 2 GE is genotype × environment variance, σ 2 E is error variance, n is number of environments and r is the number of replications (Nyquist 1991).
Using 184 polymorphic markers, a genetic linkage map was constructed for this RIL population with a total length of 2084 cM and an average interval length of 11.3 cM in our previous work (Liu et al. 2011). QTL were detected by composite interval mapping (Zeng 1994) using Windows QTL Cartographer version 2.5 (Model 6) (Wang et al. 2012). The threshold LOD value was determined with 1000 permutations at P = 0.05 level (Churchill and Doerge 1994). QTL positions were assigned underneath maximal LOD scores. Loci detected within the average interval (11.3 cM) in different environments were considered to be the same locus for each trait.
Results
Grain mineral concentration, content and yield in parents and RILs
All RILs and parents were grown to maturity in four environments: GS09, GS10, BJ09 and BJ10 (Supplementary Table S1). Grain mineral accumulation traits including concentration, content, and yield were investigated for four different micro-minerals (Cu, Fe, Mn and Zn), and three different macro-minerals (K, Mg and P). GY and KW were also evaluated.
Between the two parents, line Ye478 had significantly higher GY and KW compared to Wu312 across all environments (Table 1). For most minerals, the concentration showed similar levels in both parents, while the content and yield were higher in Ye478 with a higher KW and GY. However, Fe concentration was significantly higher in Ye478 across all environments, except in BJ09, and the P and Zn concentrations were significantly higher in Wu312 in BJ09.
Among RILs the values of all investigated traits segregated continuously and approximately fit normal distributions with absolute values of both skewness and kurtosis being less than 1.0. All traits showed transgressive segregation in both directions (lines with lower values than the lowest parent or higher values than the highest parent) (Supplementary Fig. S1). These results suggest presence of multiple genes controlling the investigated traits. Average KW was similar across environments, while GY and some minerals markedly varied among different environments (Table 2). Grain yield in GS was similar in both years and higher than that in BJ. At BJ, the lowest GY was found in 2010, likely a result from drought stress at maize silking stage (approx. 25 % less than average monthly rainfall in July 2010: Supplementary Table S2). Concentration and content of most minerals (except Cu and Zn) were similar across environments with the exception of BJ10 where higher value was found likely due to the concentrating effect by its lower GY (Table 2). Mineral yields were higher for all elements in GS than BJ.
Phenotypic variation and heritability for mineral concentration, content, and yield
The effect of genotype was significant for all traits (α = 0.05) (Table 3). Effects of environment (year and location) and genotype × environment were also significant, suggesting the presence of strong environmental effects on mineral accumulation in maize grain across these two locations and years. Within environmental effects, location had stronger effects than year for GY, KW, and all mineral yields. Cu and Zn had higher location effects for both concentrations and contents, while Mn, K and P showed stronger year effects. Fe and Mg had higher year effects for their concentrations, but lower for contents.
Despite the lower level of accumulation, Cu and Mn had higher coefficient of variation (CV %) values (18.2–28.8, 22.3–31.9, 33.4–55.3 %) for concentration, content and yield, respectively (Table 2). The heritability (H 2) of different traits varied from 48 to 85 % with an average of 70 % (Table 3). Mineral concentration and content had similar H 2 at higher levels of 72–85 %, while mineral yield had lower levels of H 2 ranging from 48 to 63 %.
Correlations of mineral concentration, content, and yield
Significant positive correlations were found for grain concentration between several minerals (Table 4). Some of the minerals were highly correlated while many had weak positive correlations. Among these minerals, P had the closest correlation to Mg concentration (r = 0.65, P < 0.01), and medium correlation to Fe, Mn, Zn and K concentrations (r = 0.39–0.48, P < 0.01). All mineral concentrations showed no significant correlation to GY or KW. Close correlations (significantly positive) were observed for content and yield between each two minerals (r = 0.33–0.96, P < 0.01). Kernel weight and GY showed positive correlations to all mineral contents (r = 0.29–0.86, P < 0.01) and yields (0.69–0.89, P < 0.01), respectively.
Significant positive correlations (r = 0.22–0.80, P < 0.01) were observed for a mineral between each two of concentration, content and yield, with an exception of a lower correlation between Zn concentration and Zn yield (r = 0.17, P = 0.013) (Supplementary Table S3). Additionally, the correlations between concentration and content were higher (r = 0.42–0.80, P < 0.01) than those either between concentration and yield (r = 0.17–0.50, P < 0.05) or between content and yield (r = 0.27–0.54, P < 0.01).
Identification of grain yield, kernel weight and grain mineral accumulation QTL
LOD threshold values were 2.9–3.3 for different traits (Supplementary Table S4). Among the 23 investigated traits, we identified 74 QTL in total for 21 traits across four environments (Fig. 1; Supplementary Table S5). No QTL were found for Cu concentration and Fe yield. The percentage of explained variance for QTL varied from 5.84 to 38.14 %. Two QTL, qZnCT1 and qMgCC10, explained more than 30 % of the phenotypic variance with 31.4 and 38.1 %, respectively. Another four QTL explained between 20 and 30 % phenotypic variance (Supplementary Table S5). Five and six QTL were identified for GY and KW, respectively. For the mineral traits, 24, 27, and 12 QTL were identified for concentration, content and yield, respectively.
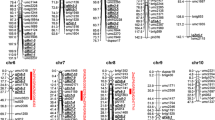
Chromosome localization of QTL for grain yield, kernel weight, and grain mineral concentration, content and yield in maize detected in Ye478 × Wu312 RIL populations. Each QTL to the left of the linkage map are above the LOD threshold for composite interval mapping (CIM) analysis. QTL are marked by its environment name with different sharp for different traits. Reference mark, hash, filled triangle, filled star, filled square, filled lower right triangle, filled circle, filled inverted triangle and filled diamond represented grain yield (GY), kernel weight (KW) and iron (Fe), manganese (Mn), zinc (Zn), copper (Cu), potassium (K), phosphorus (P) and magnesium (Mg) traits, respectively. Red, blue and yellow letters represent grain mineral concentration, content and yield, respectively. Chromosome Bins with yellow background represented important regions for controlling grain mineral traits with several QTLs co-localized here
QTL repeatedly detected across the different environments were considered as stable QTL (sQTL). About 77 % QTL (57 out of the 74 QTL) were only detected in a single environment, while other 17 QTL were detected in at least two environments. As a consequence, 8 sQTL were identified based on these 17 QTL with one (sQTL3.1) detected in three environment and 7 in two environments (Table 5). sQTL3.1 was detected for KW located at chromosome Bin 3.08/3.09 from three environments (GS09, GS10, and BJ09), explaining phenotypic variance of 20.8, 16.8, and 27.3 %, respectively (Table 5; Supplementary S5). Another sQTL(sQTL3.3) for K content was also detected at Bin 3.08/3.09, with explained phenotypic variance of 6.2 and 27.8 % in GS10 and BJ09 environments, respectively (Table 5; Supplementary Table S5). Moreover, three sQTL were identified for Mn concentration at Bin 1.04/1.05, 2.03/2.04 and 4.05/4.06 (Table 5). sQTL for Mg and Zn concentrations, Zn content were also detected.
Discussion
Genetic and phenotypic relationship between mineral concentration, content, and yield in maize grain
Enhancement of mineral accumulation (biofortification) in crop grain is one of the efficient ways to solve global nutrient malnutrition. One key step is to detect favorable QTL/genes/alleles. Several QTL mapping studies for maize grain minerals have detected QTL distributed across the ten chromosomes, and focused primarily on the most important minerals, Fe and Zn (Zhou et al. 2010; Lung’aho et al. 2011; Šimic et al. 2012). Beside Fe and Zn, QTL for Mg and P concentration were analyzed to detect three P-related QTL co-localizing on chromosome 3 (Šimic et al. 2012). A comprehensive study on 9 minerals in maize grain was also recently reported through single-kernel ionomic profile (Baxter et al. 2013). This work demonstrated the positive correlations among grain mineral concentrations with high correlation coefficients between Fe and Zn, P and Mg with no correlation found between mineral concentration and KW. In rice grain, high phenotypic correlation and co-localization of QTL for Mg and P concentration were also found (Zhang et al. 2014). Similarly, in this work, we detected significant correlations between each pair of mineral concentrations (except Cu) with the closest correlations between P and other minerals (Table 4). We also failed to show any significant correlation between mineral concentration and either KW or GY (Table 4). Moreover, we could not find any QTL co-location between mineral concentration (except Mn and Mg) and either KW or GY (Supplementary Table S6). These results indicated that the correlations of mineral concentration exist in crop grain, but correlations existing between mineral concentration and kernel mass traits were low or nonexistent. Thus, it is possible to increase several mineral concentrations as a whole without affecting grain yield in crop.
In contrast to the previous studies focused on grain concentration, we analyzed three mineral accumulation traits: concentration, content, and yield. QTL analysis for both concentration and content helped to identify co-localized concentration and content loci for each mineral. Four chromosomal regions were identified containing both concentration and content QTL (Supplementary Table S6). QTL for Mg concentration (qMgCC3.1 and qMgCC6) and content (qMgCT3 and qMgCT6) were found at Bin 3.04 and Bin 6.07/6.08, respectively. Co-localization of concentration and content QTL were also found for Mn and Zn at Bin 1.04/1.05 and Bin 5.05, respectively. Together, the fact that the favorable alleles for these four pairs QTL came from the same parent (Supplementary Table S5), suggests genetic correlations between mineral content and concentration in maize grain.
Environmental influences to mineral accumulation in maize grain
Mineral accumulation in maize grain is a complex trait affected by a number of factors, including genotype, environmental conditions and their interactions (House 1999; Baxter 2009; Baxter and Dilkes 2012). Plant breeders usually evaluate genetic materials in several environments to minimize the environmental effects and identify stable alleles (and genotypes). In this work, four environments with two locations × two years were applied to test the environmental effects on mineral accumulation in maize grain. The ANOVA showed significant (P < 0.01) effects for both year and location (Table 3) for all investigated mineral concentrations, contents and yields, in agreement with previous studies (Qin et al. 2012; Baxter et al. 2013).
Soil conditions and regional climatic factors considerably impact the environmental performance for crop production (Pacini et al. 2003). Climatic conditions during maize-growing season differed in these four experimental environments (Supplementary Table S2). BJ10 was extremely dry, which resulted in approximately 70 % yield loss compared to the average yield from the other three environments (Table 2). Higher values for all tested mineral traits were found in BJ10, which may be attributed to a concentration effect, i.e., reverse dilution effect due to lower grain yield and lower carbohydrate accumulation. However, the genetic features for the tested mineral traits in BJ10 did not differ from those in other environments because of the similar variance coefficients (Table 2), number of QTL detected (Supplementary Table S5), number of sQTL detected (Table 5), and number of co-localized QTL identified (Supplementary Table S6). Thus, BJ10 environment was able to be included for QTL analysis as a comprehensive experiment despite the presence of drought stress.
Soil properties for the two locations GS and BJ showed differences in mineral availabilities. Available Zn in BJ was higher than that in GS. Consistently, maize grain Zn accumulation in BJ was also found to be higher (Table 2). In addition, ANOVA analysis found higher location effects for both Zn concentration and content, but not for other elements (except Cu) (Table 3), further suggesting that soil Zn availability plays a critical role for its accumulation in maize grain. These results were consistent with other studies (Sharma and Bapat 2000; Chahal et al. 2005), which suggested that Zn accumulation in maize grain can be significantly improved by increasing soil Zn availability, e.g., through Zn fertilizer. Indeed, a recent work has found that Zn deficiency could be corrected by the application of Zn fertilizer to soil (Kumar and Bohra 2014).
For other elements, e.g., Cu, Fe, and Mn, the effects of soil element availability for grain mineral accumulations have not been resolved. Kumar et al. (2009) reported that Cu concentrations in leaves, grain, and straw increased significantly with an increase in the level of applied Cu in soil. Antunović et al. (2003) found that the mineral concentrations of Mn and Fe in maize grain were not in connection to the status of the corresponding elements in the experimental environments. It was presumed that interaction with other elements (e.g., Ca or Mg) could be responsible for this inconsistence for Cu, Fe, and Mn. We found that soil available Cu, Fe, and Mn were higher in GS location (Table 1). However, grain mineral accumulation in GS was similar to that in BJ (Table 3), except that BJ10 had higher mineral concentrations due to drought stress. Thus, our results suggested that soil availability of Cu, Fe, and Mn has little effect on their accumulations in maize grain.
Important QTL clusters for mineral accumulation in maize grain
Limited information is available about the genetic control and molecular mechanism contributing to high accumulation of minerals in maize grain. QTL analysis provides a powerful tool to identify chromosomal locations of genes suitable for breeding programs. In this work, we conducted a QTL analysis in four environments and found several chromosomal regions which contained stable QTL (across different environments) within a grain mineral trait or contained co-localized QTL across different traits (Fig. 1; Supplementary Table S6). In general, 52 QTL (70 %) out of the 74 detected QTL fell into 12 QTL clusters in which 3–6 QTL were grouped for different mineral traits or from different environments within a trait.
At chromosome Bin 6.07/6.08, five QTLs were detected from GS10, with one for KW and four for Zn, K, Mg and P contents (Supplementary Table S6). High phenotypic correlations between mineral contents and KW suggested the presence of corresponding QTLs for both traits in this QTL cluster. Moreover, in this region, we also detected a QTL for Mg concentration from BJ09 besides that for Mg content (Fig. 1; Supplementary Table S5, S6). Zhou et al. (2010) identified two QTL located in this region for Zn concentration using different populations. The fact of QTL co-localization for mineral concentration and content, and KW, suggested an important QTL at Bin6.07/6.08 for controlling mineral accumulation in maize grain. It also implicated that improvement with this QTL region could enhance grain mineral content via concentration and KW simultaneously.
Identified QTL for maize grain mineral accumulation traits have been always determined by specific genetic populations (Garcia-Oliveira et al. 2009; Zhou et al. 2010; Lung’aho et al. 2011; Šimic et al. 2012; Baxter et al. 2013, 2014). In this work, we identified 3 sQTL for Mn concentration at Bin1.04/1.05, 2.03/2.04 and 4.05/4.06. Among them, a sQTL (sQTL4.1) at Bin 4.05/4.06 contained 2 QTL for Mn concentration from environments GS10 and BJ10 explaining 12.3 % and 15.3 % of the phenotypic variance, respectively (Supplementary Table S5). The existence of QTL affecting Mn concentration was also reported in the same region from another maize population (Zhou et al. 2010). Moreover, a QTL for Mn concentration in rice grain has been reported on chromosome 2, which is a syntenic region of maize Bin 4.05/4.06 (Schnable et al. 2009; Zhang et al. 2014). These results suggest that Bin4.05/4.06 may contain common QTL for Mn concentration across different genetic background and even different crop species.
Author contribution statement
Conceived and designed the experiments: Lixing Yuan, Fanjun Chen and Riliang Gu. Analyzed the date: Riliang Gu, Fanjun Chen, Xin Wang and Jordon Pace. Performed field experiments: Fanjun Chen, Jianchao Liu, Pengcheng Li and Qingchun Pan. Performed lab measurement: Bingran Liu. Wrote the paper: Riliang Gu. Made the final revision: Lixing Yuan, Guohua Mi, Thomas Lübberstedt, Jordon Pace and Ayaz-Ali Soomro.
References
Abiola O, Angel JM, Avner P, Bachmanov AA, Belknap JK et al (2003) The nature and identification of quantitative trait loci: a community’s view. Nat Rev Genet 4:911–916
Anandan A, Rajiv G, Eswaran R, Prakash M (2011) Genotypic variation and relationships between quality traits and trace elements in traditional and improved rice (Oryza sativa L.) genotypes. J Food Sci 76:122–130
Antunović M, Kovačević V, Rastija M, Zdunić Z (2003) Influences of soil and genotypes on micronutrient status in maize plants. Agriculture 9:9–14
Baxter IR (2009) Ionomics: studying the social network of mineral nutrients. CurrOpin Plant Biol 12:381–386
Baxter I, Dilkes BP (2012) Elemental profiles reflect plant adaptations to the environment. Science 336:1661–1663
Baxter IR, Hermans C, Lahner B, Yakubova E, Tikhonova M et al (2012) Biodiversity of mineral nutrient and trace element accumulation in Arabidopsis thaliana. PLoS One 7:e35121
Baxter IR, Gustin JL, Settles AM, Hoekenga OA (2013) Ionomic characterization of maize kernels in the intermated B73 × Mo17 population. Crop Sci 53:208–220
Baxter IR, Ziegler G, Lahner B, Mickelbart MV, Foley R et al (2014) Single-kernel ionomic profiles are highly heritable indicators of genetic and environmental influences on elemental accumulation in maize grain (Zea may). PLoS One 9:e87628
Bouis HE, Welch RM (2010) Biofortification—a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci 50:S20–S32
Bremner JM (1996) Nitrogen-total. In: Sparks DL (ed) Methods of soil analysis. Part 3. Chemical methods. SSSA Book Ser. 5. SSSA and ASA, Madison, pp 1085–1121
Buescher E, Achberger T, Amusan I, Giannini A, Ochsenfeld C et al (2010) Natural genetic variation in selected populations of Arabidopsis thaliana is associated with ionomic differences. PLoS One 5:e11081
Cakmak I (2002) Plant nutrition research: priorities to meet human needs for food in sustainable ways. Plant Soil 247:3–24
Cakmak I, Pfeiffer WH, Mcclafferty B (2010) Biofortification of durum wheat with zinc and iron. Cereal Chem 87:10–20
Chahal DS, Sharma BD, Singh PK (2005) Distribution of forms of zinc and their association with soil properties and uptake in different soil orders in semi-arid soils of Punjab, India. Commun Soil Sci Plant Anal 36:2857–2874
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Fan MS, Zhao FJ, Fairweather-Taitc SJ et al (2008) Evidence of decreasing mineral density in wheat grain over the last 160 years. J Trace Elem Med Biol 22:315–324
Garcia-Oliveira AL, Tan LB, Fu YC, Sun CQ (2009) Genetic identification of quantitative trait loci for contents of mineral nutrients in rice grain. J Integr Plant Biol 51:84–92
Goto F, Yoshihara T, Toki S, Shigemoto N, Takaiwa F (1999) Iron fortification of rice seed by the soybean ferritin gene. Nat Biotech 17:282–286
Gregorio GB, Senadhira D, Htut H, Graham RD (2000) Breeding for trace mineral density in rice. Food Nutr Bull 21:382–386
House WA (1999) Trace element bioavailability as exampled by iron and zinc. Field Crops Res 60:115–141
Imtiaz M, Alloway BJ, Shah KH, Siddiqui SH, Memon MY, Aslam M, Khan P (2003) Zinc nutrition of wheat: growth and zinc uptake. Asian J Plant Sci 2:152–155
Kumar R, Bohra JS (2014) Effect of NPKS and Zn application on growth, yield, economics and quality of baby corn. Arch Agron Soil Sci 60:1193–1206
Kumar R, Mehrotra NK, Nautiyal BD, Kumar P, Singh PK (2009) Effect of copper on growth, yield and concentration of Fe, Mn, Zn and Cu in wheat plants (Triticum aestivum L.). J Environ Biol 30:485–488
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci Soc Am J 42:421–428
Liu J, Cai H, Chu Q, Chen X, Chen F, Yuan L, Mi G, Zhang F (2011) Genetic analysis of vertical root pulling resistance (VRPR) in maize using two genetic populations. Mol Breeding 28:463–474
Lung’aho MG, Mwaniki AM, Szalma SJ, Hart JJ, Rutzke MA, Kochian LV, Glahn RP, Hoekenga OA (2011) Genetic and physiological analysis of iron biofortification in maize kernels. PLoS One 6:e20429
Mayer JE, Pfeiffer WH, Bouis P (2008) Biofortified crops to alleviate micronutrient malnutrition. Curr Opin Plant Biol 11:166–170
McDowell SC, Akmakjian G, Sladek C, Mendoza-Cozatl D, Morrissey JB et al (2013) Elemental concentrations in the seed of mutants and natural variants of Arabidopsis thaliana grown under varying soil conditions. PLoS One 8:e63014
Nyquist WE (1991) Estimation of heritability and prediction of selection response in plant populations. Crit Rev Plant Sci 10:235–322
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Circular 939, United States Department of Agriculture, Washington
Pacini C, Wossink A, Giesen G, Vazzana C, Huirne R (2003) Evaluation of sustainability of organic, integrated and conventional faming systems: a farm and field-scale analysis. Agric Ecosys Environ 95:273–288
Pfeiffer WH, McClafferty B (2007) HarvestPlus: breeding crops for better nutrition. Crop Sci 47:S88–105
Philip JW, Martin RB (2005) Biofortifying crops with essential mineral elements. Trends Plant Sci 10:586–593
Pillen K, Zacharias A, Léon J (2003) Advanced backcross QTL analysis in barley (Hordeum vulgare L.). Theor Appl Genet 107:340–352
Qin HN, Cai LY, Liu ZZ, Wang GQ, Wang JG, Guo Y, Wang H (2012) Identification of QTL for zinc and iron concentration in maize kernel and cob. Euphytica 187:345–358
Schnable PS, Ware D, Fulton RS, Stein JC, Wei F et al (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326:1112–1115
Sharma BL, Bapat PN (2000) Levels of micronutrient cations in various parts of wheat and influenced by zinc and phosphorus application. J Indian Soc Soil Sci 48:130–134
Šimic D, Sudar R, Ledencan T, Jambrovic A, Zdunic Z, Brkic I, Kovacevic V (2009) Genetic variation of bioavailable iron and zinc in grain of a maize population. J Cereal Sci 50:392–397
Šimic D, Drinic SM, Zdunic Z, Jambrovic A, Ledencan T, Brkic J, Brkic A, Brkic I (2012) Quantitative trait loci for biofortification traits in maize grain. J Hered 103:47–54
Wang TL, Domoney C, Hedley CL, Casey R, Grusak MA (2003) Can we improve the nutritional quality of legume seeds? Plant Phys 131:886–891
Wang S, Basten CJ, Zeng ZB (2012) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182:49–84
Xue Y, Yue S, Zhang W, Liu D, Cui Z et al (2014) Zinc, iron, manganese and copper uptake requirement in response to nitrogen supply and the increased grain yield of summer maize. PLoS One 9:e93895
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Zhang M, Pinson SRM, Tarpley L, Huang XY, Lahner B, Yakubova E, Baxter I, Guerinot ML, Salt DE (2014) Mapping and validation of quantitative trait loci associated with concentrations of 16 elements in unmilled rice grain. Theor Appl Genet 127:137–165
Zhou JF, Huang YQ, Liu ZZ, Chen JT, Zhu LY, Song ZQ, Zhao YF (2010) Genetic analysis and QTL mapping of zinc, iron, copper and manganese contents in maize seed. J Plant Genet Resour 11:593–595
Acknowledgments
This study was supported by the Ministry of Science and Technology of China (2012AA100306, 2011CB100305); National Natural Science Foundation of China (31421092); the Ministry of Agriculture of China (2014ZX08003-005); Danish Strategic Research Council (NUTRIEFFICIENT 10-093498) and Chinese Universities Scientific Fund (2015ZH001).
Conflict of interest
The authors declare that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Gore.
R. Gu and F. Chen contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gu, R., Chen, F., Liu, B. et al. Comprehensive phenotypic analysis and quantitative trait locus identification for grain mineral concentration, content, and yield in maize (Zea mays L.). Theor Appl Genet 128, 1777–1789 (2015). https://doi.org/10.1007/s00122-015-2546-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-015-2546-5