Abstract
Gametocidal (Gc) chromosomes induce various types of chromosomal mutations during gametogenesis in the chromosomes of common wheat and alien chromosomes added to common wheat. However, it is not yet known whether the Gc chromosome causes aberrations at the nucleotide level because mutations caused by Gc chromosomes have been studied only by cytological screening. In order to know whether the Gc chromosome induces point mutations, we conducted PCR analysis and sequencing with the progeny of a common wheat line that is disomic for barley chromosome 2H and monosomic for Gc chromosome 2C. We analyzed 18 2H-specific EST sequences using 81 progeny plants carrying a cytologically normal-appearing 2H chromosome and found no nucleotide changes in the analyzed 1,419 sequences (in total 647,075 bp). During this analysis, we found six plants for which some ESTs could not be PCR amplified, suggesting the presence of chromosomal mutations in these plants. The cytological and PCR analyses of the progeny of the six plants confirmed the occurrence of chromosomal mutations in the parental plants. These results suggested that the Gc chromosome mostly induced chromosomal aberrations, not nucleotide changes, and that the Gc-induced chromosomal mutations in the six plants occurred after fertilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mutations, heritable changes in genetic material, occur spontaneously or can be induced by mutagens in all plant and animal species. After Stadler’s (1928) first experiment on barley, physical and chemical mutagens have been successfully used in plant breeding programs to generate artificial genetic variations leading to new varieties with improved traits (Maluszynksi 2001). Each mutagen has a specific behavior and can be used according to need for the plant improvement. Physical mutagens, such as non-ionizing (e.g. UV rays) and ionizing (e.g. X-rays, alpha rays, gamma rays, and fast and slow neutrons) radiations, induce breakage of DNA strands, resulting in large scale changes in chromosomal structure. By contrast, chemical mutagens like ethyl methane sulphonate (EMS), sodium azide, N-methyl-N-nitrosourea and hydroxylamine all of which are widely used to induce mutations in plants, affect single nucleotide pairs and cause nucleotide changes like base substitutions and frame shifts. Genetic variations caused by chemical mutagens can be screened by reverse genetic approaches, one of which is TILLING (Targeting induced local lesions in genomes). For this technique, mutant populations have been developed in many cereal crops including rice (Suzuki et al. 2008), maize (Till et al. 2004), sorghum (Xin et al. 2008), barley (Talamè et al. 2008), and tetraploid and hexaploid wheat (Slade et al. 2005; Uauy et al. 2009). Besides physical and chemical mutagens, specific DNA sequences known as mobile genetic elements or transposable elements (transposons and retrotransposons) are responsible for creating genetic variability due to their ability to cause mutations through excision, insertion, translocation, and irregular recombination (Hirochika et al. 1996; Cowan et al. 2005; Kumar and Bennetzen 1999; Havecker et al. 2004).
In addition, certain chromosomes or genes in the members of Triticeae (a tribe in the grass family including wheat) are known to induce structural changes in chromosomes. Many alien-chromosomes were introduced into common wheat (or hexaploid wheat) from its wild relatives in the genus Aegilops by backcrossing common wheat-Aegilops hybrids to common wheat. In the course of backcrossing, some of the alien chromosomes had effects like mutagenic agents in the host, namely common wheat (Endo 1990). The chromosomes or genes were named Gametocidal (Gc) chromosomes or genes, and they cause chromosomal mutations in a unique manner during gametogenesis, i.e. only in the gametes that lack the Gc chromosomes (Endo 1990, 2007). The Gc chromosome induces breakage not only in wheat chromatin but also in alien chromatin, such as the chromosomes of barley and rye introduced into the wheat genetic background as alien additions (Endo 2007).
Among the Gc chromosomes, chromosomes 2C and 3CSAT derived from Ae. cylindrica (2n = 4x = 28, DDCC) and Ae. triuncialis (2n = 4x = 28, UUCC), respectively, have been widely used to induce chromosomal breakage in wheat and alien chromosome addition lines of wheat (Endo and Gill 1996; Shi and Endo 1997, 1999, 2000; Friebe et al. 2000; Endo 2007). Structural aberrations of barley chromosomes can be identified cytologically by in situ hybridization using a barley genomic DNA probe and the barley-specific, subtelomeric repetitive sequence probe HvT01. Thus, various structural changes were singled out to develop common wheat lines carrying single barley chromatin segments, the so-called dissection lines, for individual barley chromosomes. The dissection lines have been used for cytological mapping of barley chromosomes using molecular markers, namely ESTs (Nasuda et al. 2005; Ashida et al. 2007; Sakai et al. 2009; Sakata et al. 2010; Joshi et al. 2011); however, in situ hybridization cannot reveal sub-microscopic chromosomal aberrations, much less point mutations if they occur at all. It is important to know whether the Gc chromosome induces point mutations in terms of not only clarifying the molecular mechanism of Gc action but also exploiting the Gc system for mutation breeding of wheat.
We planned this study to demonstrate whether the Gc chromosome induces changes in nucleotide sequences. We used the progeny of an alien addition line of ‘Chinese Spring’ wheat that is disomic for barley chromosome 2H and monosomic for a gametocidal chromosome 2C. We cytologically screened the progeny for plants carrying a 2H chromosome of normal appearance, with which we PCR-amplified 2H-specific EST markers and sequenced them. We did not use plants carrying 2H chromosomal segments to avoid being confused in the sequence analysis.
Materials and methods
Plant materials
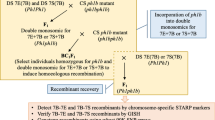
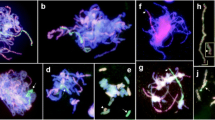
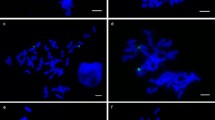
We used two alien addition lines of common wheat (Triticum aestivum L., 2n = 6x = 42, genome formula AABBDD) cultivar Chinese Spring (CS). One line is a disomic addition for barley (cv. Betzes) chromosome 2H produced by Islam et al. (1981), and the other line is a disomic addition for gametocidal (Gc) chromosome 2C produced by Endo (1988). The 2C chromosome causes chromosomal structural changes in gametes in which the 2C chromosome is not present (Endo 1988). In addition to the two addition lines, euploid CS was used as the control. We obtained these lines from the National BioResource Project-Wheat (NBRP-Wheat) (http://www.shigen.nig.ac.jp/wheat/komugi/top/top.jsp), where the lines are stored with accession numbers LPGKU2269 (euploid CS), LPGKU2088 (disomic addition of 2H in CS), and LPGKU2153 (disomic addition of 2C in CS). We made a cross between the 2H and 2C addition lines to produce an F1 hybrid (2n = 44, 21″ + 1′ 2H + 1′ 2C) and then backcrossed the F1 to the 2H addition line to obtain BC1 plants (2n = 45, 21″ + 1″ 2H + 1′ 2C). We hand-pollinated the BC1 progeny with euploid CS pollen to obtain the BC2 progeny. We found three types of chromosome constitutions in the progeny: normal 2H plus 2C, non-aberrant 2H plus no 2C, and aberrant 2H plus no 2C. For our study, we selected 81 plants with non-aberrant 2H plus no 2C to examine Gc-induced point mutations, if they occur at all, along the entire 2H chromosome. We checked for the presence of the 2C chromosome by the spike morphology of the plants; the presence of 2C was indicated by a tip-awned spike that was tougher and more slender (Fig. 1). We surveyed the 2H chromosome by fluorescence in situ hybridization (FISH) using the HvT01 probe that is specific to subtelomeric regions of all barley chromosomes (Belostotsky and Ananiev 1990) and by genomic in situ hybridization (GISH) using a total barley genomic DNA probe (Fig. 2). The procedures for chromosome preparation and simultaneous FISH/GISH were conducted as described by Sakai et al. (2009).
Selection of EST markers
Among the EST markers that had previously been mapped on the 2H chromosome (Joshi et al. 2011), we selected 18 representative markers that showed clear single PCR products of ca. 500 bp and were distributed rather evenly over the length of the 2H chromosome for sequence analysis (EST clone sequences were according to Sato et al. 2009, Stein et al. unpublished, and Biemelt et al. unpublished), and an additional 40 2H-specific EST markers (Table 1) to characterize aberrant 2H chromosomes that were found during the PCR analysis in this study. The fraction length (FL) reported in Table 1 indicates the marker position on the chromosome. The FL values, cited from Joshi et al. (2011), represent the breakpoints of the aberrant 2H chromosomes flanking the EST markers.
PCR analysis
We conducted PCR using template DNA extracted from leaves by the DNeasy Plant Mini Kit (QIAGEN), and the 58 primer sets for the EST markers (Table 1). We added 2 μl of DNA solution (ca. 15 ng/μl) as a template to a PCR mixture consisting of 10 μl of 5× PCR buffer, 1.0 μl of dNTP (10 mM each), 3.75 μl of MgCl2 (25 mM), 2.5 μl of primers (10 pmol/μl), 0.25 μl of KAPATaq Extra DNA Polymerase (5 U/μl KAPABIOSYSTEMS, USA), and 30.5 μl of dH2O. We conducted thermal cycling with an iCycler (BioRad, USA) using the following conditions: 94 °C for 2 min, 5 cycles of 94 °C for 30 s, 65 °C for 30 s (with the temperature subsequently decreased 1 °C per cycle), and 72 °C for 1 min, 35 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min, and 72 °C for 7 min. PCR products are fractionated and visualized by agarose gel electrophoresis (1.5 % Agarose S (Nippon Gene, Japan), 200 V, 40 min) and ethidium bromide staining.
Sequencing
We purified the checked PCR products of the 18 representative ESTs by PEG precipitation and sequenced the purified PCR products in both orientations at the Dragon Genomics Center, Takara-Bio Inc., Japan. Direct sequencing was performed using Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA) and analyzed with ABI 3130xl Genetic Analyzer (Applied Biosystems, USA). We analyzed the sequences with MEGA5 (Tamura et al. 2011) and aligned the sequences with CLUSTALW (Thompson et al. 1994).
Results
PCR analysis
Out of the 81 plants, 75 plants showed clear PCR amplification for all 18 markers as expected, but only six plants showed irregular patterns of PCR amplification (Table 2). Plants 2H-77 and 2H-78 lacked five markers within FL0.59–1.00 and six markers within FL0.52–1.00 on the short arm, respectively. Plant 2H-79 lacked five markers within FL0.57–1.00 on the long arm. Plant 2H-80 showed no PCR amplification for four markers within FL0.60–1.00 on the long arm. Plant 2H-76 lacked one marker close to the HvT01 sequences on the short arm, and plant 2H-81 lacked two markers close to the HvT01 sequences on the long arm. The above PCR amplification patterns suggested that two plants (2H-76 and 2H-81) had aberrant 2H chromosomes with minor deletions in the distal regions and that the other four plants (2H-77, 2H-78, 2H-79 and 2H-80) had aberrant 2H chromosomes with extensive deletions. This PCR result obviously contradicted the FISH/GISH result that had showed the presence of a normal-appearing 2H chromosome in root tip meristems of the six plants, and therefore implied that the breakage of the 2H chromosomes occurred during very early stages of differentiation after fertilization.
Analysis of the progeny of the six plants with irregular PCR amplification
We cytologically analyzed the progeny of the six plants with irregular PCR amplification and found no normal appearing of 2H chromosome that we had observed in the root tip cells of the parental plants; instead, we found aberrant 2H chromosomes (Fig. 2). We measured the FL values of the aberrant 2H chromosomes (an average of five photomicrographs for each), according to the procedure employed by Endo and Gill (1996). Chromosomes 2H-77 and 2H-78 had terminal deletions with breakpoints at FL0.53 and FL0.40 in the short arm, respectively, and chromosomes 2H-80 and 2H-81 had terminal deletions with breakpoints at FL0.58 and FL0.75 in the long arm, respectively. Chromosomes 2H-76 and 2H-79 were translocation chromosomes between 2H and wheat chromosomes with translocation points at FL0.89 in the short arm and at FL0.49 in the long arm, respectively. We further examined the progeny from a second spike for each of the six plants and found the same aberrant 2H chromosomes. These findings indicated that these 2H aberrations had occurred after fertilization, most likely after differentiation into root and shoot meristems.
The FL lengths of the aberrant 2H chromosomes in the progeny plants were consistent with the PCR result of the parental plants, i.e. the FL values were within the ranges estimated from the chromosomal positions of the 18 EST markers (Table 1). We further conducted PCR analysis of the 18 markers in the progeny of the six parental plants and obtained the same PCR results as found for the parental plants.
Plant 2H-76 lacked one representative marker (k01312), and further PCR analysis using nine additional markers in the vicinity of the breakpoint of chromosome 2H-76 in the short arm (FL0.85–1.00) divided the 11 markers, including the two representative ones (k01312 and k04629), into two groups, nine distal and two proximal to the breakpoint (FL0.89) (Fig. 3). Although the breakpoints of the other aberrant 2H chromosomes did not divide the markers, they narrowed the bin areas where the markers were located. For example, plant 2H-81 lacked two representative markers (k00932 and k00730), and also 13 additional markers located in the most distal bin (FL0.64–1.00) in the long arm (Joshi et al. 2011). This result, therefore, indicated that all 15 markers lay in a narrower bin distal to the breakpoint of chromosome 2H-81 (FL0.75) (Fig. 3). Figure 4 shows the latest version of the cytological map of chromosome 2H constructed after incorporating the data obtained in this study and those from Joshi et al. (2011).
Addition of six breakpoints to the cytological map of chromosome 2H constructed by Joshi et al. (2011). FL in parentheses: representative breakpoints flanking the 18 representative markers, FL in italics: position of C bands, ND FL non determined, EST markers (boldface italics): 18 representative markers used for PCR and sequence analyses, EST markers (regular face): additional 40 markers used to characterize the aberrant 2H chromosome
Revision of the cytological map of chromosome 2H constructed by Joshi et al. (2011). FL in parentheses: breakpoints of 72 aberrant 2H chromosomes including six obtained in this study (boldface), FL in italics: position of C bands
Sequence analysis
The amplified PCR products of the 18 representative ESTs ranged in length from 370 to 650 bp, accounting for ca. 9 kb (Table 3) altogether. We used the sequence data for BLASTN searches of the barley expressed sequence tags (ESTs) at the NCBI (National Center for Biological Information) database and found that all the sequences were in clones of Hordeum vulgare (Table 3). Chromosome 2H has been estimated to be 790 Mbp long (Suchánková et al. 2006) out of 5.1 Gbp of barley genome (Dolezel et al. 1998). We analyzed a total of 1,419 sequences of the 81 plants and the control 2H addition line to check for the occurrence of point mutations in the amplified regions. We examined the forward and reverse reads of each PCR product separately, and rechecked any ambiguous nucleotide changes found in one read in the other complementary reads. In this manner, we found no confirmed nucleotide changes, i.e. the same nucleotide change in the forward and reverse reads, in any of the sequences (Table 3).
Discussion
When present in the monosomic condition, chromosome 2C causes various kinds of chromosomal aberrations in wheat and also in wheat–barley addition lines during the gametogenesis of meiocytes (Endo and Gill 1996; Shi and Endo 1999; Sakai et al. 2009; Sakata et al. 2010; Joshi et al. 2011). Several previous studies suggested that the Gc system induces chromosomal aberrations at different developmental stages in embryos, endosperms as well as in meiocytes. For example, Nasuda et al. (1998) observed that three different Gc chromosomes (T2B-2S of Ae. speltoides, T4B-4Ssh of Ae. sharonensis and 2CcL of Ae. cylindrica, the same as the 2C chromosome used in the study) induce chromosomal breaks in the first postmeiotic mitosis of pollen grains. Tsujimoto et al. (2001) reported that the 2C chromosome induces breakage of chromosomes after the formation of megaspores and found that different chromosomal breakages occurred in embryos and endosperms. Similarly, King and Laurie (1993) observed chromosomal aberrations during early embryo and endosperm development in reciprocal crosses between ‘Chinese Spring’ monosomic 4B and an alien substitution line in which chromosome 4B is replaced by a Gc chromosome 4Sl of Ae. sharonensis. Tsujimoto and Noda (1990) also suggested that the Gc chromosome T2B-2Ssp.au of Ae. speltoides induces mutations after fertilization and ceases before differentiation of the shoot primordia.
If Gc-induced chromosomal breakage occurs exclusively during gametogenesis and stabilizes at the time of zygote formation, identical results should be obtained from cytological observations of root tip cells and PCR analysis of leaf tissues from the same plant; however, this was not always the case in this study. The contradictory results of cytological screening and PCR analysis in this study provided other positive evidence that the Gc chromosome 2C caused postzygotic chromosomal aberrations. The 2C chromosome might have affected DNA replication in postzygotic cell divisions to cause aberrations only in either of the newly replicated DNA strands or sister chromatids, and then the intact and aberrant chromatids might have moved into separate daughter cells that eventually developed into root and shoot meristems. If this was the case, the contradictions between FISH/GISH observations and PCR analysis in this study would be explicable because we used root tip cells for in situ hybridization and aerial parts to extract DNA for PCR amplification. Thus, whatever the mechanism of the Gc action of chromosome 2C, FISH/GISH screening using root tip cells could not detect a significant percentage of aberrations induced by the Gc system. Therefore, it is advisable to employ PCR, in addition to FISH/GISH, in screening for Gc-induced structural changes of alien chromosomes in common wheat, as long as some alien chromosome-specific terminal PCR markers, preferably on both chromosome arms, are available. The detection rate (7.4 %) of aberrant 2H chromosomes by PCR in this study was much higher than by FISH/GISH screening (4.4 %) conducted by Joshi et al. (2011).
Thuillet et al. (2002) directly estimated the mutation rate for microsatellite loci in Triticum turgidum ssp. durum (Durum wheat) to be 2.4 × 10−4 per allele per generation. By contrast, the frequency of induced mutations varies according to the type of mutagen and their intensity or concentration applied to plants. Ethylmethane sulphonate (EMS) induces mutations at a rate of at least one mutation per 24 and 40-kb screened in the hexaploid and tetraploid wheat TILLING populations, respectively (Slade et al. 2005). Using a similar EMS concentration, Uauy et al. (2009) reported one mutation per 49.4- and 68-kb in the hexaploid and tetraploid wheat populations, respectively. The frequency of chromosomal mutations observed in this study, 6 out of 81 plants (7.4 %), seems to be very high. By contrast, no point mutations were observed in the analysis of a ca. 650-kb sequence (ca. 8-kb region in total of the 18 loci excluding primer annealing sites of the aligned sequences for 81 Gc-subjected plants) (Table 3). We cannot rule out the possibility of point mutations in non-coding sequences that have not examined in the present study. Alternatively, the point mutations induced by the Gc chromosome 2C are so less-frequent as to be detected by the survey of the 640 kb coding sequence. It is worth mentioning that the large volume bin-mapping efforts in wheat and relatives using deletion lines induced by the Gc system with various types of DNA markers have not detected point mutations (reviewed in Endo 2007). To have a genome-wide view of point mutations induced by the Gc system, broader re-sequencing effort, which is not applicable in hexaploid wheat yet, would be required. Genome scanning by recently developed genotyping-by-sequencing approach (Poland et al. 2012) would be adopted in future experiments.
Since Gc-induced chromosomal breakage is stabilized in subsequent generations, the translocation lines carrying alien segments without point mutations would be suitable for wheat improvement programs. Although most of these translocations are between non-homoeologous segments, selection of rare compensating translocations could also be achieved by screening a large number of Gc-induced progeny plants. In this regard, employing both screening methods, namely cytological (FISH/GISH) and PCR-based screening, will ensure the identification of almost all kinds of chromosomal aberrations caused by the action of the Gc chromosome.
Abbreviations
- Gc:
-
Gametocidal
- PCR:
-
Polymerase chain reaction
- EST:
-
Expressed sequence tag
- FISH:
-
Fluorescence in situ hybridization
- GISH:
-
Genomic in situ hybridization
References
Ashida T, Nasuda S, Sato K, Endo TR (2007) Dissection of barley chromosome 5H in common wheat. Genes Genet Syst 82:123–133
Belostotsky DA, Ananiev EV (1990) Characterization of relic DNA from barley genome. Theor Appl Genet 80:374–380
Cowan RK, Hoen DR, Schoen DJ, Bureau TE (2005) Mustang is a novel family of domesticated transposase genes found in diverse angiosperms. Mol Biol Evol 22:2084–2089
Dolezel J, Greilhuber J, Lucretti S, Meister A, Lysak MA, Nardi L, Obermayer R (1998) Plant genome size estimation by flow cytometry: inter-laboratory comparison. Ann Bot 82:17–26
Endo TR (1988) Induction of chromosomal structural changes by a chromosome of Aegilops cylindrica L. in common wheat. J Hered 79:366–370
Endo TR (1990) Gametocidal chromosomes and their induction of chromosome mutations in wheat. Jpn J Genet 65:135–152
Endo TR (2007) The gametocidal chromosome as a tool for chromosome manipulation in wheat. Chromosom Res 15:67–75
Endo TR, Gill BS (1996) The deletion stocks of common wheat. J Hered 87:295–307
Friebe B, Kynast RG, Gill BS (2000) Gametocidal factor-induced structural rearrangements in rye chromosomes added to common wheat. Chromosom Res 8:501–511
Havecker ER, Gao X, Voytas DF (2004) The diversity of LTR retrotransposons. Genome Biol 5:225
Hirochika H, Sugimoto K, Otsuki Y, Tsugawa H, Kanda M (1996) Retrotransposons of rice involved in mutations induced by tissue culture. Proc Natl Acad Sci USA 93:7783–7788
Islam AKMR, Shepherd KW, Sparrow DHB (1981) Isolation and characterization of euplasmic wheat–barley chromosome addition lines. Heredity 46:161–174
Joshi GP, Nasuda S, Endo TR (2011) Dissection and cytological mapping of barley chromosome 2H in the genetic background of common wheat. Genes Genet Syst 86:231–248
King IP, Laurie DA (1993) Chromosome damage in early embryo and endosperm development in crosses involving the preferentially transmitted 4Sl chromosome of Aegilops sharonensis. Heredity 70:52–59
Kumar A, Bennetzen JL (1999) Plant retrotransposons. Annu Rev Genet 33:479–532
Maluszynksi M (2001) Officially released mutant varieties-the FAO/IAEA database. Plant Cell Tissue Organ Cult 65:175–177
Nasuda S, Friebe B, Gill BS (1998) Gametocidal genes induce chromosome breakage in the interphase prior to the first mitotic cell division of the male gametophyte in wheat. Genetics 149:1115–1124
Nasuda S, Kikkawa Y, Ashida T, Islam AKMR, Sato K, Endo TR (2005) Chromosomal assignment and deletion mapping of barley EST markers. Genes Genet Syst 80:357–366
Poland JA, Brown PJ, Sorrells ME, Jannink JL (2012) Development of high-density genetic maps for barley and wheat using a novel two-enzyme genotyping-by-sequencing approach. PLoS One. 7:e32253
Sakai K, Nasuda S, Sato K, Endo TR (2009) Dissection of barley chromosome 3H in common wheat and a comparison of 3H physical and genetic maps. Genes Genet Syst 84:25–34
Sakata M, Nasuda S, Endo TR (2010) Dissection of barley chromosome 4H in common wheat by the gametocidal system and cytological mapping of chromosome 4H with EST markers. Genes Genet Syst 85:19–29
Sato K, Nankaku N, Takeda K (2009) A high density transcript linkage map of barley derived from a single population. Heredity 103:110–117
Shi F, Endo TR (1997) Production of wheat–barley disomic addition lines possessing an Aegilops cylindrica gametocidal chromosome. Genes Genet Syst 72:243–248
Shi F, Endo TR (1999) Genetic induction of structural changes in barley chromosomes added to common wheat by a gametocidal chromosome derived from Aegilops cylindrica. Genes Genet Syst 74:49–54
Shi F, Endo TR (2000) Genetic induction of chromosomal rearrangements in barley chromosome 7H added to common wheat. Chromosoma 109:358–363
Slade AJ, Fuerstenberg SI, Loeffler D, Steine MN, Facciotti D (2005) A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat Biotechnol 23:75–81
Stadler LJ (1928) Mutations in barley induced by X-rays and radium. Science 68:186–187
Suchánková P, Kubaláková M, Kovářová P, Bartoš J, Číhalíková J, Molnár-Láng M, Endo TR, Doležel J (2006) Dissection of the nuclear genome of barley by chromosome flow sorting. Theor Appl Genet 113:651–659
Suzuki T, Eiguchi M, Kumamaru T, Satoh H, Matsusaka H, Moriguchi K, Nagato Y, Kurata N (2008) MNU-induced mutant pools and high performance TILLING enable finding of any gene mutation in rice. Mol Genet Genomics 279:213–223
Talamè V, Bovina R, Sanguineti MC, Tuberosa R, Lundqvist U, Salvi S (2008) TILLMore, a resource for the discovery of chemically induced mutants in barley. Plant Biotechnol J 6:477–485
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice”. Nucl Acids Res 22:4673–4680
Thuillet A, Bru D, David J, Toumet P, Santoni S, Sourdille P, Bataillon T (2002) Direct estimation of mutation rate for 10 microsatellite loci in durum wheat, Triticum turgidum (L.) Thell. ssp durum desf. Mol Biol Evol 19:122–125
Till BJ, Reynolds SH, Weil C et al (2004) Discovery of induced point mutations in maize genes by TILLING. BMC Plant Biol 4:12
Tsujimoto H, Noda K (1990) Mutation of five marker genes in wheat by gametocidal gene of Ae. speltoides, Gc1a. Wheat Inf Serv 71:6–9
Tsujimoto H, Yamada T, Hasegawa K, Usami N, Kojima T, Endo TR, Ogihara Y, Sasakuma T (2001) Large scale selection of lines with deletions in chromosome 1B in wheat and applications for fine deletion mapping. Genome 44:501–508
Uauy C, Paraiso F, Colasuonno P, Tran RK, Tsai H, Berardi S, Comai L, Dubcovsky J (2009) A modified TILLING approach to detect induced mutations in tetraploid and hexaploid wheat. BMC Plant Biol 9:115
Xin ZG, Wang ML, Barkley NA, Burow G, Franks C, Pederson G, Burke J (2008) Applying genotyping (TILLING) and phenotyping analyses to elucidate gene function in a chemically induced sorghum mutant population. BMC Plant Biol 8:103
Acknowledgments
The aberrant 2H lines developed in this study will be deposited with the National BioResource Project-Wheat. This work was partially supported by the National BioResource Project, MEXT Japan. Contribution number 609 from the Laboratory of Plant Genetics, Graduate School of Agriculture, Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Friebe.
Rights and permissions
About this article
Cite this article
Joshi, G.P., Endo, T.R. & Nasuda, S. PCR and sequence analysis of barley chromosome 2H subjected to the gametocidal action of chromosome 2C. Theor Appl Genet 126, 2381–2390 (2013). https://doi.org/10.1007/s00122-013-2142-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-013-2142-5