Abstract
The Kunitz trypsin inhibitor (KTi) in soybean has several polymorphic types that are controlled by multiple alleles, which behave in a co-dominant fashion. Of these, Tia and Tib, which differ by nine amino acids, are the predominant types. In order to develop a single nucleotide amplified polymorphism (SNAP) marker for the classification of the predominant KTi types, Tia and Tib, and evaluate KTi activities by differing KTi type total 451 soybean mutant lines (M12–M16 generation) were incorporated in this study. Among 451 soybean mutants, 144 and 13 mutant lines showed decreased and increased trypsin inhibitor activity when compared with the original cultivars, respectively. To identify the KTi type, we designed a SNAP marker. Among 451 mutant lines from 12 soybean cultivars and landraces, 8 mutant lines derived from cvs. Baekwoon, Paldal and Suwon115 showed a change in KTi type when compared with the original cultivars using the SNAP marker. Five mutant lines in Suwon115 changed from Tib to Tia, while two mutant lines derived from cv. Baekwoon and one mutant line derived from cv. Paldal were changed from Tia to Tib. These changes of KTi types were confirmed by sequencing of the KTi genes and non-denaturing polyacrylamide gel electrophoresis of the KTi proteins. To identify the effect of KTi activity based on the change in KTi type, we measured the KTi activity using the three cultivars and eight mutant lines that showed changes in KTi type. Two mutant lines (BW-1 and 7-2) derived from cv. Baekwoon and one mutant line (PD-5-10) from cv. Paldal that changed from Tia to Tib showed lower activity than the original cultivar. In cv. Suwon115, five mutant lines that changed from Tib to Tia showed higher activity than the original cultivar. These results indicate that the designed SNAP marker was capable of identifying the KTi type and that Tia activity was higher than Tib activity in soybean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soybeans are a major source of protein worldwide. The proteins in soybeans have traditionally been used for animal feed, but are increasingly finding their way into functional foods destined for human consumption (Donald et al. 2007). The total seed storage protein in a soybean contains approximately 6% proteinase inhibitors belonging to two major classes, the Kunitz trypsin inhibitor (KTi, 21 kDa) and the Bowman–Birk trypsin inhibitor (BBTi, 7–8 kDa) (Laskowski and Kato 1980; Wang et al. 2004). The trypsin inhibitor activities ranged from 18.2 to 71.6 trypsin inhibitor unit (TIU) in soybean meal (Mohamed and Rangappa 1992; Toledo et al. 2007). The effects of trypsin inhibitors on animal growth occur via inhibition of intestinal digestion. As a result, the presence of proteinase inhibitors in diets consisting of free amino acids leads to decreased growth (Lajolo and Genovese 2002). Additionally, KTi and BBTi were found to induce enlargement of the pancreas (hypertrophy and hyperplasia) and hypersecretion of digestive enzymes in rodents and birds. This loss in sulfur-rich endogenous proteins, trypsin and chymotrypsin would result in growth depression because soy proteins are deficient in sulfur-containing amino acids such as methionine (Lajolo and Genovese 2002).
Soybean KTi has been found to have 12 distinguishable electrophoretic forms: Tia, Tib (Singh et al. 1969), Tic (Hymowitz 1973), Tid (Zhao and Wang 1992), Tie (Wang et al. 1996, 2001), Ti-null type (Orf and Hymowitz 1979), Tif (Wang et al. 2004), Tibi5 (Wang and Li 2005), Tiaa1, Tiaa2, Tiab1 and Tig (Wang et al. 2008). These types are controlled by co-dominant multiple alleles at a single locus (Wang et al. 2008). Studies of amino acid and nucleotide sequences of polymorphic variants of KTi have revealed large variations in the sequences of KTi types, with differences in nine amino acid residues between Tia and Tib being reported (Song et al. 1993; Wang et al. 2004). The amino acid sequences of Tic, Tid and Tie differ from Tia by only one amino acid (Kim et al. 1985; Xin et al. 1999; Wang et al. 2001), while Tif differs from Tib by one amino acid (Wang et al. 2004). Kaizuma et al. (1980) suggested that the differentiation of Tia and Tib was ancient and had likely been completed prior to domestication of cultivated soybeans from wild soybeans. They considered the Tia type to be the prototype from which Tib was derived because of an absolute ascendancy of Tia in wild soybeans. Although there is no other evidence to enforce this hypothesis, the frequency of Tia in wild soybeans that originate from China and other Asian countries reported by Hymowitz and Kaizuma (1981), and Li (1993) supports this idea.
Wang et al. (2008) reported a total of 11 allelic polymorphisms at the SKTI locus in the subgenus Soja, except for Tid (Zhao and Wang 1992; Xin et al. 1999). Tia, Tib and Tic are common in both wild and cultivated soybeans, while the other seven KTi types (Tie, Tif, Tibi5, Tiaa1, Tiaa2, Tig and Tiab1) have been found in wild soybeans. Conversely, Tid was found in a cultivar that originated from China (Wang et al. 2008). Although there is a difference in KTi types, they perform a function similar to trypsin inhibitors, and their bio-function may be replaced by other similar functional proteins (Birk 1961; Birk et al. 1963; Frattali and Steiner 1968; Rachis and Anderson 1964; Yamamoto and Ikenaka 1967).
Single nucleotide polymorphisms (SNPs) have been defined as single-base changes or indels (insertions and deletions) at specific nucleotide positions (Kim et al. 2005). In soybean, a total of 280 SNPs including 233 single-base changes and 47 indels were found in a 76.3-kbp sequence from 25 different soybean genotypes (Zhu et al. 2003). Because SNPs are highly stable markers and often contribute directly to a phenotype, they can serve as a powerful tool for marker-assisted selection and map-based cloning when combined with high-throughput genotyping systems (Kim et al. 2005). SNPs can be detected using allele-specific PCR primers designed such that the 3′ nucleotide of a primer corresponds to the site of the SNP (Ugozzoli and Wallace 1991). Thus, the allele-specific primer matches exactly with the specific allele and has a 3′ mismatch with the non-specific allele because the mismatched 3′ termini are extended by DNA polymerases with a much lower efficiency than correctly matched termini (Petruska et al. 1988). However, it is difficult to use this allele-specific PCR to ensure that the two SNP alleles can be distinguished. To overcome this difficulty and increase specificity, a single nucleotide amplified polymorphism (SNAP) marker that uses a modified allele-specific primer with a mismatched base pair within four bases of the 3′ termini in addition to the 3′ termini base that is complementary to the SNP site was recently developed (Drenkard et al. 2000; Hayasi et al. 2004).
This study was conducted to determine the change in KTi type using a SNAP marker in 451 mutant lines derived from 12 soybean cultivars and landraces. In addition, we evaluated the change in KTi type to determine if it affected the activity of the trypsin inhibitor in mutant lines.
Materials and methods
Plant materials
Soybean seeds were irradiated with gamma rays generated using a 60Co gamma-irradiator (150 TBq of capacity; ACEL, ON, Canada) at the Korea Atomic Energy Research Institute (KAERI). The irradiated seeds and the controls were sown at the breeding research farm at the KAERI in 1988, 1993 and 1997. The M1 plants were harvested individually and carried forward to the M2 generation. Genetically fixed mutant lines (M12–M16 generation) were selected with excellent agricultural characteristics from 1989 to 2007. For the DNA extraction, the mutant lines, cultivars and landraces were cultivated in 2008. Specifically, 451 soybean mutant lines derived from eight cultivars (94 Seori, 95 Seori, Baekwoon, Bangsa, Hwangguem, J2, Suwon115 and Paldal) and four landraces (KAS 360-22, KAS 523-7, KAS 524-38 and KAS 636-15) were used to determine the change in KTi type using a SNAP marker (Table 1 and Supplemental data).
DNA extraction
Genomic DNA was extracted from dry seeds according to the procedure described by Motokazu and Tadahiko (2003), with some modifications. The DNA concentration was determined using the nanodrop system (Nanodrop, DE, USA). The DNA solution was then diluted to a working concentration with distilled water and stored at −20°C until use.
Design of KTi type-specific primers and SNAP-PCR
Specific primers to identify the difference between Tia and Tib sequence were designed based on sequences of the Tia and Tib that had previously been deposited in GenBank (No.X64447 and No.X64448) in the National Center for Biotechnology Information database (NCBI) (http://www.ncbi.nlm.nih.gov/) (Table 2). PCR amplification was then conducted in reaction mixtures that contained 50 ng of the genomic DNA, 2.5 pmol of each forward and reverse primer, 2.5 mM of each dNTP, 10 × PCR buffer (10 mM Tris–HCl pH 8.3, 50 mM KCl, and 1.5 mM MgCl2) and 1 U Taq polymerase in a total volume of 20 μl. The reaction mixture was subjected to the following conditions: initial denaturation at 94°C for 5 min, followed by 28 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 30 s and extension at 72°C for 30 s. The PCR products were then resolved by electrophoresis on 1.0% ethidium bromide-stained agarose gels.
Nucleotide sequencing of KTi
To identify the sequence of PCR products amplified using the KTi type-specific primers, we used KTi3 primers (Forward: 5′-ATGAAGAGCACCATCTTCTTTCT-3′ and Reverse: 5′-CACTCACTGCGAGAAAGGCCATG-3′), which were developed based on the published sequence of the soybean trypsin inhibitor KTi3 gene (GenBank accession no.S45092). This primer set amplified a 563-bp DNA fragment from soybean genomic DNA. The PCR products were cloned using a T&A Cloning kit (RBC, Bioscience). Plasmid DNA was prepared using a commercial kit (Intron, Biotechnology) and sequenced using an ABI 3130 DNA Sequencer (ABI, CA, USA).
Trypsin inhibitor assay
The inhibitory activity of a trypsin inhibitor was determined using the method described by Makoto et al. (2007), with some modification. Briefly, 80 mg of benzoyl-dl-arginine-p-nitroanilide (BAPNA, Sigma) in 2 ml of dimethylsulfoxide was diluted to 200 ml with 100 mM Tris–HCl (pH 8.2) containing 20 mM CaCl2 [substrate solution (SS)]. Next, 4 mg of trypsin (Sigma) was dissolved in 200 ml of 1 mM HCl [trypsin solution (TS)]. The total crude protein was then extracted in 5 ml of 100 mM Tris–HCl (pH 8.2) containing 20 mM calcium chloride from 100 mg of dry seeds. The suspension was then centrifuged at 13,000 rpm for 30 min, after which the clear supernatant was collected and diluted 100-fold for use in the trypsin inhibitor assay [trypsin inhibitor solution (TIS)]. Specifically, 200 μl of TS were added to 200 μl of TIS and then pre-incubated for 10 min at 37°C. Next, 500 μl of SS were added, after which the samples were incubated at 37°C for 10 min. The reaction was then terminated by adding 100 μl of 30% acetic acid, after which the absorbance of the total reaction solution was measured at 410 nm.
Nondenaturing polyacrylamide gel electrophoresis
Total crude protein was extracted from 100 mg of dry seeds in 5 ml of 100 mM Tris–HCl (pH 8.2) containing 20 mM calcium chloride. PAGE was conducted in the absence of denaturing reagent using a Bio-Rad Mini-PROTEAN 3 Cell (Bio-Rad Lab., CA, USA) according to the manufacturer’s instructions. After electrophoresis, the KTi bands were stained with Coomassie Brilliant Blue. The commercial agent, trypsin inhibitor (Cat# T6522, Sigma), was used as the Tia type marker.
Results
SNAP marker development for division of Tia and Tib
The PCR-based SNAP marker used to distinguish between the Tia and Tib KTi type was developed by changing the 112th nucleotide between the 111th and 113th identified SNP in Tia and Tib. In the Tia-specific primer, AAA was changed to ACA, while the Tib-specific primer was changed from TAG to TCG (Table 2). The KTi-specific primers were designed so that the 3′-terminal nucleotide of the primer was complementary to the SNPs, and such that the primer should contain an artificial mismatch within 6 bp of the site of the SNPs. To increase the specificity and fidelity of SNAP-PCR, cvs. Baekwoon (BW, Tia type) and Suwon115 (SW115, Tib type) genomic DNA were used to determine the optimal DNA concentration and number of amplification cycles. The KTi-specific primer set was used to test two different DNA concentrations (30 and 50 ng) and amplification cycles (25 and 28 cycles). When 25 cycles were used, the primer sets used to amplify 30 ng of genomic DNA did not generate PCR products. Additionally, 25 cycles of PCR using 50 ng of template or 28 cycles of PCR using 30 ng of template resulted in the generation of weak PCR product (563 bp). However, PCR conducted using 28 cycles and 50 ng of genomic DNA produced clearer bands than the other combinations evaluated; therefore, these were considered to be the optimum conditions for the identification of KTi type using the SNAP marker designed here (Fig. 1).
Analysis of the specificity of representative single nucleotide amplified polymorphism (SNAP) primers according to template DNA concentration and amplification cycles. Arrows indicate expected 563-bp PCR products. BW Baekwoon, SW115 Suwon115, a Tia-specific primer pair, b Tib-specific primer pair, M 100-bp ladder
SNAP marker evaluation
To assess the feasibility of using the SNAP marker for classification of the KTi type, we conducted SNAP-PCR using the genomic DNA of seven cultivars and three landraces. Among the 10 soybean cultivars and landraces evaluated, nine were found to be the Tia type and one, SW115, was Tib (Fig. 2). To confirm the results of SNAP-PCR, two Tia lines [cvs. BW and Paldal (PD)] and two Tib lines [cv. SW115 and BW-7-2 (mutant line)] were further analyzed by DNA sequencing. The DNA sequence data of the four lines was comparable with the Tia and Tib sequences of nine different sequences in the NCBI database (Fig. 3). Additionally, the Tia and Tib sequences could be distinguished based on a difference in their amino acid sequence. Specifically, BW-7-2 differed from that of Tib based on a T → C SNP at two positions, +75 and +315. When compared with cv. SW115, there was a one T → C shift in the sequence of Tib at position +99. However, these SNPs did not induce an amino acid change. The KTi nucleotide sequences of cvs. BW and PD differed from those of Tia at position +279 by a G → C SNP. However, there was no sequence difference at the loci of the SNAP marker. Taken together, these results indicate that our SNAP marker clearly defined the KTi type between Tia and Tib.
Amplification patterns of the SNAP marker for KTi type in 10 soybean cultivars and landraces. Arrow indicates expected 563-bp PCR products. 360-22 KAS360-22, 523-7 KAS523-7, 524-38 KAS524-38, SW115 Suwon115, BS Bangsa, PD Paldal, HG Hwangguem, a Tia-specific band, b Tib-specific band, M 100-bp ladder
Sequence alignment of each PCR fragment among soybean cultivars and mutant lines. Amino acids that caused the changes of nucleotides in comparison with Tia were indicated in bold. Solid triangles indicate the nucleotides that cause the changes of amino acids. Blank triangles indicate the nucleotides that do not cause the changes the amino acids. Horizontal arrows indicate the positions of primers used to classify the KTi types
Change of KTi type and KTi activity in the mutant lines
Of 12 soybean cultivars and landraces screened using the SNAP marker, cv. SW115 was the only cultivar that had Tib type (Table 1). There are no changes of KTi type in the mutant lines derived from four landraces. Among 451 mutant lines, 8 mutant lines derived from cvs. BW, PD and SW115 showed a change in KTi type when compared with the non-irradiated soybean cultivars (Fig. 4). Specifically, five mutant lines in cv. SW115 were changed from the Tib type to the Tia type, while two mutant lines derived from cv. BW and one mutant line derived from cv. PD were changed from Tia to Tib, respectively.
Amplification patterns of the SNAP marker for the KTi type in 19 soybean mutant lines and three original cultivars. a–c are cvs. Paldal (PD), Baekwoon (BW), Suwon115 (SW115) and their mutant lines, respectively. All mutant lines of a are the same as cv. PD, except for PD-5-10. All mutant lines of b are changed from Tib to Tia type. Two mutant lines, BW-1 and BW-7-2, of c are changed from Tia to Tib type and the other mutant lines are the same KTi type as the original cultivar, a Tia-specific band, b Tib-specific band, M 100-bp ladder. Arrow indicates expected 563-bp PCR products
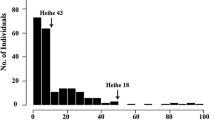
The KTi activity of 451 soybean mutant lines and 12 original cultivars and landraces was measured by incubating clarified seed homogenates with trypsin. Overall, 157 of 451 soybean lines showed changes in trypsin inhibitor activity when compared with the original cultivars and landraces. Specifically, 144 of 157 changed lines showed decreased KTi activity while 13 lines showed increased activity (Supplemental data). Of eight mutant lines that showed a change in KTi type, two (BW-1 and BW-7-2) derived from cv. BW and one mutant line (PD-5-10) derived from cv. PD that showed a change from Tia to Tib had lower activity than the original cultivar (Table 3). In SW115, five mutant lines that were changed from Tib to Tia showed higher activity than the original cultivar. In general, the activity of the Tia type was higher than that of the Tib type (Tia = 20.0 TIU and Tib = 18.2 TIU, LSD = 0.93). The five mutant lines that showed a change into the Tia type derived from SW115 had increased KTi activity.
Electrophoretic polymorphism of KTi type
Eight mutant lines that showed a change in KTi type and three original cultivars were analyzed by nondenaturing polyacrylamide gel electrophoresis (Fig. 5). The trypsin inhibitor agent used as the Tia type revealed the Tia type. Five mutant lines (SW115-5-1, SW115-9-1-1, SW115-10, SW115-11-2 and SW115-24) and two cultivars (BW and PD) were located in the same position as the Tia type marker. All seven soybean lines were identified as the Tia type. Additionally, one cultivar (SW115) and three mutant lines (BW-1, BW-7-2 and PD-5-4) showed the slightly slower electrophoretic mobility than the Tia type marker. The result of electrophoretic mobility of KTi type in cv. SW115 and three mutant lines was in agreement with the result of SNAP marker application.
Discussion
Previous studies have revealed that soybean contains 12 allelic forms of the KTi gene (Tia, Tib Tic, Tid, Tie, Ti-null type, Tif, Tibi5, Tiaa1, Tiaa2, Tiab1 and Tig), each of which encode proteins that differ by only a few amino acids (Wang et al. 2008). In wild soybean, the majority of KTi types have been found to be Tia, followed by Tib (Hymowitz 1973; Kaizuma et al. 1980). Wang et al. (2008) reported that 84.1% of 720 wild soybeans were the Tia type, while 14.03% were the Tib type. In our study, Tib type was found only in the one cultivar (SW115) among the 12 soybean cultivars and landraces using SNAP marker. Mutant lines that were derived from the four landraces have identical KTi type. Total of eight mutant lines derived from cultivars showed changes of the KTi type.
Kaizuma et al. (1980) demonstrated that differentiation of Tia and Tib was very ancient and probably occurred before domestication of cultivated soybeans from wild soybeans. Although there is no other evidence to enforce this finding, it is supported by the high frequency of Tia in wild soybeans of China and other Asian countries reported by Hymowitz and Kaizuma (1981) and Li (1993). However, in the classification of the KTi type using the SNAP marker, eight mutant lines from cvs. SW115, BW and PD were changed from Tia to Tib or vice versa. Although the two mutant lines from cv. BW, BW-1 and BW-7-2, and one mutant line, PD-5-10, from cv. PD were changed from Tia to Tib as like the opinion of Kaizuma et al. (1980), the five mutant lines from SW115, SW15-5-1, SW115-9-1-1, SW115-10, SW115-11-2, and SW115-24, were changed from Tib to Tia type. Chang et al. (2003) reported that nine mutational hotspots on which the same base was mutated simultaneously were found among the 1,941 base pairs of the sequenced region of the mnp genes of 4 mutants induced by gamma radiation. Also, Wijker et al. (1996) reported that 14% of the gamma ray induced mutations were located at the lacI gene hot spot at position 620–632. We suppose that the nine base pairs in KTi gene are sensitive to gamma ray radiation and readily substitute DNA sequences.
Two widely used types of PCR molecular markers based on SNPs are cleaved amplified polymorphic sequences (CAPS; Konieczny and Ausubel 1993) and derived CAPS (dCAPS; Neff et al. 1998). CAPS markers detect polymorphisms that occur in restriction sites, while dCAPS markers are created during PCR amplification by introduction of a restriction site at the site of an SNP using specially designed primers. SNPs can also be detected using allele-specific PCR primers designed such that the 3′ nucleotide of a primer corresponds to the site of the SNP (Ugozzoli and Wallace 1991). Thus, the allele-specific primer matches exactly with the specific allele and has a 3′ mismatch with the non-specific allele. The mismatched 3′ termini are extended by DNA polymerases with much lower efficiency than correctly matched termini (Petruska et al. 1988). Allele-specific PCR has not been widely used because a single-base pair change at the 3′ termini is often not sufficient to ensure reliable discrimination between the two SNP alleles (Kim et al. 2005). To increase specificity, a SNAP marker that uses a modified allele-specific primer with a mismatched base pair within four bases of the 3′ termini in addition to the 3′ termini base that is complementary to the SNP site was developed (Drenkard et al. 2000; Hayasi et al. 2004). There are nine SNPs between Tia and Tib according to the GenBank (No.X64447 and No.X64448) of the NCBI. Changing the 112th DNA sequence A–C in the KTi gene, we developed a SNAP marker linked to the specific allele Tia or Tib in this study. Changing the DNA sequence between two SNPs resulted in six nucleotides that were mismatched with the 3′ termini. These mismatched six nucleotides produced an allele-specific band that could be used to distinguish between Tia and Tib.
Many researchers have been investigating the polymorphism of KTi proteins by using nondenaturing polyacrylamide gel electrophoresis with three variants, Tia, Tib and Tic (Orf and Hymowitz 1979; Wang et al. 1996, 2001, 2008). In this study, in order to identify whether the developed SNAP markers can clearly classified KTi types between Tia and Tib, we conducted the non-denaturing PAGE using the mutant lines with the changed KTi types. Specifically, we identified SW115-5-1, SW115-9-1-1, SW115-10, SW115-11-2 and SW115-24, and two cultivars (BW and PD) as the Tia type based on the presence of bands at the same position as trypsin inhibitor agent that we used as the Tia type marker. Additionally, the Tib soybean lines, SW115, BW-1, BW-7-2 and PD-5-10, showed the slightly slower electrophoretic mobility than the Tia type marker. Singh et al. (1969) observed an electrophoretic polymorphism between Tia and Tib. Additionally, Wang et al. (1996) reported that the electrophoretic mobility of Tib was slower than that of Tia. In the present study, the electrophoretic mobility of four soybean lines with Tib was slower than those of seven soybean lines with Tia in the nondenaturing PAGE. These results indicate that the designed SNAP marker was capable of discriminating the KTi type.
The use of gamma irradiation as a physical mutagen can be employed to induce mutagenesis in plant breeding (Selvi et al. 2007). Among 451 mutant lines evaluated in this study, 144 were found to have lower trypsin inhibitor activity than the original cultivars, while 13 had higher activity than the original cultivars. Additionally, the average trypsin inhibitor activity of the Tia strains was higher than that of the Tib in the present study. Manjaya et al. (2007) reported that, when the soybean variety VLSoy-2 was irradiated with 250 Gy of gamma rays, a mutant line showed a low level of trypsin inhibitor. Among the 13 mutant lines observed in the present study that had higher activity when compared to the original cultivars, five had changed from Tib to Tia; however, the remaining eight did not change Ti type (Supplemental data). The higher activity of these eight mutant lines appeared to be affected by the Bowman–Birk class of trypsin inhibitor or another proteinase inhibitor following mutagenesis, or mutations not detected by a Ti class change or electrophoresis. Krishnan (2001) reported that PI. 196168, a nonsense mutant line, contained 58% trypsin inhibitor activity when compared with cv. Amsoy 71. Because PI. 196168 had a truncated protein that led to reduced accumulation of KTi, the activity detected in that strain must have been due to the presence of BBTis.
High trypsin inhibitor levels in soybeans have a negative nutritional impact in both food and feed applications (Liener 1994). In this study, we determined the change in KTi type by using a SNAP marker in the genetically fixed mutant lines mutant population induced by gamma irradiation. In addition, we demonstrated that a change in KTi type affected the activity of the trypsin inhibitor. This SNAP marker will be useful to identify KTi type followed by selection of trypsin inhibitor lacking mutant lines. Further investigation of the differences in the effects of KTi types would provide the information useful to the proteomic characterization of Tia and Tib and the difference in the biological effects of soybeans with different KTi type that are used in animal feed.
References
Birk Y (1961) Purification and some properties of a high active inhibitor of trypsin and α-chymotrypsin from soybean. Biochem Biophys Acta 54:378–381
Birk Y, Gertler A, Khalef S (1963) A pure trypsin inhibitor from soya beans. J Biochem 87:281–282
Chang HH, Lee YK, Kim JS, Kee KS, Cho KS (2003) Mutation spectrum of manganese (II) peroxidase gene in the Pleurotus ostreatus mutants induced by gamma radiation. J Microbiol 41:52–57
Donald L, Vadim B, Marina KA, Monica AS, Elio MH, Niels CN (2007) Reduction of protease inhibitor activity by expression of a mutant Bowman–Birk gene in soybean seed. Plant Mol Biol 64:397–408
Drenkard E, Richter BG, Rozoen S, Stutius LM, Angell NA, Mandrinos M, Cho RJ, Oegner PJ, Davis RW, Ausubel FM (2000) A simple procedure for the analysis of single nucleotide polymorphisms facilitates map-based cloning in Arabidopsis. Plant Physiol 124:1483–1492
Frattali V, Steiner RF (1968) Soybean inhibitor. 1. Separation and some properties of three inhibitors from commercial crude soybean trypsin. Biochemistry 7:521–531
Hayasi K, Hashimoto N, Daigen M, Ashikawa I (2004) Development of PCR-based SNP markers for rice blast resistance genes at the piz locus. Theor Appl Genet 108:1212–1220
Hymowitz T (1973) Electrophoretic analysis of SBTI-A2 in the USFA soybean germplasm collection. Crop Sci 13:420–421
Hymowitz T, Kaizuma N (1981) Soybean seed protein electrophoresis profiles from 15 Asian countries or regions; hypotheses on paths of dissemination of soybean from China. Econ Bot 35:10–23
Kaizuma N, Oikawa K, Miura M (1980) Consideration on the cause of the differential Ti alleles frequency distributions found among some regional populations of soybean (Glycine max (L.) Merrill) land varieties. J Fac Agric Iwate Univ 15:81–96
Kim SH, Hara S, Hase S, Ikenaka T, Tode H, Kitamura K, Kaizuma N (1985) Comparative study on amino acid sequence of Kunitz-type soybean trypsin inhibitors, Tia, Tib, and Tic. J Biochem 19:435–448
Kim MY, Van K, Lestari P (2005) SNP identification and SNAP marker development for a Gm NARK gene controlling supernodulation in soybean. Theor Appl Genet 110:1003–1010
Konieczny A, Ausubel F (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4:403–410
Krishnan HB (2001) Characterization of a soybean [Glycine max (L.) Merr.] mutant with reduced levels of Kunitz trypsin inhibitor. Plant Sci 160:979–986
Lajolo FM, Genovese MI (2002) Nutritional significance of lectins and enzyme inhibitors from legumes. J Agric Food Chem 50:6592–6598
Laskowski M Jr, Kato O (1980) Protein inhibitors of proteinases. Annu Rev Biochem 49:593–626
Li FS (1993) Studies on the ecological and geographical distribution of the Chinese resources of wild soybean (G. Soja). Sci Agric Sin 26:47–55
Liener IE (1994) Implications of antinutritional components in soybean foods. Crit Rev Food Sci Nutr 34:31–67
Makoto S, Daisuke I, Kosuke Y, Mitsuru A, Suguru O, Yoshie SM (2007) Kunitz soybean trypsin inhibitor is modified at its C-terminus by novel soybean thiol protease (Protease T1). Plant Prod Sci 10:314–321
Manjaya JG, Suseelan KN, Gopalakrishna T, Pawar SE, Bapat VA (2007) Radiation induced variability of seed storage proteins in soybean [Glycine max (L.) Merrill]. Food Chem 100:1324–1327
Mohamed AI, Rangappa M (1992) Screening soybean (grain and vegetable) genotypes for nutrients and anti-nutritional factors. Plant Food Hum Nutr 42:87–96
Motokazu K, Tadahiko K (2003) Rapid DNA extraction method from soybean seeds. Breed Sci 53:277–279
Neff MM, Neff JD, Chory J, Pepper AE (1998) dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J 14:387–392
Orf JH, Hymowitz T (1979) Inheritance of the absence of the Kunitz trypsin inhibitor in seed protein of soybeans. Crop Sci 19:107–109
Petruska J, Goodman MF, Boosalis MS, Sowers LC, Cheong C, Tinoco I (1988) Comparison between DNA melting thermodynamics and DNA polymerase fidelity. Proc Nat Acad Sci USA 85:6252–6256
Rachis JJ, Anderson RL (1964) Isolation of four soybean trypsin inhibitors by DEAE-cellulose chromatography. Biochem Biophys Res Commun 15:230–235
Selvi BS, Ponnuswami V, Sumathi T (2007) Identification of DNA polymorphism induced by gamma ray irradiation in Amla (Emblica Officinalis Gaertn.) grafts of V1M1 and V2M1 generation. J Appl Sci Res 3:1933–1935
Singh LC, Wilson M, Hadley HH (1969) Genetic differences in soybean trypsin inhibitor separated by disc electrophoresis. Crop Sci 9:489–491
Song SI, Kim CH, Baek SJ, Choi YD (1993) Nucleotide sequences of cDNA encoding the precursors for soybean (Glycine max) trypsin inhibitors (Kunitz type). Plant Physiol 101:1401–1402
Toledo TCF, Canniatti-Brazaca SG, Arthur V, Piedade SMS (2007) Effects of gamma radiation on total phenolics, trypsin and tannins inhibitors in soybean grains. Radiat Phys chem 76:1653–1656
Ugozzoli L, Wallace RB (1991) Allele-specific polymerase chain reaction. Methods Enzymol 2:42–48
Wang KJ, Li XH (2005) Tif type of soybean Kunitz trypsin inhibitor exists in wild soybean of northern China. In: Proceedings of the 8th national soybean research conference of China, pp 167–168
Wang KJ, Kaizuma N, Takahata Y, Hatakeyama S (1996) Detection of two new variants of soybean Kunitz trypsin inhibitor through electrophoresis. Breed Sci 46:39–44
Wang KJ, Takahata Y, Ito K, Zhao YP, Tsutsumi KI, Kaizuma N (2001) Genetic characterization of a novel soybean Kunitz trypsin inhibitor. Breed Sci 51:185–190
Wang KJ, Yamashita T, Watanabe M, Takahata Y (2004) Genetic characterization of a novel Tib-derived variant of soybean Kunitz trypsin inhibitor detected in wild soybean (Glycine soja). Genome 47:9–14
Wang KJ, Takahata Y, Kono Y, Kaizuma N (2008) Allelic differentiation of Kunitz trypsin inhibitor in wild soybean (Glycine soja). Theor Appl Genet 117:565–573
Wijker CA, Lafleur MVM, Steeg H, Mohn GR, Retèl J (1996) γ-Radiation-induced mutation spectrum in the episomal lacI gene of Escherichia coli under oxic conditions. Mutat Res 349:229–239
Xin H, Xie KF, Dong AW, Uan QY, Gu QM (1999) The amino acid sequence determination of a new variant of Kunitz soybean trypsin inhibitor (SBTi-A2). Soybean Genet Newslett (online Journal). http://www.soybgenetics.org/aricles/sgn1999004.html (accessed 24 Mar 1999)
Yamamoto M, Ikenaka T (1967) Studies on soybean trypsin inhibitor. Purification and characterization of two soybean trypsin inhibitors. J Biochem 62:141–149
Zhao SW, Wang H (1992) A new electrophoretic variant of SBTi-A2 in soybean seed protein. Soyb Genet Newsl 19:22–24
Zhu YL, Song QJ, Hyten SM, Fickus EW, Young ND, Cregan PB (2003) Single-nucleotide polymorphism in soybean. Genetics 163:1123–1134
Acknowledgments
This work was supported by a grant (Code 308020051SB030) from the Agricultural R&D Promotion Center, Korea Rural Economic Institute, and a grant from the Korea Atomic Energy Research Institute (KAERI) and Ministry of Education, Science and Technology (MEST), Korea.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by I. Rajcan.
D. S. Kim and K. J. Lee contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, D.S., Lee, K.J., Kim, JB. et al. Identification of Kunitz trypsin inhibitor mutations using SNAP markers in soybean mutant lines. Theor Appl Genet 121, 751–760 (2010). https://doi.org/10.1007/s00122-010-1346-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-010-1346-1