Abstract
In hybrid maize (Zea mays L.) breeding, doubled haploids (DH) are increasingly replacing inbreds developed by recurrent selfing. Doubled haploids may be developed directly from S0 plants in the parental cross or via S1 families. In both these breeding schemes, we examined 2 two-stage selecting strategies, i.e., considering or ignoring cross and family structure while selection among and within parental crosses and S1 families. We examined the optimum allocation of resources to maximize the selection gain ΔG and the probability P(q) of identifying the q% best genotypes. Our specific objectives were to (1) determine the optimum number and size of crosses and S1 families, as well as the optimum number of test environments and (2) identify the superior selection strategy. Selection was based on the evaluation of testcross progenies of (1) DH lines in both stages (DHTC) and (2) S1 families in the first stage and of DH lines within S1 families in the second stage (S1TC-DHTC) with uniform and variable sizes of crosses and S1 families. We developed and employed simulation programs for selection with variable sizes of crosses and S1 families within crosses. The breeding schemes and selection strategies showed similar relative efficiency for both optimization criteria ΔG and P (0.1%). As compared with DHTC, S1TC-DHTC had larger ΔG and P (0.1%), but a higher standard deviation of ΔG. The superiority of S1TC-DHTC was increased when the selection was done among all DH lines ignoring their cross and family structure and using variable sizes of crosses and S1 families. In DHTC, the best selection strategy was to ignore cross structures and use uniform size of crosses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optimum allocation of test resources is of crucial importance for the efficiency and competitiveness of breeding programs. With limited test resources, a plant breeder has to strike a balance among the number of crosses, test candidates within each cross, as well as test environments and replications within environments. Selection among crosses enables breeders to discard inferior crosses in early stages of line development and to assign the resources to the promising ones (cf., Schnell 1982). This selection is generally based on the mean performance of the crosses, but this entails the risk of discarding individual superior candidates within the rejected crosses. Therefore, some researchers favored selection among the total number of test candidates disregarding their cross structure (cf., Lush 1947).
In hybrid maize (Zea mays L.) breeding, doubled haploids (DH) are increasingly becoming popular, replacing conventionally developed inbred lines. Alternative breeding schemes for recurrent selection with DH, disregarding cross structures, were optimized by Gordillo and Geiger (2008). However, there is no such study on the identification of inbred lines for utilization in hybrid breeding as well as the comparison of selection strategies considering or ignoring cross structures. Further, Gordillo and Geiger (2008) used approximations to simplify the estimation of the selection gain (ΔG, Falconer and Mackay 1996).
A constant number of lines or families within crosses is generally considered in literature (cf., Baker 1984; Bernardo 2003). Under this assumption, Longin et al. (2007) and Wegenast et al. (2008) developed and applied theory for ΔG in breeding schemes for various situations in maize breeding. In practical maize breeding, the numbers of S1 families and DH lines vary among crosses. Larger numbers of S1 families or DH lines are produced in promising crosses than others, based on prior information of their parents or their mean performance in earlier testing stages. The same is true for DH lines within S1 families. Thus, a higher proportion of the resources is allocated to the more promising crosses. However, no formula or simulation program is available in literature to compute the optimum allocation of test resources and estimates of ΔG under this situation, which is an apparent gap between theory and practical breeding.
Besides ΔG, progress from selection has been quantified by the probability P(q) of identifying the q% superior genotypes (cf., Keuls and Sieben 1955). Longin et al. (2006a, b) found similar optimum allocations for both these criteria in a DH breeding scheme, but they considered only one cross.
We developed and employed simulation programs to assess the optimum allocation of test resources for DH line development and their evaluation in testcrosses to maximize ΔG or P(q). Two breeding schemes were considered in which the selection was based on the performance of testcrosses of (1) DH lines derived fromseveral crosses at two stages (DHTC) or (2) S1 families fromseveral crosses in the first and of DH lines within S1 families, in the second stage (S1TC-DHTC). In both breeding schemes, we investigated the effect of different selection strategies on the optimum allocation of test resources. Selection strategies used in earlier studies (Longin et al. 2007; Wegenast et al. 2008) were extended to include (a) selection among and within crosses and S1 families disregarding cross structures and (b) variable sizes of crosses and S1 families. Our specific objectives were to (1) determine the optimum number and sizes of crosses and S1 families as well as the optimum number of test environments for maximizing ΔG and P(q) and (2) identify the superior selection strategy for each breeding scheme with respect to ΔG and P(q).
Materials and methods
Breeding schemes
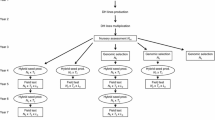
We considered two breeding schemes, DHTC and S1TC-DHTC (Wegenast et al. 2008, Supplementary Fig. S1). In DHTC, DH lines are produced from S0 plants randomly taken from a parental cross. In S1TC-DHTC, S1 families are developed from similarly taken S0 plants and evaluated in test crosses, then DH lines are produced in selected S1 families. Further, parental crosses for a new breeding cycle are selected on the basis of the parental testcross mean in the preceding breeding cycle. For selection among parental crosses, a phenotypic correlation (ρ P ) of the mean performance of the parental lines, known from preceding breeding cycles with the mean genotypic value of the testcross performance of their progenies, was assumed to be 0.71 or 0.50 (Wegenast et al. 2008). The selection at both stages was based on the phenotypic mean of testcross performance of the candidates with a fixed given tester, evaluated at L j test locations, where j refers to the selection stages 1 and 2. The target variable throughout this study is the genotypic value of the testcross performance for grain yield.
Selection strategies
For each breeding scheme, we evaluated two selection strategies (Table 1). In strategy 1, we selected first among parental crosses and then among DH lines within crosses in DHTC–1 and additionally among S1 families in S1 TC-DHTC–1. In the second strategy, selection at both stages was performed among all DH lines disregarding the cross structure in DHTC–2. In S1TC-DHTC–2, selection at the first stage was performed first among crosses and then among S1 families; at the second stage, selection was performed among all DH lines disregarding the cross and family structure. The second selection strategy had three variations as described in "Size of crosses and S1 families".
Size of crosses and S1 families
Three different procedures were used to determine the number of DH lines or S1 families per cross and DH lines per S1 family (hereafter referred to as size of crosses and S1 families; Table 1). In selection strategies 1 and 2a, we assumed a uniform size of crosses and a uniform size of S1 families in each stage. In selection strategies 2b and 2c, variable sizes of crosses and S1 families were assumed. In DHTC–2b and 2c, the size of the crosses depended on their rank, calculated from their performance in the parental selection. In S1TC-DHTC–2b and 2c, the size of the crosses and S1 families within crosses in the second stage depended on their rank, calculated from their performance at the first selection stage. In both breeding schemes, with poorer rank, the size of crosses and S1 families decreased moderately in strategy 2b and strongly in strategy 2c (an example is given in Supplementary Fig. S1). In some allocations, the available test candidates could not be fully allocated to the crosses or S1 families within crosses. In these cases, the remaining small number of DH lines were assigned to the best cross and S1 family within crosses.
Test locations common to both stages (L c ) were assumed such that L c = min(L 1, L 2). Without restrictions on L j in stage j, ΔG is maximum for one replication per test location for both stages of selection (cf., Bernardo 2002; Melchinger et al. 2005). Thus, we considered the number of replications equal to one. After two stages of selection, the best N f = 10 DH lines were selected. In strategy 1, the best 10 DH lines with the highest testcross performance within the best cross (and S1 family within that cross) were selected, based on an earlier study showing that this approach maximized ΔG (Wegenast et al. 2008).
Economic frame and quantitative-genetic parameters
A fixed total budget for the production and evaluation of the test candidates in two selection stages was defined in terms of testcross plot equivalents. An equal plot size at both selection stages was assumed. In DHTC, the budget equals N 1[K DH + L 1(1 + K T )] + N 2 L 2(1 + K T ), where N j refers to the total number of test candidates available in stage j, L j to the total number of test locations at stage j, K DH to the production costs of one DH line and K T to the production costs of testcross seed for one plot. In S1TC-DHTC, the budget equals N 1[K F + L 1(1 + K T )] + N 2 [K DH + L 2(1 + K T )], where K F refers to the production costs of one S1 family. All costs are based on actual costs in the maize breeding program of the University of Hohenheim. We assumed that K DH = 1/2, K T = 1/25, and K F = 1/12 testcross plot equivalents. Three budgets were compared with a total of 10,000, 20,000 and 40,000 testcross plot equivalents available for line development in a heterotic pool.
The values of variance components (σ 2 G , σ 2G×y , σ 2G×l , σ 2G×l×y , σ 2 e ) were obtained from the evaluation of DH populations for grain yield in maize programs of Central Europe (Wegenast et al. 2008), where σ 2 G is the genotypic variance among testcrosses of the candidate lines with a given tester, σ 2G×y the variance of the genotype × year interactions, σ 2G×l the variance of the genotype × location interactions, σ 2G×l×y the variance of the genotype × location × year interactions, and σ 2 e the variance of the residual error. The index G in the variance component ratios refers to the respective test candidates, i.e., crosses (C), DH lines within crosses (DH/C), S1 families within crosses (F/C) or DH lines within S1 families (DH/F). In scenario VC1, we assumed a variance component ratio σ 2 G :σ 2G×y :σ 2G×l :σ 2G×l×y :σ 2 e = 0.5:0.125:0.125:0.25:1 for C and DH/C, respectively, and 0.25:0.0625:0.0625:0.125:1 for F/C and DH/F, respectively (Wegenast et al. 2008). In other scenarios, the contribution of σ 2 G was kept constant, but the non-genetic variances were doubled (VC2) and quadrupled (VC3).
Simulation model
The selection strategies were investigated by Monte Carlo simulations. Since grain yield is a quantitative trait, we assumed a Gaussian distribution of the genotypic and phenotypic values. Parental selection was based on the genotypic values of the crosses, assuming N(0, σ 2 C ).
In DHTC, the phenotypic value of a DH line was modeled by
with
where c and dh are the effects of the crosses and DH lines within crosses, respectively, m c as well as m dh/c the effects masking the former effects, and e the residual error. σ 2 C ′ is the genotypic variance among crosses after parental selection (Wegenast et al. 2008).
In S1TC-DHTC, the phenotypic value of an S1 family in stage j = 1 was modeled by
with c, m c , and e as defined above and
and
where f/c is the effect of the S1 families within crosses and m f/c their masking effect. The phenotypic value of a DH line in stage j = 2 in S1TC-DHTC was modeled by
with c, f/c, m c , m f/c and e as defined above and
where dh/f is the effect of the DH lines within S1 families and m dh/f their masking effect. The covariance between the phenotypic values of both selection stages was determined as \(\sigma^2_G+\frac{L_c \sigma^2_{G \times l}} {L_1L_2}.\)
The number of simulation runs required to have an accuracy of 0.01 for ΔG was determined on the basis of the standard error of the arithmetic mean as (3SD/0.01)2 (Berry and Lindgren 1996). Thus, between 9,000 and 29,000 simulation runs were performed. The simulation programs were written in C and implemented in the statistical software R (R Development Core Team 2006).
Optimum allocation of test resources and optimization criteria
The optimum allocation of test resources for per-cycle selection gain (\( \Updelta \widehat{G})\) or the probability of identifying superior genotypes \((\widehat{P}(q))\) as well as their standard deviations (\({\text{SD}}_{\Updelta \widehat{G}}\) and \({\text{SD}}_{\widehat{P}(q)}\)) were estimated by extending the approach of Longin et al. (2006b). The value of q, the q% best genotypes, considered was 5, 1, and 0.1%. For example, \(\widehat{P}(5\%)\) corresponds to the probability that the selected DH lines comprise the fraction of the 5% DH lines with the highest genotypic value of testcrosses in the unselected base population of all crosses considered before parental selection. Additionally, we calculated the average coefficient of coancestry (\(\bar{\Uptheta}\)) among the selected DH lines for each allocation (Longin et al. 2009). The allocation of test resources refers to tuples (N j , L j ) for both stages j. It was considered optimum if it maximized the corresponding optimization criterion. The optimum allocation as well as the corresponding optimization criteria are denoted by an asterisk, e.g., \(L_1^*, \Updelta \widehat{G}^*.\) This optimum allocation was obtained for each scenario by a grid search in the space of all admissible resource allocations. Since \(\Updelta \widehat{G}\) was only estimated with a precision of 0.01, the optimum allocation (N * j , L * j ) was determined such that (1) the number of locations was minimum (Utz 1969; Longin et al. 2006b) and (2) the number of DH lines per cross or S1 family was minimum among all allocations within a 0.01 drop-off of \(\Updelta \widehat{G},\) to facilitate the conduct of field trials.
Results
The breeding schemes and selection strategies showed similar relative efficiency for both optimization criteria ΔG and P(0.1%). S1TC-DHTC was distinctly superior to DHTC, the superiority being more pronounced for \(\widehat{P}(0.1\%)^*\) than for \(\Updelta \widehat{G}^*\) (Table 2). The optimization criteria, \(\widehat{P}^*(5\%)\) and \(\widehat{P}^*(1\%)\), showed only minor differences between the two breeding schemes and various selection strategies, because all values were close to unity (data not shown). Consequently, results were presented only for \(\Updelta \widehat{G}^*\)
and \(\widehat{P}(0.1\%)^*.\) The highest values of both optimization criteria were observed for S1TC-DHTC in strategy 2c, and for DHTC in strategy 2a. For \(\Updelta \widehat{G}^*\) in S1TC-DHTC–2c, the optimum allocation was 12 (L *1 ) and 15 (L *2 ) test locations, four crosses \({(N^{*}_{1_{C}})}\) and 153 S1 families \({(N^{*}_{1_{F/C}})}\) in each cross at the first stage, and 764 DH lines (N *2 ) at the second stage. In DHTC–2a, N *1 was larger, while N *2 and L *1 were smaller than the corresponding numbers for S1TC-DHTC–2c. For \(\widehat{P}(0.1\%)^*\) in S1TC-DHTC–2c, \({N^{*}_{1_{C}}}\) was about 20% larger and L *1 was smaller in comparison with \(\Updelta \widehat{G}^*.\) In DHTC–2a, the optimum allocation was similar for both optimization criteria. The standard deviation, \(\hbox{SD}_{\Updelta \widehat{G}^*}\) was larger in S1TC-DHTC compared with DHTC, but \(\hbox{SD}_{\widehat{P}(0.1\%)^*}\) was smaller. The average coancestry coefficient \(\bar{\Uptheta}\) was 50–100% larger for both optimization criteria in S1TC-DHTC than in DHTC, but differed only slightly between the optimization criteria.
For both optimization criteria, with an increasing number of crosses in the first stage \({(N_{1_{C}})}\), selection response increased up to 3–4 crosses and decreased thereafter in all selection strategies (Fig. 1). Deviations from the optimum \({N_{1_{C}}}\) led to a smaller decrease in S1TC-DHTC than in DHTC for \(\Updelta \widehat{G}.\) For S1TC-DHTC, response curves were almost flat in the vicinity of the maximum. Furthermore, differences for the optimization criteria among the selection strategies increased with increasing \({N_{1_{C}}}\) in DHTC.
Selection gain (\(\Updelta \widehat{G}\)) and the probability of selecting superior genotypes (\(\widehat{P}(0.1\%)\)) as a function of the number of crosses in the first stage \((N_{1_C})\) for selection strategies 1 (open square), 2a (open circle), 2b (open triangle) and 2c (open diamond) in breeding scheme DHTC (solid symbols) and S1TC-DHTC (hollow symbols)
The effect of varying budgets, variance component ratios and ρ P on the optimization criteria and the optimum allocation of test resources are presented for the best selection strategy in both breeding schemes, namely DHTC–2a and S1TC-DHTC–2c (Table 3). An increase in the budget (from 10,000 to 40,000 plot equivalents) resulted in higher values of N *1 and N *2 , and increased optimization criteria in both breeding schemes. An increase in the non-genetic variances (from VC1 to VC3) generally reduced N *1 and N *2 values, with the exception of N *2 in S1TC-DHTC-2c. Furthermore, the rise in non-genetic variances decreased both optimization criteria, but caused an increase in L * j . A reduction in ρ P (from 0.71 to 0.50) resulted in increased \({N^{*}_{1_{C}}}\) values for both breeding schemes and both optimization criteria. On the other hand, the reduction in ρ P did not result in considerable changes in N *2 values, though they were increased for \(\Updelta \widehat{G}\) and decreased for \(\widehat{P}(0.1\%)^*\). However, the reduction in ρ P affected the changes in optimization criteria, by reducing them in case of DHTC–2a and by improving them in case of S1TC-DHTC–2c.
Discussion
Our results on the optimum allocation of test resources and estimates of ΔG considering breeding schemes S1TC-DHTC and DHTC were in conformity with the earlier studies of Longin et al.(2007) and Wegenast et al. (2008). In particular, S1TC-DHTC was superior to DHTC for \(\Updelta \widehat{G}\). The optimum number of crosses at the first stage \({(N^{*}_{1_{C}})}\) increased with an increasing budget and ρ P and decreasing non-genetic variances; however, in all situations of both breeding schemes, \(N_{1_C}^* \le 12\). A larger proportion of the test resources was allocated to the second selection stage in S1TC-DHTC in comparison with DHTC. The production cost for an S1 family K F hardly influenced allocation of the budget to the selection stages and the estimates of both optimization criteria (data not shown). An increasing budget or ρ P had a larger impact on \(\Updelta \widehat{G}^*\) in DHTC than in S1TC-DHTC. In DHTC, deviations from \(N_{1_C}^*\) led to a larger reduction in ΔG in comparison with S1TC-DHTC.
Comparison of the breeding schemes
As in the case of ΔG, breeding scheme S1TC-DHTC was superior to DHTC for \(\widehat{P}(0.1\%)\), considering non-hierarchical selection, and variable cross and family sizes (Tables 2 and 3; Fig. 1). A comparison of the respective selection strategies in the breeding schemes showed S1TC-DHTC to have more than 15% higher ΔG * and 34% higher \(\widehat{P}(0.1\%)^*\) (Table 2). However, the longer cycle length of S1TC-DHTC than DHTC was not considered in the present study, because per-year estimation cannot be made for P(q). The higher \(SD_{\Updelta \widehat{G}^*}\) in S1TC-DHTC than in DHTC (Tables 2, 3) was mainly attributable to a larger SD at the first selection stage (data not shown). In consequence, the deployment of S1TC-DHTC instead of DHTC offers the chance of having a larger mean ΔG *, but a larger variation in these estimates.
The selfing of the S0 generation in S1TC-DHTC led to larger average coancestry coefficient \(\bar{\Uptheta}\) among the selected candidates in comparison with DHTC (Tables 2, 3). A larger \(\bar{\Uptheta}\) indicates a reduced genetic variance among the selected candidates, leading to a reduced ΔG in a long-term recurrent selection program. On the other hand, the S1 development offers an additional generation for recombinations, thereby increasing the genetic variance, which results in larger response to long-term selection (Bernardo 2009) The present study focused on the identification of DH lines for the development of hybrids. Thus, it is recommended to employ S1TC-DHTC. For recurrent selection, DH lines from additional crosses could be selected and intermated for the next breeding cycle to ensure a long-term breeding success.
Comparison of selection strategies
We compared two selection strategies: (1) selection first among and then within crosses in DHTC–1 and additionally within S1 families in S1TC-DHTC–1 and (2) selection among DH lines disregarding the cross and family structure in DHTC–2 and S1TC-DHTC–2. Normally, not all candidates within the topmost cross are superior to the best candidates of other crosses (cf., Lush 1947), because the distributions of the test candidates of different crosses overlap. In the present study, in selection strategy 2, superior DH lines from crosses or S1 families irrespective of parental performance were selected; thus, no superior genotype was rejected due to the mean performance of its cross or S1 family, improving \(\Updelta \widehat{G}^*\) and \(\widehat{P}(0.1\%)^*\) (Table 2; Fig. 1).
In S1TC-DHTC, selection strategy 2c with variable size of crosses and S1 families was superior and achieved higher \(\Updelta \widehat{G}^*\) and \(\widehat{P}(0.1\%)^*\), and both criteria had SDOC and \(\bar{\Uptheta}\) equal or lower than the other selection strategies (Table 2; Fig. 1). In strategy 2c, at least half of the test candidates belonged to the best cross after the first selection stage and one quarter of the test candidates to the second best cross (Table 1; Supplementary Fig. S1). In strategy 2b, however, only 7/25 of the test candidates belonged to the best and about 1/6 to the second best cross after the first selection stage. Thus, it was worth to have variable sizes of crosses and S1 families and devote a large budget to the better cross. Positive effects of variable sizes of families on ΔG were also observed in animal breeding (Toro and Nieto 1984; Toro et al. 1988; Toro and Pérez-Encisco 1990). In DHTC, the application of variable sizes of crosses did not improve the values for the optimization criteria. The dimension of the crosses was calculated before the first selection stage based solely on parental information. Therefore, a more effective use of the parental cross and family information, e.g., using best linear unbiased prediction (BLUP, Bernardo 1996) might further improve the use of variable cross and family sizes.
Considering the best selection strategy in each breeding scheme, the relative superiority of S1TC-DHTC–2c over DHTC–2a increased with a decreasing budget and ρ P for both optimization criteria (Table 3). On comparing S1TC-DHTC–2c with DHTC–2a for varying variance component ratios, opposite trends were found for \(\Updelta \widehat{G}^*\) and \(\widehat{P}(0.1\%)^*\): smaller non-genetic variances led to an increase in the relative superiority of S1TC-DHTC–2c over DHTC–2a for \(\Updelta \widehat{G}^*\), but a decreasing relative superiority for \(\widehat{P}(0.1\%)^*\). Most likely, the low impact of small non-genetic variances on \(\widehat{P}(0.1\%)^*\) in S1TC-DHTC was due to the fact that for \(\widehat{P}(0.1\%)^*\), the values were already very high and, consequently, there was little scope for improvement.
The reason for the lower SD OC in strategy 2 than in strategy 1 in S1TC-DHTC (Table 2) might be that progress from selection is less variable when selection in the second stage is performed among all DH lines and not only among the DH lines of selected crosses and S1 families. In DHTC, the decrease in \(\hbox{SD}_{\Updelta \widehat{G}^*}\) in selection strategy 2 compared with strategy 1 was stronger than in S1TC-DHTC. This may be attributable to the non-hierarchical selection among all DH lines in both selection stages.
The lower (11%) value of \(\bar{\Uptheta}\) in S1TC-DHTC–2, as compared with S1TC-DHTC–1 (Table 2), is attributable to the final selection of all DH lines of the best S1 family within the best cross in strategy 1. On the other hand, in S1TC-DHTC–2, the finally selected DH lines originate mostly from more than one S1 family and cross (data not shown). In animal breeding, variable family sizes also led to a decrease in the inbreeding coefficient (Toro and Nieto 1984.) If DH lines in S1TC-DHTC–1 were finally selected out of more than one cross or S1 family within crosses, \(\bar{\Uptheta}\) could be reduced. However, this will reduce \(\Updelta \widehat{G}^*\) (Wegenast et al. 2008).
Comparison of the optimization criteria
The estimate of \(\Updelta \widehat{G}\) reflects the superiority of the population generated by intermating the selected genotypes in comparison with the genotypic mean of the base population, whereas \(\widehat{P}(q)\) quantifies the chance to develop superior varieties without reference to the mean of the whole selected group (Wricke and Weber 1986). To have a realistic chance of success in identifying a superior genotype, P(q) should be greater than 0.75 (Longin et al. 2006a, b), which can be achieved in both breeding schemes for \(\widehat{P}(5\%)^*\) and \(\widehat{P}(1\%)^*\) (data not shown). However, the probability of having the top 0.1% genotypes under evaluation, actually included in the selected fraction, i.e., P(0.1%), is distinctly higher with S1TC-DHTC than with DHTC (Tables 2, 3). The optimization criteria did not affect the ranking of the breeding schemes or selection strategies. However, the differences among the breeding schemes, selection strategies, as well as the assumptions concerning the total budget and the variance component ratios, were relatively more pronounced for \(\widehat{P}(0.1\%)^*\) than for \(\Updelta\widehat{G}^*\).
The relative decrease in the optimization criteria due to non-optimum allocation in both breeding schemes was larger for \(\widehat{P}(0.1\%)\) than for \(\Updelta\widehat{G}\) (Fig. 1). The higher sensitivity of \(\widehat{P}(0.1\%)\) to non-optimal allocation in comparison with \(\Updelta\widehat{G}\) might be due to the binomial character of P(q): for the calculation of \(\widehat{P}(q)\), selected genotypes that belong to the upper 0.1% quantile are recorded as 1 and those that are below the 0.1% quantile are recorded as 0. For the calculation of \(\Updelta \widehat{G}\), however, the continuously distributed phenotypic values of the genotypes are used.
The binomial nature of \(\widehat{P}(q)\) with genotypes surpassing the defined threshhold or not also influences its SD. The values of \(SD_{\widehat{P}(q)}\) assume their maximum value for \({\widehat{P}(q)}=0.5\) and their minimum for \({\widehat{P}(q)}=0\) and \({\widehat{P}(q)}=1\) (Longin et al. 2006b). This explains the considerably lower \(SD_{\widehat{P}(0.1\%)^*}\) in S1TC-DHTC in comparison with DHTC.
Optimum allocation of test resources
An increase in the number of test candidates in the first stage (N *1 ) at the expense of a reduced optimum number of test candidates in the second stage (N *2 ) enhanced \(\widehat{P}(0.1\%)^*\) in S1TC-DHTC (Tables 2, 3). This is consistent with results of previous studies (Robson et al. 1967; Johnson 1989; Knapp 1998). In DHTC, however, N * j was hardly influenced by the choice of the optimization criteria. This might be due to the very high N *1 ; thus, a further increase in N *1 would not have any additional positive effect on \(\widehat{P}(0.1\%)^*\).
In S1TC-DHTC, selection strategy 2 led to a decrease in L *1 at the expense of an increase in N *2 in comparison with strategy 1 (Table 2). The reason for the increase in N *2 being that an increase in the total number of genotypes enhances the chance to select superior DH genotypes irrespective of the cross performance. In DHTC–2, N *2 decreased in favor of an increased N *1 or L *1 in comparison with DHTC–1. However, the allocation of the test resources to the first and second selection stage was not affected by the selection strategy. Furthermore, in DHTC–2, selection among all DH lines disregarding the cross structure was also applied in the first stage; thus, a shift of the budget from the first to the second stage did not result in more effective selection.
Response curves of S1TC-DHTC were flat in the vicinity of the maximum (Fig. 1): for all strategies, when the number of crosses in the first stage (\(N_{1_C}\)) did not exceed 15, and the decrease in the value of the optimization criteria was less than 4% in comparison with the maximum in S1TC-DHTC. For these calculations, the allocation of all other factors except \(N_{1_C}\) was optimized. The reason for the lower sensitivity of S1TC-DHTC to a non-optimal allocation might be that a loss in response to selection among parents is compensated by an increase in the response to selection among S1 families in the first stage of selection without reducing the selection intensity in the second stage of selection.
In S1TC-DHTC–2, a larger part of the resources was allocated to the second selection stage as compared with S1TC-DHTC–1 (Table 2). The reason for this additional shifting of the test resources to the second stage in S1TC-DHTC–2 might be that the topmost DH lines disregarding their cross and family structure, were selected and, it proved beneficial to test these lines more extensively.
Conclusions
Breeding scheme S1TC-DHTC had larger \(\Updelta\widehat{G}^*\) and \(\widehat{P}(0.1\%)^*\) than DHTC. The superiority of S1TC-DHTC was further enhanced when selection was done among all DH lines disregarding their cross and family structure, as well as the use of variable instead of uniform sizes of crosses and S1 families. With variable sizes of crosses and S1 families, the allocation of a large fraction of the budget to the crosses on top after the first selection stage was the superior strategy. Although the ranking was not altered with the use of ΔG * or P(0.1%)*, differences between breeding schemes were higher for P(0.1%)*. Thus, P(q) offers a very sensitive tool to differentiate among breeding schemes and selection strategies. Further investigations are warranted to examine whether selection progress under various breeding schemes and selection strategies can be upgraded by giving optimal weights to parental information using BLUP approaches.
References
Baker RJ (1984) Quantitative genetic principles in plant breeding. In: Gustafson JP (ed) Gene manipulation in plant improvement. Plenum Press, New York, pp 147–176
Bernardo R (1996) Best linear unbiased prediction of maize single-cross performance. Crop Sci 36:50–56
Bernardo R (2002) Breeding for quantitative traits in plants. Stemma Press, Woodbury, Minnesota, pp 152–156
Bernardo R (2003) Parental selection, number of breeding populations, and size of each population in inbred development. Theor Appl Genet 107:1252–1256
Bernardo R (2009) Should maize doubled haploids be induced among F1 or F2 plants? Theor Appl Genet 119:255–262
Berry DA, Lindgren BW (1996) Statistics. Duxberry Press, New York, p 371
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Longman Scientific and Technical Ltd, Essex, England, pp 184–194, 230–231
Gordillo GA, Geiger HH (2008) Alternative recurrent selection strategies using doubled haploid lines in hybrid maize breeding. Crop Sci 48:911–922
Johnson B (1989) The probability of selecting genetically superior S2 lines from a maize population. Maydica 34:5–14
Keuls M, Sieben JW (1955) Two statistical problems in plant selection. Euphytica 4:34–44
Knapp SJ (1998) Marker-assisted selection as a strategy for increasing the probability of selecting superior genotypes. Crop Sci 38:1164–1174
Longin CFH, Maurer HP, Melchinger AE, Frisch M (2009) Optimum allocation of test resources and relative efficiency of alternative procedures of within-family selection in hybrid breeding. Plant Breed 128:213–216
Longin CFH, Utz HF, Melchinger AE, Reif JC (2006a) Hybrid maize breeding with doubled haploids: comparison between selection criteria. Acta Agron Hung 54:343–350
Longin CFH, Utz HF, Reif JC, Schipprack W, Melchinger AE (2006b) Hybrid maize breeding with doubled haploids: I. One-stage versus two-stage selection for testcross performance. Theor Appl Genet 112:903–912
Longin CFH, Utz HF, Reif JC, Wegenast T, Schipprack W, Melchinger AE (2007) Hybrid maize breeding with doubled haploids: III. Efficiency of early testing prior to doubled haploid production in two-stage selection for testcross performance. Theor Appl Genet 115:519–527
Lush JL (1947) Family merit and individual merit as bases for selection. Part 1. Am Nat 81:241–261
Melchinger AE, Longin CFH, Utz HF, Reif JC (2005) Hybrid maize breeding with doubled haploid lines: quantitative genetic and selection theory for optimum allocation of resources. In: Proceedings of the Forty First Annual Illinois Corn Breeders’ School 2005, Urbana-Champaign, Illinois, USA, pp 8–21
R Development Core Team (2006) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org
Robson DS, Powers L, Urquhart NS (1967) The proportion of genetic deviates in the tails of a normal population. Genet Breed Res 37:205–216
Schnell FW (1982) A synoptic study of the methods and categories of plant breeding. Z Pflanzenzuecht 89:1–18
Toro MA, Nieto BM (1984) A simple method for increasing the response to artificial selection. Genet Res Camb 44:347–349
Toro MA, Nieto B, Salgado C (1988) A note on minimization of inbreeding in small-scale selection programmes. Livest Prod Sci 20:317-323
Toro M, Pérez-Encisco M (1990) Optimization of selection response under restricted inbreeding. Genet Sel Evol 22:93–107
Utz HF (1969) Mehrstufenselektion in der Pflanzenzüchtung. (In German) Arbeiten der Universität Hohenheim, vol 49. Verlag Eugen Ulmer, Stuttgart, Germany
Wegenast T, Longin CFH, Utz HF, Melchinger AE, Maurer HP, Reif JC (2008) Hybrid maize breeding with doubled haploids IV Number versus size of crosses and importance of parental selection in two-stage selection for testcross performance. Theor Appl Genet 117:251–260
Wricke G, Weber WE (1986) Quantitative genetics and selection in plant breeding. Walter de Gruyter, Berlin, New York, pp 172–194
Acknowledgments
This research was supported by funds from DFG, Grant No 1070, International Research Training Group “Sustainable Resource Use in North China” to T. Wegenast. The authors appreciate the editorial work of Dr. J. Muminović, whose suggestions considerably improved the style of the manuscript. We greatly appreciate the helpful comments and suggestions of the anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Becker.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00122-010-1428-0
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wegenast, T., Utz, H.F., Longin, C.F.H. et al. Hybrid maize breeding with doubled haploids: V. Selection strategies for testcross performance with variable sizes of crosses and S1 families. Theor Appl Genet 120, 699–708 (2010). https://doi.org/10.1007/s00122-009-1187-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-009-1187-y