Abstract
Grapevine molecular maps based on microsatellites, AFLP and RAPD markers are now available. SSRs are essential to allow cross-talks between maps, thus upgrading any growing grapevine maps. In this work, single nucleotide polymorphisms (SNPs) were developed from coding sequences and from unique BAC-end sequences, and nested in a SSR framework map of grapevine. Genes participating to flavonoids metabolism and defence, and signal transduction pathways related genes were also considered. Primer pairs for 351 loci were developed from ESTs present on public databases and screened for polymorphism in the “Merzling” (a complex genotype Freiburg 993–60 derived from multiple crosses also involving wild Vitis species) × Vitis vinifera (cv. Teroldego) cross population. In total 138 SNPs, 108 SSR markers and a phenotypic trait (berry colour) were mapped in 19 major linkage groups of the consensus map. In specific cases, ESTs with putatively related functions mapped near QTLs previously identified for resistance and berry ripening. Genes related to anthocyanin metabolism mapped in different linkage groups. A myb gene, which has been correlated with anthocyanin biosynthesis, cosegregated with berry colour on linkage group 2. The possibility of associating candidate genes to known position of QTL is discussed for this plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grapevine genomics has improved: genetic maps are available (Doligez et al. 2002; Grando et al. 2003; Doucleff et al. 2004; Fischer et al. 2004; Riaz et al. 2004; Doucleff et al. 2004; Lowe and Walker 2006; Di Gaspero et al. 2007), mainly based on microsatellites produced by the international Vitis Microsatellites Consortium and in part by the France Genomic National Program Genoplante (Adam-Blondon et al. 2004). Recently, a SSRs reference genetic linkage map based on five different segregating populations has been published by Doligez et al. (2006) and a dense SNP-based genetic linkage map anchoring Pinot Noir BAC contigs (http://genomics.research.iasma.it) has been published by Troggio et al. (2007). The grapevine community can now use concrete molecular tools (Donald et al. 2002; Barker et al. 2005; Adam-Blondon et al. 2005). In the last four years, a number of sequencing initiatives, coordinated by the International Grapevine Genome Program (http://www.vitaceae.com and http://www.intl-pag.org/13/13-grape.html), have produced more than 316,000 grapevine ESTs deposited in international databases (http://www.ncbi.nih.nlm.gov and www.tigr.org). This large assembly of available sequences, resulting in roughly 27,000 unigenes, is a source of data for developing single nucleotide polymorphism (SNP) markers from coding sequences. Single base substitution and/or small insertion–deletion polymorphisms represent the most abundant type of DNA variation (Rafalski 2002). SNPs in grapevine are quite frequent: they occur every 47 bp when clones of different Vitis species are compared (Salmaso et al. 2004), or every 64 bp when the comparison is intraspecific (Lijavetzky et al. 2007). More recently, the genome sequence data from the highly heterozygous genome of Pinot Noir, and the discovery of more than 2 millions of mapped SNPs, extended the evaluation of nucleotide variation to the entire genome rather than to limited resequenced DNA regions. It was found that the SNP frequency had an average value of 4.0 per kilobase across the grape genetic map, with several regions showing SNP frequency peaks between 5 and 7.5 per 1 kb (Velasco et al. 2007). The high frequency of SNPs, both in coding and non-coding regions, enables to develop SNP based maps, including them in a SSRs reference framework to allow comparative mapping (Rieseberg et al. 1995; Lai et al. 2005a, b).
In this paper, progress towards a transcript map for grapevine, based on the localization of expressed genes and unique genomic sequences, are described. The mapping experiment is based on a F1 population (the hybrid “Merzling” × Vitis vinifera L, cv Teroldego) which was selected due to the large number of segregating traits, including tolerance to fungal pathogens, colour and quality of anthocyanins, resistance to Phylloxera vastatrix, shape and compactness of the bunch and high versus low quality of berry metabolic profiles.
Public sequence sources (Moser et al. 2005; http://www.tigr.org) were screened taking particular care to select also genes involved in three metabolic pathways: flavonoid metabolism, defence response and signal transduction. Transcription factors putatively involved in the control of agronomic traits were also considered. Particular attention was focussed on anthocyanins, secondary metabolites which constitute the major subgroup of grape flavonoids. In V. vinifera, besides their natural role as pigments, these compounds are associated to other putative features, such as anti-oxidant, potential anticancer and anti-arteriosclerosis (Hou 2003; Kahkonen and Heinonnen 2003; Navindra et al. 2003; Passamonti et al. 2003).
Colour segregation as a simple Mendelian trait justified a deeper analysis of the last five enzymes of the anthocyanin pathway: chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), dihydroflavonol 4-reductase (DFR), leucoanthocyanidin dioxygenase (LDOX) and UDP glucose-flavonoid 3-o-glucosyl transferase (UFGT) (Sparvoli et al. 1994) and, in addition of some myb transcription factors proposed as regulators of the phenylpropanoid pathway (Kobayashi et al. 2002). We have investigated the localization of these genes on the map and any correlation with the berry colour by co-localization with putative berry colour genetic loci (Doligez et al. 2002, 2006; Grando et al. 2003; Kobayashi et al. 2004; Lijavetzky et al. 2006; This et al. 2007). Concerning other traits having QTL components, we have performed QTL-gene co-localization with the possibility of generating information on QTL candidate genes. Recent proposals have adopted a similar meta-QTL approach (Arcade et al. 2004; Chardon et al. 2004).
The results provided demonstrate that comparison of genetic maps, and the detection of the SNPs in coding regions which allows to identify candidate genes, open perspectives to future grapevine genomic approaches.
Materials and methods
Plant material and DNA extraction
An interspecific F1 population of 89 individuals derived from the cross between “Merzling” (F) (complex hybrid of V. vinifera descending from Vitis rupestris and Vitis lincecumii, with a white berry and tolerant to several pathogens) and V. vinifera cv Teroldego (T) (high quality traits, susceptible to pathogens and with black berry) was used for linkage analysis. The cross was developed at the Istituto Agrario di San Michele all’Adige (IASMA). The progeny segregated for disease resistance, berry colour and other quality traits.
DNA was isolated from young leaves following the Doyle and Doyle (1990) procedure with a few modifications as in Grando et al. (2003). After RNase digestion (10 μg/μl Rnase A), samples were resuspended in sterile water.
Simple sequence repeats (SSRs)
A total of 177 primer pairs were used on the “Merzling” × Teroldego cross: 25 VVMD (Bowers et al. 1996, 1999); 5 VVS (Thomas and Scott 1993); 2 scuVV (Scott et al. 2000); 9 VrZAG (Sefc et al. 1999); 39 VVI loci (Merdinoglu et al. 2005); 3 UDV (Di Gaspero et al. 2005) and 94 markers developed by the Vitis Microsatellite Consortium (VMC), managed by Agrogene SA, Moissy Cramayel, France. The SSR markers were selected to be well-spread over the 19 linkage groups according to the last available version of the reference map of Doligez et al. (2006).
PCR was carried out in a standard reaction of 12.5 μl. Ten nanograms of template DNA were added to the reaction mixture containing 0.25 μM of each primers, 100 μM dNTPs, 1 mM MgCl2, 1 U of Taq polymerase and 1× Taq polymerase buffer. Amplification was carried out on the two parents of the map using a Biometra T gradient thermocycler, programmed as follows: 4 min at 94°C followed by 30 cycles of 1 min at 94°C, 30 s at 54 or 58°C, 1 min and 30 s at 72°C followed by a final stage of 10 min at 72°C. After PCR, the presence of amplification products was tested using agarose gel electrophoresis. Once the melting temperature was optimised for primer pairs showing polymorphism, the entire population was screened at the optimal temperature. The primers were labelled with ABI fluorescent dyes at the 5′-ends of “forward” primers and analysed using capillary electrophoresis with an automatic 3100 ABI sequencer. Chromatograms were analysed using the software GENESCAN 3.7 (Applied Biosystems); allele calling was carried out using GENOTYPER (Applied Biosystems).
Single nucleotide polymorphisms (SNPs)
cDNA sequences were identified from two libraries of V. vinifera cv. Regent—developing inflorescence (IN: 187) and shoot tips (GR: 32); from five cDNA libraries of V. vinifera cv Pinot noir—berry (BA: 26); root (RA: 10); bud (GE: 7); and two leaf libraries (F1 and F2: 2) (Moser et al. 2005); from a BAC library of V. vinifera cv. Pinot noir (BAC-ends: 80) (Faes 2004; Adam-Blondon et al. 2005) and from public database (CHI, F3H, LDOX, DFR, UFGT, MybA, MybB). Primer pairs have been deduced using Primer3 software (Rozen and Skaletski 2000) and used to amplify the corresponding genomic sequences. Primers were designed to have an average length of 20 nucleotides, melting temperatures of 58–62°C, and to amplify 200–300 bp. Gene homology was deduced by comparison to known genes present in the public database.
Gene fragments belonging to three metabolic pathways (anthocyanin metabolism, defence response and signal transduction) were considered including sugar metabolism and transcription factors putatively controlling agronomic traits. Primer pairs deduced from libraries and gene putative functions are available at the web site http://genomics.research.iasma.it/iasma/marker_vite/snp.html and in Salmaso 2003 and in Faes 2004. They are reported in Table 1. Primer pairs of genes related to the anthocyanin pathway were:
CHI (chalcone isomerase) (primers as in Salmaso et al. 2004): primer forward AGTTCAGGTCGAGAACGTCC; primer reverse CCATCTCTCCTTCAACCACC); F3H (flavanone 3-hydroxylase), primer forward TAC AGG AGG AAG ATG AGC AA; primer reverse TTA AAG ATG GTC CAA GAT GAA C; DFR (Dihydroflavonol 4-reductase), primer forward GAT GAC CTC TGC AAT GCT CA; primer reverse CCA TGC AGA GAC CAC CTT G; LDOX (leucoanthocyanidin dioxygenase), primer forward AAG GTT CCC CAG CCT GAA T; primer reverse AGC AGG CAG AGA CAA ACA TA; UFGT (UDP-glucose-flavonoid 3-o-glucosyl transferase) (primers as in Salmaso et al. 2004: primer forward TTCTTGGAGAAGACCAGAGG; primer reverse TCCAAACAGGTGGTACAAGC); MybA (VvmybA1 transcription factor) (primers from Kobayashi et al. 2004); MybB (VlmybB1-2 transcription factor), primer forward GGT AAG AGC TCC TTG TTG CG; primer reverse GAG AAT TCA CTG GAG GAC GG.
PCR amplification was carried out in 25 μl reaction containing 0.2 mM dNTPs, 1.5 mM MgCl2, 0.2 mM of each primer and 0.2 U Taq DNA polymerase. The amplification protocol consisted of 35 cycles of 45 s at 94°C, 30 s from 55 to 60°C and 90 s at 72°C, preceded by denaturation of 4 min at 94°C and followed by a 10 min extension at 72°C. After amplification in the two parents of the map, the presence of amplification products was tested on agarose gel electrophoresis. Once the melting temperature was optimised, the genes selected were screened on the entire population at the optimal temperature.
Four approaches were used to score for polymorphisms: single strand conformational polymorphism (SSCP; Orita et al. 1989); cleaved amplified polymorphic sequences (CAPS; Konieczny and Ausubel 1993; Neff et al. 2002); DNA fragment length polymorphysm (DFLP; Schneider et al. 1999); and microsequencing (Syvanen et al. 1990). SSCP was modified as in Salmaso et al. (2004). For the development of CAPS, the software dCAPS finder 2.0 (Neff et al. 2002) allowed to search for SNPs cleaveable in the parental lines. Digestion was carried out as follows: 500 ng of PCR product were added to 2 U of restriction enzymes and 1× digestion buffer. Digestion was carried out at 37° for 30 min and polymorphic patterns on 2% agarose gels were recorded. Polymorphisms of DFLP were determined in 2% agarose gel. Microsequencing was carried out as in Troggio et al. (2008).
Linkage analysis
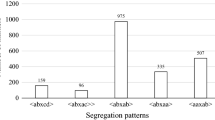
The pseudo-testcross strategy (Weeden 1993; Grattapaglia et al. 1995) was followed to produce separate linkage maps for both parental lines. The two maps were aligned each other based on co-dominant or doubly heterozygous dominant markers present in both parental genotypes resulting into the single “Merzling” × Teroldego map. The initial linkage analysis was carried out using MAPMAKER/EXP 3.0 (Lander et al. 1987), excluding bands heterozygous in both parents (ab × ab segregation type). A framework map for each parent was obtained using first the “SUGGEST SUBSET” command (LOD 3.0, minimum distance 2.0, minimum individuals 50) to define linkage groups and then the “COMPARE” command to determine the probable order of all markers in each linkage group (minimum LOD 2.0). Markers shown to depart from Mendelian segregation were also used for map construction, but their position was defined after establishing the order for other markers. Markers with distorted segregations have an asterisk indicating the level of distortion (*P < 0.05, **P < 0.01, ***P < 0.005). Integrated linkage analysis was carried out with Joinmap 3.0 (Van Ooijen and Voorrips 2001), using the Kosambi function for the estimation of map distances (Kosambi 1944), LOD ≥4.0 as a thresholds for determination of linkage groups, and 0.4 recombination fractions. Three rounds of mapping were carried out for six of the 20 linkage groups identified in “Merzling”, and for four of the 21 linkage groups identified in Teroldego. Homologous chromosome pair maps were integrated into the consensus map: the “FIXED SEQUENCE” command was used to determine the order of markers relative to the order obtained from the initial MAPMAKER analysis.
Results
SSRs
Out of the 177 primer pairs used to amplify DNA loci, 117 resulted polymorphic (13 VVMD, 4 VVS, 1 scuVV, 9 VrZAG, 29 VVI, 3 UDV and 58 VMC). Eight SSR primer pairs amplified two DNA fragments per genotype, and two SSR primer pairs amplified three, thus increasing the number of polymorphic loci to 129. These extra loci were distinguished with the letters a, b or c. Marker segregations were tested against expected segregation ratios using a chi-square test. 31 SSR loci segregated in “Merzling” (P1), 28 in Teroldego (P2) and 70 in both parents, these representing bridges anchoring homologous linkage groups of the two genetic maps. Of 89 markers showing distorted segregation ratios, ten were discarded due to the high distortion at P < 0.001 and eight because affecting the order of neighbour marker loci. Eighty SSRs used in this study have also been placed in the framework-integrated grape map of Doligez et al. (2006), while 66 SSRs are in common with the Syrah × Pinot Noir genetic map of Troggio et al. (2007) (Table 3, Supplementary Fig. 1).
SNPs and phenotypic traits
A total of 351 primer pairs were tested for amplification on the two parents of the map. Of these 259 yielded single PCR product and were further considered in SNP discovery, while 92 failed to amplify any fragment. Seven primers pairs amplified two DNA fragments of identical size identified as unique for a specific genotype by the SSCP analysis and were considered in the analysis for multilocus SNP, loci distinguished with the letters a and b. The 259 primer combinations were assayed for SNP discovery on the two parents with different technique. Of these, 76 markers were polymorphic at SSCP analysis, 100 at microsequencing analysis, four at CAPs and two at DFLP. Eighty-four were discarded because monomorphic at the four techniques.
A total of 182 markers were evaluated for segregation by a chi-square test. A total of 70 SNP markers segregated in P1, 76 in P2 and 36 in both parents, the last representing bridges anchoring homologous linkage groups of the two genetic maps. Of 92 markers showing distorted segregation ratios, seven were discarded due to the high distortion at P < 0.001 and 14 because affecting the order of neighbour marker loci.
Because of our interest in specific genes, seven primer pairs were used to amplify the flavonoid related genes CHI, DFR, LDOX, F3H, UFGT, VvmybA1 and VlmybB1-2. Berry colour segregated as a simple mendelian trait and scoring data were also included in the marker matrix.
Ninety-five out of 139 SNPs are in common with the highly dense SNP-based grape genetic map of Troggio et al. (2007) (Table 3, Supplementary Fig. 1).
Linkage maps
Assembly of the maps by linkage/recombination analysis was carried out with Mapmaker/EXP 3.0 and JoinMap 3.0. Following a pseudo-testcross strategy, marker sets from both parents were processed separately and the maps aligned with each other based on markers segregating in both parents (Table 2).
After a first round of calculation at LOD ≥4, eight SSR markers and 14 SNPs were excluded because affecting the order of neighbouring marker loci or excessively increased the linkage group end distances.
A total of 26 SSRs and 58 SNPs were mapped only in the “Merzling” map, 25 SSRs and 61 SNPs only in the Teroldego map, whereas and 56 SSRs and 26 SNPs in both maps (Table 2). The “Merzling” map consisted of 166 loci (82 SSRs and 84 SNPs) covering 20 linkage groups and 914 cM, with an average marker interval of 5.5 cM. The Teroldego map consisted of 168 loci (81 SSRs, 87 SNPs and one morphological marker) distributed on 21 linkage groups covering 1,173.7 cM, with an average marker interval of 7.0 cM. There were 17 and 18 unlinked loci, respectively for the “Merzling” and Teroldego maps.
A consensus map was produced based on integrated dataset and using the Joinmap v3.0 program (Fig. 1). A total of 247 loci (108 SSRs and 139 SNPs) were mapped to 20 linkage groups (linkage group 18 in fact splitted in groups 18a and 18b), covering 1,309.2 cM, with an average marker distance of 5.4 cM (Table 2). Linkage groups were numbered according to the map of Doligez et al. (2006) and Troggio et al. (2007). A small group was identified because of common SSRs with the published maps (group T1b). In general, marker distribution was fairly even: no pronounced clustering of any marker type was evident, also when genes participating to the same biochemical pathway were considered. Few markers with segregation distortion (*P < 0.05, **P < 0.01, ***P < 0.005) were mapped to linkage groups 1, 3, 5, 6, 7, 8, 10,13, 14, 16, 17, 18a, 18b, 19 (Table 3). Large gaps of 20 or more cM were present on three linkage groups of the consensus map (FT4, FT12, FT18a, FT19). Marker order and marker intervals were generally consistent between homologs from the parental and the consensus maps, with local inversions of closely linked markers more evident when “Merzling” and the consensus map are compared (F3, F5, F7, F8, F10, F14, T10, T13).
“Merzling” (F), Teroldego (T) and consensus “Merzling” × Teroldego (F × T) maps. Linkage groups of the “Merzling”, Teroldego and consensus map are numbered from 1 to 19 with the prefixes F, T and FT. Markers with distorted segregations have asterisks indicating the level of distortion (*P < 0.05, **P < 0.01, ***P < 0.005). Marker positions are reported based on recombination distances (cM). Genes belonging to anthocyanin biosynthesis are underlined. Linked markers which excessively increased the linkage group end distances or which affected the order of neighbours in the group were not included in the map and listed below each linkage group
Marker order in the two parental maps (based on 66 SSR and 95 SNP markers) was in general consistent with the one observed in the consensus linkage map of V. vinifera Syrah × Pinot Noir cross (Troggio et al. 2007), expect for few local inversions, mainly in the “Merzling” map (Supplementary Fig. 1).
Genes related to the flavonoid pathway did not position in one or few clusters: F3H and VvmybA1 mapped in the Teroldego map in two different linkage groups (LG 4 and LG 2, respectively). CHI (LG 13), DFR (LG 18b) and LDOX (LG 2) were assigned to the “Merzling” map. UFGT (LG 16) and VlmybB1-2 (LG 5) were assigned both in Teroldego and “Merzling” maps. Berry colour mapped to linkage group 2 in the Teroldego map at the same locus as VvmybA1 (Fig. 1).
Discussion
SSR markers made possible to create a marker framework including SNPs related to functional genes. In rare cases, SSRs clustered (e.g. LG 1), but in general they were well distributed along linkage groups and maintained the position occupied in the recently published grapevine map (for instance Doligez et al. 2006; Troggio et al. 2007) with few exceptions.
The map contains 139 new functional gene markers which represents an average of 7.3 new markers per each of the 19 linkage groups. Marker order in the consensus map differed from the parental maps due to local inversions and to the mapping position of 20 loci. Marker order differences may depend from local variation in recombination frequency, from the segregation of specific markers in only one parent and from synteny disruption in the two parents of the map. The maps in fact derive from a complex V. vinifera hybrid parent (“Merzling”), such that a possible chromosomal rearrangement of the Vitis spp. genomes should not be excluded (Doucleff et al. 2004; Lowe and Walker 2006). Although discussion on grape genome duplication remain still open, a possible explanation could be find in duplications of loci in Vitis spp. genomes followed by mutations in primer regions (Jaillon et al. 2007; Velasco et al. 2007). Microsatellite sequences resulted randomly distributed throughout the map. Random distribution of SSR loci has also been reported by other authors for grapevine fruit-tree maps (Testolin et al. 2001; Doligez et al. 2006; Troggio et al. 2007). In contrast to microsatellite markers, the SNP markers, selected in this study from expressed genome regions, were supposed to map in high-coding euchromatic regions. We have not found evidence of a particular clustering of SNPs, to the point of suggesting the presence, in grapevine, of gene-rich chromosomal regions. The low density of our SNP markers may have, in this sense, contributed. Gaps on linkage groups 4, 12, 18a and 19 were noted, besides in this paper also in the Syrah × Grenache map (Adam-Blondon et al. 2004), supporting, for the Vitis spp. genome, the possible presence of local heterogeneity in recombination. Differences in recombination rates, either global or restricted to particular genomic regions, have been reported for several plant and animal species (Karp and Jones 1983; Causse et al. 1996; Simianer et al. 1997) and recently addressed in grapevine during the construction of a dense linkage map anchoring Pinot Noir BAC-contig (Troggio et al. 2007; http://genomics.research.iasma.it).
SNPs are the most frequent polymorphisms present in an eukaryotic genome. This would suggest to concentrate mapping efforts on SNPs markers. One target of the present study was to map genes participating to anthocyanin metabolism responsible for the colour of the berry. CHI, F3H, DFR, LDOX and UFGT, involved in this metabolic pathway, mapped to different linkage groups. Only LDOX was positioned in the linkage group to which belong the genetic factor supporting berry colour (LG 2) (see also Grando et al. 2003). However, the distance of 39.0 cM between the two loci excludes a direct functional correlation between LDOX and LG 2. The same locus supporting berry colour has been mapped to the same genomic region by Fischer et al. (2004), Doligez et al. (2002) and Doligez et al. (2006), revealing a coincidence with the location we propose. In the “Merzling” × Teroldego segregating population, berry colour co-segregated with a myb gene mapping to linkage group 2. Evidence of cosegregation between the expression of VvmybA1 and skin colour in the grapevine has been demonstrated by Kobayashi et al. (2004) analysing Vitis species. In the work of Kobayashi (2004), white-skinned genotypes had an insertion of the retrotransposon Gret1 in the 5′-flanking region of VvmybA1. The Gret1 insertion inhibits the expression of the myb transcription factor and, consequently, do not induce the expression of UFGT, the last gene of the phenylpropanoid pathway. The genetic link between this myb transcription factor and anthocyanin biosynthesis, as proposed at molecular level by Kobayashi et al. (2004), has been demonstrated here in V. vinifera in agreement with recently published papers (Lijavetzky et al. 2006; This et al. 2007). Our work is the first demonstration of co-localization of Myb with the colour starting from a cross population segregating for the berry colour.
Our map provides a resource for the identification of candidate genes supporting known QTL positions. In fact, co-segregation of SNPs derived from coding genes and a marker linked to QTLs is a logic strategy for the identification of genes underlying important traits (Wright et al. 2005). Data reporting the position of grapevine QTLs (Doligez et al. 2002; Fischer et al. 2004; Fanizza et al. 2005), when interpreted on the light of our map, show cases where ESTs, credited to have a specific function, map in proximity to QTLs (Fig. 2). However, in most cases the exercise did not reveal an obvious match between the trait and the putative function of the concerned gene. We identified two genes (RA0493 and BE1074L06R) that may contribute to the berry ripening. Kobayashi et al. (2002) have previously shown that mybB transcripts are present in all stage of grape berry development, but they increase noticeably at the colouring stage. RA0493, homologue to mybB transcription factor, maps coincident with a QTL controlling begin of berry ripening (OIV code 303, http://www.genres.de) in the interval defined by markers VVMD6 and VMC7A4 (Fischer et al. 2004). While the grape has been classified as a non-climacteric fruit whose ripening is thought to be ethylene independent, it has been showed that a transient increase of endogenous ethylene production occurs just before veraison (i.e. inception of ripening). The observation that ethylene perception is required for the increase of berry diameter, the decrease of berry acidity and for anthocyanin accumulation implies that grape contains a functional network of ethylene signalling at the onset of ripening, in part necessary for the ripening process (Chervin et al. 2004). BE1074L06R is homologous to a kinase/phosphatase MAP putatively involved in ethylene biosynthesis (Kim et al. 2003). This is consistent with the specificity of the QTL associated with it. An additional gene, IN0860, encoding a putative Ca2+-binding protein associated with the hypersensitive reaction of the plant to a pathogen (Jakobek et al. 1999), maps close to the VVMD27 SSR locus, in a position coincident with a “minor” QTL for downy mildew resistance (Fischer et al. 2004). The hypersensitive reaction (HR) is an inducible plant response associated with disease resistance. It is characterized by rapid and localized cell death at the site of infection. Although the mechanisms by which grapevine cells operate to reduce disease incidence caused by the downy mildew fungus Plasmopara viticola are not fully elucidated, an accumulation or conversion of phenolic compounds and a hypersensitive response seems to be associated with an enhanced disease resistance of the plant (Kortekamp and Zyprian 2003). This Ca2+-binding protein, mapped in this population, provides a functional link with the co-mapping QTL.
Summary of associations between previously mapped QTLs (Fischer et al. 2004) and ESTs markers with similar candidate functions. QTLs are drew on the right of the linkage group around the closest SSR (underlined) in QTL mapping population. On the left of the linkage groups there is the candidate function of the EST mapped based on homology
The associations we have highlighted need to be functionally proved using proper tools. In any case, as observed in the sunflower (Lai et al. 2005a, b), identified EST/QTL associations represent an important step in identifying genes underlying important traits.
References
Adam-Blondon AF, Roux C, Claux D, Butterlin G, Merdinoglu D, This P (2004) Mapping 245 SSR markers on the Vitis vinifera genome: a tool for grape genetics. Theor Appl Genet 109(5):1017–1027
Adam-Blondon AF, Bernole A, Faes G, Lamoureux D, Pateyron S, Grando MS, Caboche M, Velasco R, Chalhoub B (2005) Construction and characterization of BAC libraries from major grapevine cultivars. Theor Appl Genet 110(8):1363–1371
Arcade A, Labourdette A, Falque M, Mangin B, Chardon F, Charcosset A, Joets J (2004) BioMercator: integrating genetic maps and QTL towards discovery of candidate genes. Bioinformatics 20:2324–2326
Barker CL, Donald T, Pauquet J, Ratnaparkhe MB, Bouquet A, Adam-Blondon AF, Thomas MR, Dry I (2005) Genetic and physical mapping of the grapevine powdery mildew resistance gene, Run1, using a bacterial artificial chromosome library. Theor Appl Genet 111:370–377
Bowers JE, Dangl GS, Vignani R, Meredith CP (1996) Isolation and characterization of the new polymorphic simple sequence repeat loci in grape (Vitis vinifera L.). Genome 45:1142–1149
Bowers JE, Dangl GS, Meredith CP (1999) Development and characterization of additional microsatellite DNA markers for grape. Am J Enol Vit 50:243–246
Causse M, Santoni S, Damerval C, Maurice A, Charcosset A, Deatrick J, de Vienne D (1996) A composite map of expressed sequences in maize. Genome 39:418–432
Chardon F, Virlon B, Moreau L, Falque M, Joets J, Decousset L, Murignaux A, Charcossot A (2004) Genetic architecture of flowering time in maize as inferred from quantitative trait loci meta-analysis and synteny conservation with the rice genome. Genetics 168:2169–2185
Chervin C, El-Kereamy1 E, Roustan JP, Latché A, Lamon J, Bouzayen M (2004) Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci 167:1301–1305
Di Gaspero G, Cipriani G, Marrazzo MT, Andreetta D, Prado Castro MJ, Peterlunger E, Testolin R (2005) Isolation of (AC)n-microsatellites in Vitis vinifera L. and analysis of genetic background in grapevines under marker assisted selection. Mol Breed 15:11–20
Di Gaspero G, Cipriani G, Adam-Blondon A-F, Testolin R (2007) Linkage maps of grapevine displaying the chromosomal locations of 420 microsatellite markers and 82 markers for R-gene candidates. Theor Appl Genet 114(7):1249–1263
Donald TM, Pellerone F, Adam-Blondon AF, Bouquet A, Thomas MR, Dry IB (2002) Identification of resistance gene analogs linked to a powdery mildew resistance locus in grapevine. Theor Appl Genet 104:610–618
Doucleff M, Jin Y, Gao F, Riaz S, Krivanek AF, Walker MA (2004) A genetic linkage map of grape, utilizing Vitis rupestris and Vitis arizonica. Theor Appl Genet 109(6):1178–1187
Doligez A, Bouquet A, Danglot Y, Lahogue F, Riaz S, Meredith CP, Edwards KJ, This P (2002) Genetic mapping of grapevine (Vitis vinifera L.) applied to the detection of QTLs for seedlessness and berry weight. Theor Appl Genet 105:780–795
Doligez A, Adam-Blondon AF, Cipriani G, Di Gaspero G, Laucou V, Merdinoglu D, Meredith CP, Riaz S, Roux C, This P (2006) An integrated SSR map of grapevine based on five mapping populations. Theor Appl Genet 113:369–382
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus Biotech 12:13–15
Faes G (2004) Analisi della struttura del genoma di Vitis vinifera. Ph.D. dissertation, University of Udine, Italy, 100 pp
Fanizza G, Lamaj F, Costantini L, Chaabane R, Grando MS (2005) QTL analysis for fruit yield components in table grapes (Vitis vinifera). Theor Appl Genet 111:658–664
Fischer B, Salakhutdinov I, Akkurt M, Eibach R, Edwards KJ, Töpfer R, Zyprian EM (2004) Quantitative trait locus analysis of fungal disease resistance factor on a molecular map of grapevine. Theor Appl Genet 108:501–515
Grando MS, Bellin D, Edwards KJ, Pozzi C, Stefanini M, Velasco R (2003) Molecular linkage maps of Vitis vinifera L. and Vitis riparia Mchx. Theor Appl Genet 106:1213–1224
Grattapaglia D, Bertolucci FL, Sederoff RR (1995) Genetic mapping of QTLs controlling vegetative propagation in Eucalyptus grandis and E. urophylla using a pseudo-testcross strategy and RAPD markers. Theor Appl Genet 90:933–947
Hou DX (2003) Potential mechanisms of cancer chemoprevention by anthocyanins. Curr Mol Med 3(2):149–159
Jaillon O, Aury J, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, Vezzi A, Legeai F, Hugueney P, Dasilva C, Horner D, Mica E, Jublot D, Poulain J, Bruyère C, Billault A, Segurens B, Gouyvenoux M, Ugarte E, Cattonaro F, Anthouard V, Vico V, Del Fabbro C, Alaux M, Di Gaspero G, Dumas V, Felice N, Paillard S, Juman I, Moroldo M, Scalabrin S, Canaguier A, Le Clainche I, Malacrida G, Durand E, Pesole G, Laucou V, Chatelet P, Merdinoglu D, Delledonne M, Pezzotti M, Lecharny A, Scarpelli C, Artiguenave F, Pè M, Valle G, Morgante M, Caboche M, Adam-Blondon A, Weissenbach J, Quétier F, Wincker P, The French–Italian Public Consortium for Grapevine Genome Characterization (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449:463–467
Jakobek JL, Smith-Becker JA, Lindgren PB (1999) A bean cDNA expressed during a hypersensitive reaction encodes a putative calcium-binding protein. Mol Plant Microbe Interact 12:712–719
Karp A, Jones RN (1983) Cytogenetics of Lolium perenne. Part 2. Chiasma distribution. Theor Appl Genet 64:137–145
Kahkonen MP, Heinonen M (2003) Antioxidant activity of anthocyanins and their aglycons. J Agric Food Chem 51(3):628–633
Kim CY, Liu Y, Thorne ET, Yang H, Fukushige H, Gassmann W, Hildebrand D, Sharp RE, Zhang S (2003) Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant cell (11):2707–2718
Kobayashi S, Ishimaru M, Hiraoka K, Honda C (2002) Myb-related genes of Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta 215:924–933
Kobayashi S, Yamamoto NG, Hirochika H (2004) Retrotransposon-induced mutations in grape skin color. Science 14;304(5673):982
Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4:403–410
Kortekamp A, Zyprian E (2003) Characterization of Plasmopara-resistance in grapevine using in vitro plants. J Plant Physiol 160(11):1393–1400
Kosamby DD (1944) The estimation of map distance from recombination values. Ann. Eugen 12:172–175
Lai Z, Livingstone K, Zou Y, Church SA, Knapp SJ, Andrews J, Rieseberg LH (2005a) Identification and mapping of SNPs from ESTs in sunflower. Theor Appl Genet 111(8):1532–1534
Lai Z, Nakazato T, Salmaso M, Burke JM, Tang S, Knapp SJ, Rieseberg LH (2005b) Extensive chromosomal repatterning and the evolution of sterility barriers in hybrid sunflower species. Genetics 171:291–303
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newberg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Lijavetzky D, Ruiz-Garcia L, Cabezas JA, De Andres MT, Bravo G, Ibanez A, Martinez-Zapater J (2006) Molecular genetics of berry colour variation in table grape. Mol Genet Genomics 276(5):427–435
Lijavetzky D, Cabezas J, Ibanez A, Rodriguez V, Martinez-Zapater J (2007) High throughput SNP discovery and genotyping in grapevine (Vitis vinifera L.) by combining a re-sequencing approach and SNPlex technology. BMC Genomics 8:424
Lowe KM, Walker MA (2006) Genetic linkage map of the interspecific grape rootstock cross Ramsey (Vitis champinii) × Riparia Gloire (Vitis riparia). Theor Appl Genet 112(8):1582–1592
Merdinoglu D, Butterlin G, Bevilacqua L, Chiquet A, Adam-Blondon A-F, Decroocq S (2005) Development and characterization of a large set of microsatellite markers in grapevine (Vitis vinifera L.) suitable for multiplex PCR. Mol Breed 15:349–366
Moser C, Segala C, Fontana P, Salakhudtinov I, Gatto P, Pindo M, Zyprian E, Toepfer R, Grando MS, Velasco R (2005) Comparative analysis of expressed sequence tags from different organs of Vitis vinifera L. Funct Integr Genomics 5:208–217
Navindra PS, Yanjun Z, Muraleedharan GN (2003) Inhibition of proliferation of human cancer and cyclooxygenase enzymes by anthocyanidins and chatechins. Nutr Cancer 46(1):101–106
Neff MM, Turk E, Kalishman M (2002) Web-based primer design for single nucleotide polymorphism analysis. Trends Genet 18:613–615
Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T (1989) Detection of polymorphisms of human DNA by gel electrophoresis as single strand conformational polymorphisms. Proc Natl Acad Sci USA 86:2766–2770
Passamonti S, Vrhovsek U, Vanzo A, Mattivi F (2003) The stomach as a site for anthocyanins absorption from food. FEBS Lett 544:210–213
Rafalski A (2002) Applications of single nucleotide polymorphisms in crop genetics. Curr Opin Plant Biol (2):94–100
Riaz S, Dangl GS, Edwards KJ, Meredith CJ (2004) A microsatellite marker based framework linkage map of Vitis vinifera L. Theor Appl Genet 108:864–872
Rieseberg LH, Van Fossen C, Desrochers AM (1995) Hybrid speciation accompanied by genomic reorganization in wild sunflower. Nature 375:713–727
Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, pp 365–386
Salmaso M (2003) Analysis of genome diversity and construction of a functional map in Vitis spp. Ph.D. Dissertation, University of Padova, Italy, 108 pp
Salmaso M, Faes G, Segala C, Stefanini M, Salakhutdinov I, Zyprian E, Toepfer R, Grando MS, Velasco R (2004) Genome diversity and gene haplotypes in the grapevine (Vitis vinifera L.), as revealed by single nucleotide polymorphisms. Mol Breed 14:385–395
Schneider K, Borchardt DC, Schafer-Pregl R, Nagl N, Glass C, Jeppsson A, Gebhardt C, Salamini F (1999) PCR-based cloning and segregation analysis of functional gene homologues in Beta vulgaris. Mol Gen Genet. 262(3):515–524
Scott KD, Eggler P, Seaton G, Rossetto M, Ablett EM, Lee LS, Henry RJ (2000) Analysis of SSR derived from grape ESTs. Theor Appl Genet 100:723–726
Sefc KM, Regner F, Turetschek E, Glössl J, Steinkellner H (1999) Identification of microsatellite sequences in Vitis riparia and their applicability for genotyping of different Vitis species. Genome 42:367–373
Simianer H, Szyda J, Ramon G, Lien S (1997) Evidence for individual and between-family variability of the recombination rate in cattle. Mamm Genome 8:830–835
Sparvoli F, Martin C, Scienza A, Gavazzi G, Tonelli C (1994) Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.). Plant Mol Biol. 24(5):743–755
Syvanen AC, Aalto-Setala K, Harju L, Kontula K, Soderlund H (1990) A primer-guided nucleotide incorporation assay in the genotyping of apolipoprotein E. Genomics 8:684–692
Testolin R, Huang WG, Lain O, Messina R, Secchione A, Cipriani G. (2001) A kiwifruit (Actinidia spp.) linkage map based on microsatellites and integrated with AFLP markers. Theor Appl Genet 103:30–36
This P, Cadle-Davidson M, Lacombe T, Owens CL (2007). Wine grape (Vitis vinifera L.) color associates with allelic variation in the domestication gene VvmybA1. Theor Appl Genet 114:723–730
Thomas MR, Scott NS (1993) Microsatellite repeats in grapevine reveal DNA polymorphisms when analysed as sequence-tagged sites (STSs). Theor Appl Genet 86:985–99l
Troggio M, Malacarne G, Coppola G, Segala C, Cartwright DA, Pindo M, Stefanini M, Mank R, Moroldo M, Morgante M, Grando MS, Velasco R (2007) A dense single-nucleotide polymorphism-based genetic linkage map of grapevine (Vitis vinifera L.) anchoring Pinot Noir bacterial artificial chromosome contigs. Genetics 176(4):2637–2650
Troggio M, Malacarne G, Vezzulli S, Faes G, Salmaso M, Velasco R (2008) Methods for polymorphism detection and genotyping within expressed regions in grapevine genome. Vitis 47(1):21–30
Van Ooijen JW, Voorrips RE (2001) JoinMap 3.0. Software for the calculation of genetic linkage maps. Plant Research International, Wageningen, The Netherlands
Velasco R, Zharkikh A, Troggio M, Cartwright DA, Cestaro A, Pruss D, Pindo M, FitzGerald LM, Vezzulli S, Reid J, Malacarne G, Iliev D, Coppola G, Wardell B, Micheletti D, Macalma TM, Facci M, Mitchell JT, Perazzolli M, Eldredge G, Gatto P, Oyzerski R, Moretto M, Gutin N, Stefanini M, Chen Y, Segala C, Davenport C, Demattè L, Mraz A, Battilana J, Stormo K, Costa F, Tao Q, Si-Ammour A, Harkins T, Lackey A, Perbost C, Taillon B, Stella A, Solovyev V, Fawcett JA, Sterck L, Vandepoele K, Grando MS, Toppo S, Moser C, Lanchbury J, Bogden R, Skolnick M, Sgaramella V, Bhatnagar SK, Fontana P, Gutin A, Van de Peer Y, Salamini F, Viola R (2007). High quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2(12):e1326
Weeden NF (1993) Approaches to mapping in horticultural crops. Plant genome analysis. CRC Press Inc., Boca Raton, pp 7–68
Wright SI, Bi IV, Schroeder SG, Yamasaki M, Doebley JF, McMullen MD, Gaut BS (2005) The effects of artificial selection on the maize genome. Science 308:1310–1314
Acknowledgments
This work has been supported by the Cass di Risparmio di Trento e Rovereto Foundation. We thank Rosalba Grillo for providing technical support and Cinzia Segala for processing ESTs. Particular thanks to Francesco Salamini and Silvia Vezzulli for the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Guiderdoni.
Marzia Salmaso and Giulia Malacarne contributed equally to the present work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salmaso, M., Malacarne, G., Troggio, M. et al. A grapevine (Vitis vinifera L.) genetic map integrating the position of 139 expressed genes. Theor Appl Genet 116, 1129–1143 (2008). https://doi.org/10.1007/s00122-008-0741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-008-0741-3