Abstract
During the domestication of bread wheat (Triticum aestivum L.), evolutionary modifications that took place in seed dispersal mechanisms enhanced its suitability for agricultural production. One of these modifications involved the evolution of the free-threshing or hulless characteristic. In this study, we studied quantitative trait loci (QTL) affecting components of the free-threshing habit (threshability and glume tenacity) on chromosome 2D in a recombinant inbred line (RIL) population developed by the International Triticeae Mapping Initiative (ITMI) as well as the tenacious glumes 1 (Tg1) gene in F2 progeny (CS/CS2D F2) of a cross between Chinese Spring and the 2D2 substitution line [Chinese Spring (Ae. tauschii 2D)]. In the ITMI population, two QTL affected threshability (QFt.orst-2D.1 and QFt.orst-2D.2) and their location coincided with QTL affecting glume tenacity (QGt.orst-2D.1 and QGt.orst-2D.2). In the CS/CS2D F2 population, the location of QTL that affected glume tenacity (QGt.orst-2D.1), the size of a glume base scar after detachment (QGba.orst-2D), and Tg1 (12-cM interval between Xwmc112 and Xbarc168) also coincided. Map comparisons suggest that QFt-orst-2D.1, QGt.orst-2D.1, and QGba.orst-2D correspond to Tg1 whereas QFt.orst-2D.2 and QGt.orst-2D.2 appear to represent separate loci. The observation of coincident QTL for threshability and glume tenacity suggests that threshability is a function of glume adherence. In addition, the observation of the coincident locations of Tg1 and QTL for the force required to detach a glume and the size of a glume base scar after detachment suggests that Tg1’s effect on both glume tenacity and threshability resides on its ability to alter the level of physical attachment of glumes to the rachilla of a spikelet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hexaploid wheat (Triticum aestivum L., 2n = 42, AABBDD) arose ∼8,000 years ago from a spontaneous hybridization event between tetraploid wheat (T. turgidum L. 2n = 28, AABB) and the weedy diploid goatgrass Aegilops tauschii Cosson (2n = 14, DD) (Huang et al. 2002). During the domestication of wheat, changes in spike morphology and development enhanced its suitability for agricultural practices. These changes included modifications of rachis fragility, spikelet disarticulation, awn development, pubescence, grain size, glume tenacity, and threshability. Of these modifications, one that has been investigated because of its evolutionary significance and its importance in the practical utilization of the wheat grain, is the free-threshing habit. Genotypes with non-tenacious glumes that require limited mechanical action during the de-hulling process are considered free-threshing (hulless) whereas genotypes with tenacious glumes that are not readily detached represent non-free-threshing (hulled) wheats.
Hexaploid wheat has been historically subdivided into several subspecies based on rachis fragility and the free-threshing trait (Kimber and Sears 1983). Free-threshing forms with a tough rachis include T. aestivum ssp. aestivum, ssp. sphaerococcum, and ssp. compactum whereas ssp. spelta, ssp. macha, and ssp. vavilovii have fragile rachises and are hulled and non-free threshing (Leighty and Boshnakian 1921; Sears 1946; Unrau 1950; Kabarity 1966; Feldman 2001). Mac Key (1966) proposed that rachis brittleness and glume tenacity are the product of the interplay between two genetic systems. One component is polygenic and the other is represented by a modifier of the polygenic system. This modifier is Q, an APETALA2-like transcription factor (Faris et al. 2003; Simons et al. 2006), on chromosome 5A (Mac Key 1954; Sears 1954; Muramatsu 1963, 1986; Kuckuck 1964; McFadden and Sears 1946) and the polygenic system involves genes on homoeologous group 3 chromosomes that primarily control rachis brittleness (Watanabe and Ikebata 2000; Watanabe et al. 2005; Nalam et al. 2006), genes on homoeologous group 2 chromosomes that affect glume adherence or tenacity (Sears 1968; Kerber and Rowland 1974; Chen et al. 1999; Simonetti et al. 1999; Taenzler et al. 2002; Jantasuriyarat et al. 2004), and QTL on other chromosomes (Simonetti et al. 1999: Jantasuriyarat et al. 2004). This model where Q’s effects on glume tenacity and rachis fragility is not only pleiotropic but depends on interactions with loci that control these characteristics directly is still well accepted (Luo et al. 2000).
Kerber and Dyck (1969) originally reported the existence of a factor in the D genome that affected threshability and glume tenacity in hexaploid wheat. This was later confirmed by Kerber and Rowland (1974) whose studies showed that the non-free-threshing trait of synthetic hexaploids, irrespective of whether their tetraploid parent carried Q or q, was due to the Tg1 (tenacious glumes 1) gene on the short arm of chromosome 2D. Because non-free-threshing hexaploids were produced when free-threshing tetraploids were crossed with Ae. tauschii, the authors concluded that the dominant Tg1 allele derived from Ae. tauschii interfered with or avoided the effect of Q.
When the free-threshing habit was studied in a recombinant inbred line population developed from a cross between a spring wheat, Opata-85, and a synthetic hexaploid wheat, W-7984, QTL on chromosomes 2A, 2B, 2D, 5A, 6A, 6D and 7B were found to affect the free-threshing character (Jantasuriyarat et al. 2004). In the study by Jantasuriyarat et al. (2004), the QTL on the short arm of chromosomes 2D (corresponding to Tg1) and the long arm of chromosome 5A (corresponding to Q) had the largest effects on the trait. Overall, the free-threshing habit was predominantly affected by Tg1 and to a lesser extent by Q. Investigations with hexaploid wheat aneuploids (Sears 1954), tetraploid wheat (Simonetti et al. 1999), and T. monococcum (Taenzler et al. 2002) also suggest that genes on group 2 chromosomes primarily influence the free-threshing habit by their direct effects on glume tenacity. Thus, the free-threshing phenotype of hexaploid wheat is largely the result of interactions between tenacious glumes loci on group 2 chromosomes and Q on chromosome 5A.
Q has been the subject of intense research leading to its precise localization and isolation (Faris et al. 2003; Simons et al. 2006). On the other hand, there has been a scarcity of studies involving genes that affect glume tenacity like Tg1. Thus, the aim of our research is to use map-based methods to study Tg1 as an initial step towards its eventual isolation and characterization. Previously, we used quantitative trait mapping to regionally localize Tg1 on chromosome 2D (Jantasuriyarat et al. 2004). In this study, we placed Tg1 in a microsatellite-based linkage map of chromosome 2D, determined its chromosome bin location, assessed its relationship to a QTL described by Jantasuriyarat et al. (2004) and other genes in group 2 chromosomes, and performed a preliminary assessment of its phenotypic effect.
Materials and methods
Plant material
The localization of factors affecting threshability and glume tenacity was achieved using two mapping populations. The first mapping population consisted of recombinant inbred lines (RIL) developed by a collaborative mapping project of the International Triticeae Mapping Initiative (ITMI) (Nelson et al. 1995a, b, c; Marino et al. 1996; Van Deynze et al. 1995). Seed of the ITMI RIL population and the two parents were provided by Dr. C. Qualset (University of California, Davis). Opata-85, W-7984, and 110 ITMI RILs were previously grown in three sites (University East Farm, West Greenhouse, and Hyslop Farm Field Laboratory, Corvallis, ON) for two years (1999 and 2000) to study traits associated with the free-threshing habit (Jantasuriyarat et al. 2004). For this study, the ITMI population and its parents were again planted in un-replicated 5 m row plots at Hyslop Farm Field Laboratory in 2001.
The second mapping population (CS/CS2D) used in this study consisted of F2 and F2:3 progeny from a cross between Chinese Spring and the 2D2 substitution line. The 2D2 line is a substitution line where chromosome 2D from Chinese Spring was substituted by chromosome 2D from Ae. tauschii [Chinese Spring (Ae. tauschii 2D)]. Seed for the 2D2 substitution line was provided by Dr. Jan Dvorak (University of California, Davis). Chinese Spring, the 2D2 substitution line, and 93 F2 individuals were planted at West Greenhouse, Oregon State University, in 2003. Chinese Spring, the 2D2 substitution line, and 93 F2:3 families were planted in un-replicated 5-m row plots at Hyslop Farm Field Laboratory in 2005.
Homoeologous group 2 cytogenetic stocks were used to assign markers to chromosomes and chromosome segments. These stocks included Chinese Spring nullisomic–tetrasomic (N2AT2B, N2BT2D, and N2DT2A), ditelosomic (Dt2DS and Dt2DL), and four deletion lines (2DS1, 2DS5, 2DL3 and 2DL9). The group 2 cytogenetic stocks were provided by Dr. B.S. Gill (Kansas State University, Manhattan). Karyotypes detailing chromosome deletion breakpoints can be found in GrainGenes’ wEST database (http://wheat.pw.usda.gov/west/binmaps/wheat2_rice.html).
DNA isolation, marker analysis, and map construction
About 30–50 mg of leaf tissue from lines from the ITMI and CS/CS2D populations and the parental lines were used for DNA extraction. DNA was extracted as described by Riera-Lizarazu et al. (2000). Microsatellite markers previously placed on chromosome 2D by various research groups were used in this study (Röder et al. 1998; Pestsova et al. 2000; Somers et al. 2004). In addition, PCR-based markers for three restriction fragment length polymorphism (RFLP) probes were developed. These sequence tagged site (STS) markers were based on EST accessions AJ440662 (PSR928), BE438756 (BCD175), and BE438952 (BCD1970) (http://www.ncbi.nlm.nih.gov/). The locus Xorw2 (primers 5′-CGTCGTTTAAACAAGACATC-3′ and 5′-CATGTGGCAGTCATCGTACA-3′) was based on AJ440662, Xorw3 (primers 5′-TCGACCTCCAGGTCAAGGAG-3′ and 5′-GTCTCAGGTATCACCCGCGC-3′) was based on BE438756, and Xorw4 (primers 5′-TTGCCCCATCTGTAAAAAGG-3′ and 5′-TTGGGAGGAGGAAAAGAGGT-3′) was based on BE438952.
Seven microsatellite primer sets (gwm455, gwm296, gwm261, gdm107, gwm484, gwm102, and gwm515) that had been previously used to map loci on chromosome 2DS were used to genotype the parents and the 110 ITMI RILs. This was necessary since only a subpopulation of 60–70 individuals had been genotyped previously with the markers of interest (Röder et al. 1998; Pestsova et al. 2000). Publicly available marker data for at least 100 ITMI RILs on chromosome 2D was obtained from GrainGenes 2.0 (http://wheat.pw.usda.gov/cgi-bin/graingenes/browse.cgi). Twenty-three molecular markers were used to construct a linkage map of chromosome 2D using the CS/CS2D F2 population. Polymerase chain reaction (PCR) amplification of microsatellite and STS loci and agarose gel-based separation of PCR products was performed as described by Nalam et al. (2006). For molecular markers that did not have easily discernible polymorphisms on agarose gels, fluorescent detection of PCR amplification products was achieved using one primer labeled with either 5-carboxy-fluroscein (5-FAM) or 4,7,2′,4′,5′,7′-hexafloro-6-carboxyrhodamin (HEX). Amplification products were electrophoresed and detected in an ABI Prism™ 3100 DNA sequencer at the Core Labs, Center for Genome Research and Biocomputing, Oregon State University.
Linkage maps for chromosome 2D were constructed using Mapmaker/Exp 3.0 (Lander et al. 1987) and JoinMap 3.0 (Van Ooijen and Voorrips 2001). Recombination fractions were converted into map distances (cM) using the Kosambi mapping function. Chromosome maps were drawn with MapChart 2.1 (Voorrips 2002).
Phenotypic assessment
The ITMI population and their parental lines (W-7984 and Opata) were evaluated for the free-threshing habit by measuring percent threshability and glume tenacity. Percent threshability was measured by processing eight randomly chosen mature spikes of each line through a gasoline-powered thresher and collecting both threshed and unthreshed seeds. Threshability was then calculated as the percentage of completely threshed seeds out of all seeds harvested. To measure glume tenacity (or adherence), a LKG-1 Hunter force gauge (AMETEK, Inc., Hatfield, PA, USA) was used to measure the force (N, Newton) required to detach a glume at its base. Glume tenacity was measured in four randomly selected spikelets of four spikes per line. Chinese Spring and the 2D2 substitution line were similarly evaluated for percent threshability and glume tenacity. The CS/CS2D F2 population and their F2:3 families were only evaluated from glume tenacity. In the case of F2:3 families, four randomly selected spikelets of ten different spikes per family were evaluated. The ITMI RILs and the CS/CS2D F2 individuals were also evaluated qualitatively for glaucousness (waxiness/glossiness) of stems and leaves. These evaluations were used to map Iw2, a dominant inhibitor of glaucousness, segregating in both populations.
The glume bases of Chinese Spring, the 2D2 substitution line, and CS/CS2D F2 population were also studied (Fig. 1). Namely, we measured the size (area in mm2) of the scar at the base of a glume after physical detachment. We evaluated two pairs of detached glumes from one to three centrally-located spikelets per line. All detached glumes were placed in florist foam and visualized using a Leica MZ6 stereo dissecting microscope (Leica Microsystems GmbH, Wetzlar, Germany) equipped with a Photometrics CoolSNAP digital camera (Roper Scientific, Inc., Trenton, NJ, USA). Digital images of glume bases were captured using RSImage 1.09 (Roper Scientific, Inc.) and ImageJ (Abramoff et al. 2004) was used to measure the size (area in mm2) of detachment scars. Analysis of variance, mean comparisons, and linear regression were performed using SAS 9.1 (SAS Institute 2003).
Glume base detachment scars (arrows) from Chinese Spring (a), the 2D2 substitution line (b) and the association of Tg1 genotype with glume tenacity (c) and detachment scar size (d) in the CS/CS2D F2 population. In a and b, four pairs of representative glumes are shown. CS had an average detachment scar of 0.221 mm2 whereas 2D2 had an average scar of 0.582 mm2. Bar scale in a and b represents 1 mm. c Column graph showing the association of Tg1 genotype with the average force (N) required to detach a glume (glume tenacity) in the CS/CS2D F2 population. Glumes from Tg1Tg1 individuals required an average of 4.241 N of force for detachment whereas individuals with the Tg1tg1 and tg1tg1 genotypes required 2.333 and 1.076 N, respectively. Vertical bars in c represent Fisher’s least significant difference (LSD) of 0.368 N (P < 0.0001). d Column graph showing the association of Tg1 genotype with the average detachment scar size/area (mm2) in the CS/CS2D F2 population. After glume detachment, Tg1Tg1 individuals had an average glume base scar size of 0.445 mm2 whereas individuals with the Tg1tg1 and tg1tg1 genotypes had average scar sizes of 0.372 and 0.259 mm2, respectively. Vertical bars in d represent a LSD of 0.037 mm2 (P < 0.0001)
QTL analysis
Phenotypic data and linkage maps were used for quantitative trait locus (QTL) analysis using QTL Cartographer 2.5 (Basten et al. 1999). For analyses involving the ITMI population, a whole genome map with ∼500 loci described by Jantasuriyarat et al. (2004) was used in conjunction with the map of chromosome 2D that was constructed for this study. Least square trait means from each environment (except for un-replicated experiments) and means across environments were analyzed. QTL were mapped using composite interval mapping (CIM) (Zeng 1993, 1994) with a maximum of five co-factors selected using the forward-selection backward-elimination stepwise regression procedure. For QTL analyses involving the CS/CS2D population, glume tenacity and detached glume base scar size (area) measurements and a map of chromosome 2D were used. In all cases, a 5-cM scan window was used for all analyses and the likelihood ratio (LR) statistic was computed every 1 cM. Based on previous work (Jantasuriyarat et al. 2004), a LOD score (Z) of 3.0 or likelihood ratio (LR) of 14 (Basten et al. 1994, 1999) was deemed an adequate threshold for QTL identification.
Results
Phenotypic data
The mean trait values for percent threshability and glume tenacity for the ITMI population are presented in Table 1. W-7984 is a synthetic wheat with highly tenacious glumes that required a minimum of ∼11 N of force to either detach or break a glume from a spikelet. Consequently, W-7984 was not easily threshed (33.6% threshability). Opata-85, on the other hand, required only 2.4 N of force to achieve glume detachment and was found to be very threshable (97.9% threshability). Glume tenacity measured as the force (N) necessary to separate glumes from their spike rachises ranged from 0.75 to 7.53 N in the ITMI population. Percent threshability for the ITMI RILs ranged from 11.76 to 98.76%.
Chinese Spring was easily threshed (94.5%) whereas the 2D2 substitution line showed less threshability (66.8%) (Table 1). This was consistent with the observation that the 2D2 substitution line had more tenacious glumes (5.9 N) than Chinese Spring (1.5 N). Glume tenacity values for the CS/CS2D F2 population ranged from 0.50 to 4.50 N. With respect to Chinese Spring and the 2D2 line, we did not observe obvious differences in the overall shape or texture of their glumes. On the other hand, there were marked differences on the level of adherence of a given glume to the base of a spikelet when we studied the size of detached glume scars (Fig. 1a, b). The 2D2 substitution line had significantly (P < 0.001) larger glume base scars (0.582 mm2) than Chinese Spring (0.221 mm2). Glume base scar size for the CS/CS2D F2 population ranged from 0.162 to 0.546 mm2 (Table 1). Glume tenacity and glume scar size were positively correlated (R 2 = 0.52, P < 0.0001) in the CS/CS2D F2 population.
QTL analysis
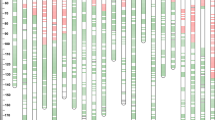
Two QTL that affected percent threshability were identified on chromosome 2D (Table 2). The QTL were designated QFt.orst-2D.1 and QFt.orst-2D.2 (Table 2). QFt.orst-2D.1 explained from 22 to 61% of the phenotypic variance. In the analysis over three environments, QFt.orst-2D.1 explained 42% of the phenotypic variance. QFt.orst-2D.2 explained 19–51% of the phenotypic variance. A combined analysis across environments showed that QFt.orst-2D.2 explained 38% of the phenotypic variance. The locus most closely associated with QFt.orst-2D.1 was Xgwm261 and with QFt.orst-2D.2 was Xgwm455.
Two QTL that affected glume tenacity, designated QGt.orst-2D.1 and QGt.orst-2D.2, were also detected on chromosome 2D (Table 2). QGt.orst-2D.1 explained 18–25% of the phenotypic variance in the environments tested. In the analysis across environments, QGt.orst-2D.1 explained 46% of the phenotypic variance. QGt.orst-2D.2 explained 13–31% of the phenotypic variance. This QTL explained 31% of the phenotypic variance across environments. The loci most closely associated with QGt.orst-2D.1 and QGt.orst-2D.2 were Xgwm261 and Xgwm455, respectively. The peaks of the two QTL were separated by a distance of ∼12 cM. W-7984 contributed the higher value allele for QTL that affected glume tenacity while percent threshability increased with the Opata-85 alleles at these QTL.
QTL analysis, with glume tenacity data from the CS/CS2D F2 population, was also carried out (Table 3). A single major QTL that explained 67% of the phenotypic variance was placed in an interval flanked by Xgwm261 and Xbarc168. Because the location of this QTL coincided with QGt.orst-2D.1 and QFt.orst-2D.1 (Fig. 2a, b), we have also named this QTL QGt.orst-2D.1. A QTL that affected the size of glume base scars after detachment (QGba.orst-2D) was also detected on chromosome 2D. QGba.orst-2D, flanked by Xgwm261 and Xbarc168, explained 55% of the phenotypic variance. The location of QGba.orst-2D coincided with the location of QGt.orst-2D.1. QTL analysis showed that the mode of inheritance for glume tenacity and glume base scar size was additive with some dominance. The 2D2 line contributed the higher value alleles for all QTL (Table 3). Markers associated with these QTL were all placed in the deletion bin 2DS5-0.47–1.00 (Fig. 2c).
Genetic linkage maps of chromosomes 2D showing the location of QTL for free-threshing habit associated traits and the tenacious glumes 1 locus (Tg1) as well as their relationship to chromosome deletion bins. a Genetic linkage map of chromosome 2D and QTL for free-threshing habit associated traits using the ITMI RIL population. Open bars indicate the 2-LOD interval for the QTL that affected percent threshability (QFt.orst-2D.1 and QFt.orst-2D.2). Solid bars indicate the 2-LOD interval for QTL that affected glume tenacity (QGt.orst-2D.1 and QGt.orst-2D.2). b Genetic linkage map of chromosome 2D using the CS/CS2D F2 mapping population showing the location of a QTL that affected glume tenacity and glume base scar size (area) as well as the location of the tenacious glumes 1 (Tg1) gene. A solid bar indicates the 2-LOD interval for a QTL that affected glume tenacity (QGt.orst-2D.1) and a thatched bar indicates the 2-LOD interval for a QTL that affected the size (area) of detached glume base scars (QGba.orst-2D). STS loci Xorw2, Xorw3, and Xorw4 may correspond to the RFLP loci Xpsr928, Xbcd175, and Xbcd1970, respectively. The lines between linkage maps a and b connect common markers. c Deletion bin map of chromosome 2D. Arrows indicate deletion breakpoints. Lines and arrows show the deletion bin location in c for loci in map b. Dark bands on the chromosome indicate the location of C-bands (Gill et al. 1991)
Genotyping and localization of Tg1
QTL analysis suggested that a major factor with mostly additive gene action was responsible for differences in glume tenacity and detached glume base scar size in the CS/CS2D population. In order to genetically localize this factor that represents Tg1, the glume tenacity of the CS/CS2D F2 population and their F2:3 derivatives were evaluated. The distribution of glume tenacity values of the F2 population grown in the greenhouse (2003) was continuous and is consistent with earlier descriptions of Tg1 as a semi-dominant gene (Kerber and Rowland 1974). Thus, classification of all individuals with respect to their genotype at the Tg1 locus in this generation was not possible. Glume tenacity assessments of F2:3 progeny planted at Hyslop farm in 2005, did allow the classification of F2 plants from which they were derived. If the glumes of a F2:3 family were uniformly tenacious the genotype of the F2 progenitor was deduced to be homozygous for the dominant Tg1 allele (Tg1Tg1) whereas the F2 progenitor of a F2:3 family with uniformly non-tenacious glumes was classified as being homozygous for the recessive tg1 allele (tg1tg1). Finally, the F2 progenitor of a F2:3 family that segregated for glume tenacity was classified as a heterozygote (Tg1tg1). This progeny test allowed the localization of Tg1 to a 12-cM interval between the markers Xwmc112 and Xbarc168 (Fig. 2b).
The relationships between genotype at the Tg1 locus and both glume tenacity and glume base scar size are shown in Fig. 1c and d, respectively. All genotypic classes (Tg1Tg1, Tg1tg1, and tg1tg1) required significantly (P < 0.0001) different levels of force to effect glume detachment. Individuals with the Tg1Tg1 genotype required the greatest force (3.24 N) for glume detachment followed by individuals with the Tg1tg1 (2.33 N) and tg1tg1 (1.08 N) genotypes (Fisher’s least significant difference, LSD, was 0.37 N). Similarly, the size of glume base scars was greatest (P < 0.0001) for individuals with the Tg1Tg1 genotype (0.45 mm2) followed by those from individuals with the Tg1tg1 (0.37 mm2) and tg1tg1 (0.26 mm2) genotypes (LSD 0.04 mm2).
Discussion
In this study, we used two mapping populations and quantitative trait mapping to localize factors affecting components of the free-threshing character on chromosome 2D of common wheat (T. aestivum). In analyses using the ITMI RIL population, two QTL that affected both glume adherence and percent threshability were identified. This is consistent with a previous study (Jantasuriyarat et al. 2004) showing that threshability and glume tenacity were negatively correlated and suggesting that threshability was a function of glume adherence. If glume adherence is high, threshability is low and vice versa. The location of QGt.orst-2D.1 and QFt.orst-2D.1, in the ITMI population map, and Tg1, in the CS/CS(2D) map, suggest that these QTL represent the action of Tg1 (Fig. 2). In a previous study involving the ITMI population, Tg1 had been regionally localized on chromosome 2D but the presence of a second set of QTL (QGt.orst-2D.2 and QFt.orst-2D.2) was overlooked (Jantasuriyarat et al. 2004). It is likely that our inability to detect these coincident QTL in an earlier study was due to missing data, a more sparse coverage of the chromosomal regions in question, and the reported difficulty of separating linked QTL (Haley and Knott 1992; Whittaker et al. 1996). Nonetheless, our results are in line with a report of an additional gene/factor on chromosome 2D, besides Tg1, that also affects glume adherence in wheat (Ternowskaya and Zhirov 1993). Whether or not this factor corresponds to the QTL identified in our study needs further study.
The presence of factors that affect glume architecture on homoeologous group 2 chromosomes dates back to Sears’ (1954) observations that plants that were nullisomic for chromosomes 2A, 2B, or 2D had papery glumes while plants that were tetrasomic for these chromosomes had glumes that were stiffer than normal disomic plants. Other genetic studies have also shown that factors on homoeologous group 2 chromosomes directly affect hulledness and the free-threshing trait by controlling glume adherence or tenacity (Sears 1968; Kerber and Rowland 1974; Simonetti et al. 1999; Chen et al. 1999; Taenzler et al. 2002; Jantasuriyarat et al. 2004). However, the relationship between these loci has not been studied. Evidence for a possible orthologue of Tg1 was presented by Taenzler et al. (2002) who mapped a soft glume (Sog, Dorofeev and Navruzbekov 1982) locus on chromosome 2Am of T. monococcum. However, recent genetic and chromosome bin mapping work suggest that the Sog gene is linked to the centromere (Sood et al. 2007) whereas Tg1 occupies a more distal segment in the short arm of chromosome 2D. Thus, Tg1 and Sog do not appear to be orthologous genes. Another difference between Sog and Tg1 is that the dominant Sog allele increases spike compactness significantly whereas Tg1 does not have that effect (Jantasuriyarat et al. 2004). Simonetti et al. (1999) found a QTL on chromosome 2B that predominantly affected threshability in a RIL population derived from a cross between a T. turgidum ssp. durum and T. turgidum ssp. dicoccoides. This QTL was attributed to a locus named tenacious glumes 2 (Tg2) that was interpreted to be a homoeologue of Tg1. Due to the sparse marker coverage of the pertinent region on chromosome 2B, we could not determine whether Tg2 was orthologous to Tg1 or QGt.orst-2D.2. Thus, more precise mapping of Tg2 is needed to clarify their relationship.
Tg1 was localized to an interval flanked by Xwmc112 and Xbarc168. This chromosomal interval is near the genetic location of two well-characterized genes that affect plant development as well as adaptation and agronomic performance. These are Rht8, a gene that affects plant height, and Ppd-D1, a gene that affects photoperiod response (Korzun et al. 1998). A barley homoeologue of Ppd-D1 has been isolated recently (Turner et al. 2005) but the identity of Rht8 is still unknown. Thus, the Tg1 region is particularly interesting because it harbors major genes involved in wheat domestication, adaptation, and improvement. With current map-based methods and the availability of various genomic resources in wheat, the isolation of genes in this region is now possible. In order to gauge the feasibility of using map-based methods to this end, we mapped the pertinent markers using chromosome 2D Chinese Spring deletion stocks. Ten markers associated with the Tg1 region were placed in deletion bin 2DS5-0.47–1.0 that spans the terminal 53% of the short arm of chromosome 2D (Fig. 2c). Erayman et al. (2004) showed that the gene-containing fraction (29%) of the wheat genome was organized in 18 major and 30 minor gene-rich regions (GRRs). The location of Tg1 with respect to consensus GRRs could not be unambiguously determined because the RFLP loci, Xbcd102 and Xcdo1379, flanking Tg1 have been placed in GRRs 2S0.9 and 2S0.8, respectively. The GRR 2S0.9 spans a physical segment of 39 Mb with a recombination frequency of 1.3 Mb/cM whereas the GRR 2S0.8 represents a physical segment of ∼7 Mb with a recombination frequency of 215 Kb/cM (Erayman et al. 2004). Regardless of which GRR harbors Tg1, cloning a gene in these areas of the chromosome using map-based methods should be feasible.
Synthetic hexaploids derived from hybridizations between tetraploid durum wheat (T. turgidum) and Ae. tauschii are non-free-threshing despite being homozygous for the dominant Q allele (Kerber and Dyck 1969; Kerber and Rowland 1974). In this study, W-7984 (a synthetic hexaploid) exhibited extremely tenacious glumes requiring at least 11 N of force to effect glume detachment or breakage. Consequently, the threshability of this genotype was quite low (34%) compared to the common wheat varieties Opata 85 (98%) and Chinese Spring (95%). The 2D2 substitution line also exhibited greater glume tenacity (5.9 N) compared to the common wheats (Opata 85 and Chinese Spring) but its glumes were less tenacious than W-7984 (Table 1). Thus, the 2D2 substitution line had threshability values that were lower than common wheats but higher than W-7984. In the case of W-7984, the free-threshing trait results from the action and interaction of Tg1, Q, and various other genetic loci (Jantasuriyarat et al. 2004). On the other hand, our analysis suggests that differences between Chinese Spring and the 2D2 substitution line with respect to glume tenacity and threshability are due to the action of one factor, Tg1, on chromosome 2D because they are nearly identical for the rest of the genome.
The glumes of the 2D2 substitution line (Tg1Tg1) and Chinese Spring (tg1tg1) were indistinguishable with respect to overall shape and appearance. On the other hand, when we looked at glume bases we found that the 2D2 substitution line had significantly larger detachment scars compared to Chinese Spring (Fig. 1a, 1b; Table 1) showing that its glumes were more firmly attached than those of Chinese Spring. When we studied the CS/CS2D population, we found that QTL for the force required to detach a glume (QGt.orst-2D.1), glume base scar size (QGba.orst-2D), and Tg1 had coincident locations (Fig. 2b; Table 3). In addition, our analysis showed an association between genotype at the Tg1 locus and both glume tenacity and glume base scar size (Fig. 1c, d). Individuals with the Tg1Tg1 genotyped required the greatest force to effect glume detachment (3.241 N) and had the largest glume base scars after detachment (0.445 mm2) followed by individuals with the Tg1tg1 (2.333 N and 0.372 mm2, respectively) and tg1tg1 (1.076 N and 0.259 mm2, respectively) genotypes. These observations suggest that QGt.orst-2D.1 and QGba.orst-2D represent the action of Tg1 and that Tg1’s effect on glume tenacity appears to reside on its ability to alter the level of physical attachment of glume bases to the rachilla of a spikelet.
Morphological and anatomical studies in the genus Triticum have shown that glumes are not deciduous tissues and that abscission layers do not form at the point of their attachment to the rachilla of the spikelet (Morrison 1994). Consequently, glume tenacity or adherence appears to be principally a function of the lignification of parenchyma cells at the base of the glume. In the case of common wheat (T. aestivum), non-tenacious glumes at maturity are only partially lignified and there is shrinkage of tissue at the glume base (Schröder 1931 cited by Morrison 1994). Conversely, genotypes with tenacious glumes show greater parenchyma cell lignification and reduced glume base tissue contraction. Thus, we suspect that Tg1’s effect on glume adherence and threshability might be mediated by its ability to modulate the lignification of parenchyma cells at the base of a given glume. Further research is needed to explore this possibility.
References
Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with imageJ. Biophotonics Int 11:36–42
Basten CJ, Weir BS, Zeng ZB (1994) Zmap–a QTL cartographer. In: Smith C, Gavora JS, Chesnais BBJ, Fairfull W, Gibson JP, Kennedy BW, Burnside EB (eds) Proceedings of the 5th world congress on genetics applied to livestock production: computing strategies and software, vol 22. Guelph, ON, Canada, pp 65–66
Basten CJ, Weir BS, Zeng ZB (1999) QTL Cartographer Version 1.13, a reference manual and tutorial for QTL mapping. http://www.statgen.ncsu.edu/qtlcart/cartographer.html
Chen Q-F, Yen C, Yang J-L (1999) Chromosome location of the gene for the hulled character in the Tibetan weedrace of common wheat. Genet Resour Crop Evol 46:543–546
Dorofeev VF, Navruzbekov NA (1982) Genetic aspects of easy threshing and rachis strength in naked-grained wheats. Doklady Vsesoyuznoi Ordena Lenina i Ordena Trudovogo Krasnogo Znameni Akademii Sel’skokhozyaistvennykh Nauk Imeni V.I. Lenina 2:3–6
Erayman M, Sandhu D, Sidhu D, Dilbirligi M, Baenziger PS, Gill KS (2004) Demarcating gene-rich regions of the wheat genome. Nucleic Acid Res 32:3546–3565
Faris JD, Fellers JP, Brooks SA, Gill BS (2003) A bacterial artificial chromosome contig spanning the major domestication locus Q in wheat and identification of a candidate gene. Genetics 164:311–321
Feldman M (2001) Origin of cultivated wheat. In: Bonjean AP, Angus JW (eds) The world wheat book. Lavosier, Paris, pp 3–58
Gill BS, Friebe B, Endo TR (1991) Standard karyotype and nomenclature system for description of chromosome bands and aberrations in wheat (Triticum aestivum). Genome 34:830–839
Haley CS, Knott SA (1992) A simple method for mapping quantitative trait loci in line crosses using flanking markers. Heredity 69:315–324
Huang S, Sirikhachornkit A, Faris J, Gill BS, Haselkorn R, Gornicki P (2002) Phylogenetic analysis of the acetyl-CoA carboxylase and 3-phosphoglycerate kinase loci in wheat and other grasses. Plant Mol Biol 48:805–820
Jantasuriyarat C, Vales MI, Watson CJW, Riera-Lizarazu O (2004) Identification and mapping of genetic loci affecting the free-threshing habit and spike compactness in wheat (Triticum aestivum L.). Theor Appl Genet 108:261–273
Kabarity A (1966) On the origin of the new cultivated wheat II. Cytogenetical studies on the karyotypes of some Triticum macha varieties. Beitr Biol Pflanzen 42:339–346
Kerber RE, Dyck PL (1969) Inheritance in hexaploid wheat of leaf rust resistance and other characters derived from Aegilops squarrosa. Can J Genet Cytol 11:639–647
Kerber RE, Rowland GG (1974) Origin of the threshing character in hexaploid wheat. Can J Genet Cytol 16:145–154
Kimber, G, Sears ER (1983) Assignment of genome symbols in Triticeae. In: Proceedings of the 6th international wheat genet symposium Kyoto, Japan, pp 1195–1196
Korzun V, Röder MS, Ganal MW, Worland AJ, Law CN (1998) Genetic analysis of the dwarfing gene (Rht8) in wheat. Part I. Molecular mapping of Rht8 on the short arm of chromosome 2D of bread wheat (Triticum aestivum L.). Theor Appl Genet 96:1104–1109
Kuckuck H (1964) Experimentelle Untersuchungen zur Entstehung der Kulturweizen. Z Pflanzenzuchtg 51:97–140
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic maps of experimental and natural populations. Genomics 1:174–181
Leighty CE, Boshnakian S (1921) Genetic behavior of the spelt form in crosses between Triticum spelta and Triticum aestivum. J Agri Res 7:335–364
Luo MC, Yang ZL, Dvorak J (2000) The Q locus of Iranian and European spelt wheat. Theor Appl Genet 100:602–606
Mac Key J (1954) Neutron and X-ray experiments in wheat and a revision of speltoid problem. Hereditas 40:65–180
Mac Key J (1966) Species relationships in Triticum. In: Proceedings of the 2nd international wheat genet symposium (Lund) 1963, Sweden. Hereditas (suppl) 2:237–276
Marino CL, Nelson JC, Lu YH, Sorrels ME, Leroy P, Lopes CR, Hart GE (1996) RFLP-based linkage maps of the homoeologous group 6 chromosomes of hexaploid wheat (Triticum aestivum L. em. Thell). Genome 39:359–366
McFadden ES, Sears ER (1946) The origin of Triticum spelta and its free-threshing hexaploid relatives. J Hered 37:81–116
Morrison LA (1994) Reevaluation of systematic relationships in Triticum L. and Aegilops L. based on comparative morphological and anatomical investigations of dispersal mechanisms. Ph.D. Thesis, Oregon State University
Muramatsu M (1963) Dosage effect of the spelta gene q of hexaploid wheat. Genetics 48:469–482
Muramatsu M (1986) The vulgare super gene, Q: its universality in durum wheat and its phenotypic effects in tetraploid and hexaploid wheats. Can J Genet Cytol 28:30–41
Nalam VJ, Vales MI, Watson CJW, Kianian SF, Riera-Lizarazu O (2006) Map-based analysis of genes affecting the brittle rachis character in tetraploid wheat (Triticum turgidum L.). Theor Appl Genet 112:373–381
Nelson JC, Sorrells ME, Van Deynze AE, Lu YH, Atkinson M, Bernard M, Leroy P, Faris JD, Anderson JA (1995a) Molecular mapping of wheat: major genes and rearrangements in homoeologous groups 4, 5, and 7. Genetics 141:721–731
Nelson JC, Van Deynze AE, Autrique E, Sorrells ME, Lu YH, Merlino M, Atkinson M, Leroy P (1995b) Molecular mapping of wheat homoeologous group 2. Genome 38:516–524
Nelson JC, Van Deynze AE, Autrique E, Sorrells ME, Lu YH, Negre S, Bernard M, Leroy P (1995c) Molecular mapping of wheat homoeologous group 3. Genome 38:525–533
Pestsova E, Ganal MW, Röder MS (2000) Isolation and mapping of microsatellite markers specific for the D genome of bread wheat. Genome 43:689–697
Riera-Lizarazu O, Vales MI, Ananiev EV, Rines HW, Phillips RL (2000) Production and characterization of maize chromosome 9 radiation hybrids derived from an oat-maize addition line. Genetics 156:327–339
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
SAS Institute (2003) SAS user’s guide, version 9.1. SAS Institute Inc., Cary, NC, USA
Schröder E (1931) Anatomische Untersuchungen an den Spindeln der Triticum- und Aegilops-Arten zur Gewinnung neuer Gesichtspunkte für die Abstammung und Systematik der Triticum-Arten. Beih Bot Zentralbl 48:333–403
Sears ER (1946) The sphaerococcum gene in wheat. Rec Genet Soc Am 15:65–66
Sears ER (1954) The aneuploids of common wheat. Columbia, Mo., University of Missouri, pp 3–58
Sears ER (1968) Relationships of chromosome 2A, 2B, and 2D with their rye homoeologue. In: Finlay KW, Shepherd KW (eds) Proceedings of the 3rd international wheat genet symposium, Canberra, Australia, pp 53–61
Simonetti MC, Bellomo MP, Laghetti G, Perrino P, Simeone R, Blanco A (1999) Quantitative trait loci influencing free-threshing habit in tetraploid wheats. Genet Res Crop Evol 46:267–271
Simons KJ, Fellers JP, Trick HN, Zhang Z, Tai Y-S, Gill BS, Faris JD (2006) Molecular characterization of the major wheat domestication gene Q. Genetics 172:547–555
Somers DJ, Issac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Sood S, Kuraparthy V, Bai G, Dhaliwal HS, Gill BS (2007) Molecular mapping of soft glume (Sog) gene in diploid wheat. In: Abstracts of plant and animal genome XV, 13–17 January, San Diego, CA. P282
Taenzler B, Esposti RF, Vaccino P, Brandolini A, Effgen S, Heun M, Schafer-Pregl R, Borghi B, Salamini F (2002) Molecular linkage map of Einkorn wheat: mapping of storage-protein and soft-glum genes and bread-making quality QTLs. Genet Res Camb 80:131–143
Ternowskaya TK, Zhirov EG (1993) Bread wheat genome D. Genetic control of tender glume and depression at its base. Tsitologiya I Genetica 27:78–83
Turner A, Beales J, Faure S, Dunford RP, Laurie DA (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310:1031–1034
Unrau J (1950) The use of monosomic and nullisomics in cytogenetic studies of common wheat. Sci Agr 30:66–89
Van Deynze AE, Dubcovsky J, Gill KS, Nelson JC, Sorrels ME, Dvořák J, Gill BS, Lagudah ES, McCouch SR, Appels R (1995) Molecular-genetic maps for group 1 chromosomes of Triticeae species and their relation to chromosomes in rice and oat. Genome 38:45–59
Van Ooijen JW, Voorrips RE (2001) JoinMap 3.0, software for the calculation of genetic linkage maps. Plant Research International, Wageningen
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Watanabe N, Ikebata N (2000) The effects of homoeologous group 3 chromosomes on grain color dependent seed dormancy and brittle rachis in tetraploid wheat. Euphytica 115:215–220
Watanabe N, Takesada N, Fujii Y, Martinek P (2005) Comparative mapping of genes for brittle rachis in Triticum and Aegilops. Czech J Genet Plant Breed 41:39–44
Whittaker J, Thompson R, Visscher PM (1996) On the mapping of QTL by regression of phenotype on marker-type. Heredity 77:23–32
Zeng Z (1993) Theoretical basis for separation of multiple linked gene effects in mapping quantitative trait loci. Proc Natl Acad Sci USA 90:10972–10976
Zeng Z (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Acknowledgments
We would like to thank Tanya Filichkin for translating Russian papers and Dr. Jeff Leonard and Kelsey Allen for technical assistance. We would also like to thank Drs. C. Qualset, J. Dvorak and B.S. Gill for providing seed from various genetic stocks. Financial support from the Oregon Agricultural Experiment Station, the National Science Foundation Research for Undergraduates Program, and USDA-CSREES National Research Initiative Plant Genome Program (Award No. 2006-55606-16629) are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D.A. Hoisington.
Rights and permissions
About this article
Cite this article
Nalam, V.J., Vales, M.I., Watson, C.J.W. et al. Map-based analysis of genetic loci on chromosome 2D that affect glume tenacity and threshability, components of the free-threshing habit in common wheat (Triticum aestivum L.). Theor Appl Genet 116, 135–145 (2007). https://doi.org/10.1007/s00122-007-0653-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-007-0653-7