Abstract
The APA (Arcelin/Phytohemagglutinin/α-Amylase inhibitor) gene family is composed of various members, present in Phaseolus species and coding for lectin and lectin-related seed proteins having the double role of storage and defense proteins. Here members of the APA family have been identified by immunological, functional, and molecular analyses and representative genes were sequenced in nine wild species of Phaseolus. All taxa possessed at least one member of the true lectin gene. No arcelin type sequences have been isolated from the species examined. Among the wild species studied, only P. costaricensis contained an α-amylase inhibitor (α-AI). In addition P. augusti, P. maculatus, P. microcarpus, and P. oligospermus showed the presence of the lectin-related α-amylase inhibitor-like (AIL) genes and α-AI activity. Data from Southern blot analysis indicated the presence of only one lectin gene in P. parvulus and P. filiformis, while an extensive gene duplication of the APA locus was found in the other Phaseolus species. Phylogenetic analysis carried out on the nucleotide sequences showed the existence of two main clusters and clearly indicated that lectin-related genes originated from a paralogous duplication event preceding the development of the ancestor to the Phaseolus genus. The finding of detectable α-AI activity in species containing AIL genes suggests that exploiting APA genes variability in the Phaseolus genus may represent a valuable tool to find new members that may have acquired insecticidal activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seeds of species belonging to the Phaseolus genus contain, in addition to the well-known sugar-binding lectin phytohemagglutinin (PHA), other related proteins that show sequence homology to the true lectins but have lost the capability to bind carbohydrates, or may have retained only a weak binding activity (Pueyo and Delgado-Salinas 1997). They are referred to as lectin-related proteins and include a small group of proteins termed arcelins (ARC) or arcelin-like (ARL), when present in species different from common bean (P. vulgaris L.), and α-amylase inhibitors (α-AI) or α-amylase inhibitor-like (AIL), according to their biological activity or inactivity, respectively (Lioi et al. 2003).
The major interest towards these proteins is that, beside being storage proteins, that account for up to about 50% of total seed proteins, they are recognised agents for seed protection, particularly against the attacks of insect larvae (Sharon and Lis 1990; Chrispeels and Raikhel 1991). Functionally, PHA, the common bean lectin so called for its property to agglutinate blood cells, binds to glycans in the intestinal epithelium of mammals and insects, while arcelins are poorly digestible and may cause an alteration of insect gut structure (Bardocz et al. 1995; Minney et al. 1990; Paes et al. 2000). α-Amylase inhibitor binds to and inhibits specific animal and insect α-amylases but has no effect on those from plants (Franco et al. 2002). The protective effects of α-AIs that have been demonstrated in transgenic plants also under field conditions, suggest that genes coding for this type of proteins could be exploited for crop protection (Chrispeels et al. 1998; Morton et al. 2000).
In P. vulgaris, lectin and lectin-related seed proteins are encoded at a single locus, the so-called APA (Arcelin/Phytohemagglutinin/α-Amylase inhibitor) locus on linkage group B4 (Gepts 1999). At the DNA level, genes are between 732 and 825 bp, share identity above 77% and are intronless. The differences in functionality among APA members are mainly derived from sequence variations that cause structural changes via the elimination of one, two or three loops in the tertiary protein structure (Rougé et al. 1993, Santimone et al. 2004).
In the evolutionary pathway leading to P. vulgaris, an extensive evolution of the APA locus has occurred: a lectin ancestor gene underwent a paralogous duplication event giving rise to the progenitor of the true lectin and to the progenitor of the other lectin-related genes. This latter evolved generating the gene coding for the biologically active form of α-AI and, through a second duplication event, the arcelin genes, found only in some wild accessions (Lioi et al. 2003). On the contrary, in Lima bean (P. lunatus L.) lectin gene evolution followed a different pathway. The ancestor lectin type gene duplicated to yield the lectin gene (LBL, Lima bean lectin) and the progenitor of ARL genes, that further evolved into AIL genes: so ARL represents an evolutionary intermediate form preceding AIL (Sparvoli et al. 2001).
Although most of the species belonging to the Phaseolus genus contain lectin and lectin-related polypeptides in their seeds (Schmit and Debouck 1991; Pueyo and Delgado-Salinas 1997), these proteins and their corresponding genes have been partially characterized only in P. coccineus L. (runner bean) and P. acutifolius A. Gray (tepary bean), two other cultivated species of the genus in addition to P. vulgaris and P. lunatus (Morgan and Manen 1981; Reynoso-Camacho et al. 2003; Mirkov et al. 1994; Lioi et al. 2006). In tepary bean, a complete set of genes comprising PHA, ARL, AIL, and α-AI has been reported (Mirkov et al. 1994; Yamada et al. 2005).
No data are available about these APA protein coding genes in wild Phaseolus species, with the exception of an AIL sequence from P. maculatus Scheele (Mirkov et al. 1994). Therefore, the study of the presence of these insecticidal proteins in different wild Phaseolus species may lead to the identification of new factors, potentially active against different insect pests, that could be used for crop protection purposes. Moreover, isolation of new APA genes from different Phaseolus sources would help understanding the evolutionary pathway of the APA locus in Phaseolus species other than common and Lima beans. To this purpose we looked for lectin and lectin-related genes in nine wild Phaseolus species. The species were chosen according to the phylogenetic analysis carried out by Delgado-Salinas et al. (1999) that showed nine monophyletic species clades within the Phaseolus lineage, designated as P. vulgaris (clade 9), P. lunatus (clade 8), P. polystachios (clade 7), P. leptostachyus (clade 6), P. pauciflorus (clade 5), P. filiformis (clade 4), P. tuerckheimii (clade 3) and P. pedicellatus (clade 2), and P. microcarpus (clade 1). Lectin and lectin-related genes amplified by PCR on genomic DNA were cloned, sequenced and then used for a molecular evolutionary analysis. The evolutionary pathway followed by the APA genes and the relationships among the members of this multigene family in the different species, as well as their relation with the detection of α-amylase inhibitory activity in the seed extracts, are discussed.
Materials and methods
Plant materials
Phaseolus accessions were kindly provided by CIAT (Centro Internacional de Agricultura Tropical), Colombia (G prefix), the National Botanic Garden of Belgium (NI prefix), and by the IPK (Institute of Plant Genetics and Crop. Plant Research), Germany (PHA prefix). The list of wild species used for the analyses and their relative accession identification codes are reported in Table 1. Seeds from P. vulgaris cv. Taylor’s Horticultural (Asgrow), and cultivated forms of P. coccineus (NI16), P. lunatus (PHA8067) morphotype Sieva, and P. acutifolius var. latifolius G. Freeman (NI558) were used for comparison of APA proteins and genomic organisation.
Protein extraction, SDS-PAGE and protein blotting
Total seed proteins were extracted from dry seeds by homogenisation and separated by electrophoresis on an 15% sodium dodecylsulfate-polyacrylamide gel (SDS-PAGE) as described by Bollini and Chrispeels (1978). Gels were stained with Coomassie Brilliant Blue R-250 or blotted on a supported nitrocellulose membrane (BIO-RAD). Immunoblot analysis was performed according to Burnette (1981) using rabbit antibodies against P. vulgaris α-AI at 1:1000 dilution. Peroxidase-linked anti-rabbit IgG was used as the secondary antibody.
Assay of α-amylase inhibitory activity
Inhibitory activity against human salivary α-amylase (EC 3.2.1.1; Type IX-A, Sigma) was measured by the increase of iodine staining after the action of the α-amylase enzyme on soluble starch as described in Altabella and Chrispeels (1990). Different volumes (from 5 to 50 μl) of seed extracts in (diluted from 10 to 400 times) were preincubated with a fixed amount of enzyme (0.16 U) for 30 min at room temperature in a final volume reaction of 0.250 ml. Then, an equal volume of 0.15% potato starch solution was added and after 5 min at room temperature the reaction was stopped by adding 1 ml of Iodine reagent (Varner and Mense 1972) and absorbance measured at 620 nm.
Isolation of lectin nucleotide sequences and Southern hybridisation
Genomic DNA was extracted from leaf tissue using the “GenElute Plant Genomic DNA Miniprep Kit” (SIGMA) according to the manufacturer’s instructions. Lectin sequences were amplified using the degenerate primers P1 and P2 as described by Mirkov et al. (1994). The resulting PCR products were gel-purified and cloned in pGem-T (Promega) plasmid. Clones were screened according to the presence/absence of restriction sites for AfaI, AvaI, EcoRV, PstI. At least ten selected clones were sequenced in both directions with an automatic sequencer. The nucleotide sequences obtained are available in the EMBL (European Molecular Biology Laboratory) Nucleotide Database with the accession numbers listed in Table 1. Specific primers for the amplification of α-AI genes, two internal (P3 and P4) and one at the 5′ end (P5), were designed on the basis of P. vulgaris α-AI-1 sequence as follow: P3: 5′ AGG GTT TGA CAG AGA AAC CGA GAA 3′; P4: 5′ ATG AAT ATC CGC ACT CAC CGC CAA 3′; P5: 5′ ATG GCT TCC TCC AAG TTA CTC TCC C. The internal primers P3 and P4 were used in combination with P1 (3′ end) and P5 (5′ end) terminal primers, respectively. PCR conditions were as in Mirkov et al. (1994).
For Southern hybridisation, 3 μg of genomic DNA were digested with the restriction enzyme EcoRI, size-separated and transferred onto a nylon membrane. The insert of the Pv3-36 clone (PHA-E) (Lioi et al. 2003) was labelled with α-[32P]-dCTP and used for filter hybridisation as described in Sparvoli et al. (1996). The filter was washed in 1.5 mM sodium citrate pH 7.0, 15 mM NaCl, 0.5% SDS (0.5× SSC), at 65°C.
Sequence comparison and molecular evolution methods
The new lectin and lectin-related nucleotide sequences, in addition to those from Phaseolus species already present the EMBL databases, were multialigned according to their corresponding amino acid sequence alignments by using the ClustalW program and hand optimised by SEAVIEW graphic tool (Galtier et al. 1996). Phylogenetic analysis was carried out by the MrBayes v3.0b4 program (Huelsenbeck and Ronquist 2001) by using the Dayhoff model for protein sequences, with the invariant site plus Gamma options (four categories). For Bayesian analysis, one cold and three incrementally heated chains were run for 1,000,000 generations and trees were sampled every 100 generations. For the analyses of amino acid sequences the results of the initial 200,000 generations were discarded (burnin) after stationarity of the InL had been reached. The PAUP* 4.04b program was then used to construct a majority role consensus tree with the results of the remaining 800,000 generations (8,000 reconstructed trees), thus allowing the calculation of the Bayesian posterior probabilities at the different nodes (Swofford 1998).
Results
SDS-PAGE, immunoblot analysis and α-amylase inhibitor activity in wild and cultivated beans
In order to obtain information on the complexity and abundance of lectin and lectin-related polypeptides in seeds of the different Phaseolus species, an immunological analysis was performed. Total protein samples from seeds belonging to the nine wild species examined (Table 1) and samples of the four major cultivated species, P. vulgaris, P. coccineus, P. acutifolius, and P. lunatus morphotype Sieva, used for comparison, were submitted to SDS-PAGE under reducing conditions and immunologically analysed with polyclonal antibodies raised against P. vulgaris recombinant α-AI (Fig. 1a, b).
SDS-PAGE and immunoblot analysis of total seed protein extracts from different Phaseolus species: 1 P. vulgaris (Pv); 2 P. coccineus (Pcoc); 3 P. costaricensis (Pcos); 4 P. acutifolius (Pa); 5 P. lunatus (Pl); 6 P. augusti (Pau); 7 P. maculatus (Pma); 8 P. leptostachyus (Ple); 9 P. parvulus (Ppa); 10 P. filiformis (Pfi); 11 P. oligospermus (Pol); 12 P. glabellus (Pgl); 13 P. microcarpus (Pmi). a Polypeptides stained with Coomassie Brilliant Blue. b Lectin and lectin-related polypeptides detected with an antiserum against P. vulgaris recombinant α-AI. Molecular weights (M) are indicated on the left
Several cross-reacting polypeptides were recognised (Fig. 1b). The antibodies used are able to cross-react with α-AI as well as with lectin and lectin-related polypeptides, since these share common epitopes (Ceriotti et al. 1989; Sparvoli et al. 1998). In protein blot analysis the antiserum mainly detected polypeptides in the 33–45 kDa range in all the seed extracts analysed. In particular, in common bean, the well known E and L subunits of PHA, around 35 kDa, as well as the α-AI polypeptides, in the 15–18 kDa range, were well detected (Fig. 1b, lane1) (Vitale et al.1984; Santino et al. 1992).
Cross-reacting polypeptides in the range of 31–35 kDa were found in the cultivated species P. coccineus and P. acutifolius (Fig. 1b, lanes 2, 4), as well as in wild P. costaricensis, P. oligospermus, P. glabellus and P. microcarpus (Fig. 1b, lanes 3, 11–13). In Lima bean, lectin (LBL) and lectin-related AIL and ARL, have been well characterised and they strongly cross-react with the antiserum in the range of 34–44 kDa (Fig. 1b, lane 5) (Sparvoli et al. 2001). Similar patterns were present also in P. augusti, P. maculatus and P. leptostachyus in which several strongly reacting polypeptides were detected (Fig. 1b, lanes 6–8).
Seed extracts from P. parvulus and P. filiformis evidenced very faint cross-reacting polypeptides (Fig. 1b, lanes 9, 10), suggesting that lectin type polypeptides are poorly represented in their seeds. Indeed, the corresponding SDS-PAGE pattern (Fig. 1a, lanes 9, 10) showed the presence of only a pair of major polypeptides, of about 40–50 kDa, that cross-reacted with antibodies against phaseolin, indicating that they correspond to vicilin storage proteins (not shown).
Among the APA proteins, α-AI is the only one that has been reported to undergo a proteolytic cleavage during its accumulation in the storage compartment. This event gives rise to a mature and active α-AI made up of subunits of 15–18 kDa (Pueyo et al. 1993). These polypeptides were detected, besides in P. vulgaris, also in P. coccineus, P. costaricensis, and in P. acutifolius seeds (Fig. 1b, lanes 1–4). This last contains slightly larger α-AI polypeptides, as already shown by Mirkov et al. (1994) and Pueyo and Delgado-Salinas (1997).
To complement the immunological results, seed extracts from all the wild and cultivated species above analysed were also tested for their ability to inhibit human saliva α-amylase (Table 2). Phaseolus coccineus and P. costaricensis seed extracts showed levels of α-AI activity comparable to that present in P. vulgaris (cv. Taylor’s). A much lower activity (about 1% of that measured in P. vulgaris) was detected in P. acutifolius, P. maculatus, P. oligospermus and P. microcarpus seed extracts and was comparable to that measured in P. vulgaris accession G12949 which contains the α-AI type 2, whose specificity to mammalian α-amylases is 100 times lower than that against Zabrotes subfasciatus α-amylase (Santino et al. 1993). All the remaining species listed in Table 1, did not show any inhibitory activity, even at extract concentration 100-fold higher than that of P. vulgaris.
Genomic organisation of the APA locus in the Phaseolus genus
To define the complexity of the APA locus in the Phaseolus genus a Southern blot analysis was carried out. Genomic DNA from all taxa was digested with EcoRI and hybridisation patterns were obtained using the labelled insert of Pv3-36 PHA clone as probe (Fig. 2). The hybridisation was performed at low stringency (0.5XSSC) in order to detect all lectin and lectin-related genes. P. parvulus and P. filiformis gave very simple hybridisation patterns, with only one major fragment detected, thus suggesting the presence of only one lectin type gene (Fig. 2, lanes 9, 10). Two major hybridising fragments of 17.5 and 5.7 kb were detected in P. microcarpus (Fig. 2, lane 13), while more complex hybridisation patterns were observed in the other species, indicating the presence of several genes and suggesting a more extensive evolutionary process for the APA locus.
Southern blot of genomic DNA digested with EcoRI restriction enzyme and probed with the insert of Pv3-36 clone (P. vulgaris PHA). DNA size markers (kb) are indicated on the left. Abbreviations on the top as reported in Fig. 1
Lectin and lectin-like genes isolation
To shed light at the molecular level on the evolution of the APA gene family in the Phaseolus genus, corresponding genes were isolated from the nine wild species listed in Table 1, representative of the nine monophyletic species clades described by Delgado-Salinas et al. (1999). Several DNA genomic clones were obtained by PCR with degenerate oligonucleotide primers corresponding to the N-terminal and C-terminal portions of lectin polypeptides (Mirkov et al. 1994). Clones were screened according to their sizes and to the presence/absence of cleavage sites for specific restriction enzymes. This approach resulted in the identification of several sequences (Table 1). Clones coding for lectins were isolated from all species examined. Nucleotide identities among the different species ranged between 84 and 97%.
A FASTA search of the five different putative lectin genes isolated from P. costaricensis, against the EMBL nucleotide database, revealed a high similarity (95%) with the PHA-L gene of P. coccineus (Lioi et al. 2006). A high percentage of similarity (98%) also resulted between the lectin isolated from P. augusti and the LBL genes from P. lunatus (Sparvoli et al. 1998, 2001). In both P. leptostachyus and P. oligospermus, the co-presence of two types of lectin sequences was found (Table 1).
Besides lectins, lectin-related clones, classified as AIL (α-amylase inhibitor-like) on the basis of their similarity with previously reported sequences, were identified in four of the nine wild species examined, P. augusti, P. maculatus, P. microcarpus, and P. oligospermus (Table 1). The highest number of sequences coding for AIL proteins was present in P. maculatus, where ten different clones were isolated. Four of them turned out to be pseudogenes with a stop codon present in their sequences, while the other six presumably code for functional proteins. Also in P. oligospermus the AIL gene found is probably not functional, in fact the loss of two nucleotides (after nucleotide 304) gives rise to a shift in the open reading frame that produces a shorter polypeptide of 181 amino acids.
The multiple alignment of the deduced amino acid sequences of the presumed functional lectin and lectin-related ones (Electronic supplementary material) shows that residues important for sugar binding were strictly conserved (Sharon and Lis 1990; Loris et al. 1998) except for P. filiformis and P. parvulus, where the Gly130 was substituted by Pro and Ala, respectively. Most lectins possess two putative glycosylation sites along their sequence, however, this number was higher in AILs, with two AIL forms of P. maculatus (PmaAIL7 and PmaAIL10) having up to seven glycosylation sites. All the AIL genes present a specific and conserved deletion, defined GAP1 by Finardi-Filho et al. (1996), and only P. augusti AIL also showed GAP3, typically found in ARL and ARC genes (Sparvoli et al. 1998, 2001).
Using P1 and P2 primers, no α-AI genes were identified in any species, even in those, like P. coccineus and P. costaricensis, where α-AI was expected since α-AI polypeptides were immunodetected. Therefore, specific internal primers P3 and P4, coupled to the P1 (3′ end) or P5 (5′ end) terminal primers, were used to identify α-AI genes. In this way, a complete α-AI gene was isolated from P. coccineus and P. costaricensis, while no amplification product was detected in all the other taxa examined.
The α-AI genes from P. coccineus and P. costaricensis were very similar to each other and to P. vulgaris α-AI-1 (99% identity), while a lower level of identity (89%) was shared with P. vulgaris α-AI-2. The deduced amino acid sequence of P. coccineus α-AI differed by only one amino acid residue from the complete peptide sequence of α and β subunits determined by Sawada et al. (2002) and by four amino acid residues from that determined by de Azevedo Pereira et al. (2006).
Evolutionary analysis
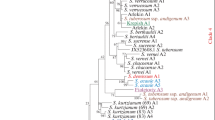
The Bayesian method, at deduced amino acid sequences from lectin and lectin-related genes isolated of wild Phaseolus, was used to construct a phylogenetic tree (Fig. 3). The soybean lectin gene (gene bank accession no. K00821) was used as an outgroup for inferring relationships within gene families and among Phaseolus species.
Phylogenetic tree calculated on lectin and lectin-related deduced amino acid sequences isolated in Phaseolus taxa, using the Bayesian method. Numbers close to the nodes represent posterior probabilities for Bayesian analysis. Accession numbers of the sequences used for the analysis are indicated beside the clone name
Our analyses showed that lectin and lectin-related genes can be divided into two main clusters. This indicates that a paralogous duplication event, that occurred during the evolution of the Phaseolus ancestor before speciation events, separated the precursor of lectin genes from a precursor common to the other lectin-related genes of AIL, ARC, ARL and α-AI types.
In the cluster that grouped all the true lectins, a statistically significant node supported the separation of the P. vulgaris subcluster (called clade 9 by Delgado-Salinas et al. (1999) and comprising P. vulgaris, P. coccineus, P. costaricensis and P. acutifolius) from the other subcluster grouping lectins of the other Phaseolus species (Fig. 3 lower part). These appeared to be much closer to the soybean lectin used as an outgroup than to the lectins of the P. vulgaris group. Three subclusters were clearly detected in the P. vulgaris group. The more ancient was the one containing P. vulgaris lectins [PvLEC(3) and PvLEC(4)], then a duplication event occurred producing the actual PHA-E and PHA-L genes that are found in P. vulgaris as well as in the other species belonging to this group: P. coccineus, P. costaricensis and P. acutifolius. This clustering suggests that the diversification between PHA-L and PHA-E occurred before speciation for these closely related species. In addition, among the PHA genes the one of P. acutifolius (PaPHA) seems to be the most distant from the other ones.
The second subcluster grouped the lectins of other wild Phaseolus species and cultivated P. lunatus. Three groups were present in this second subcluster: one group contained the lectins of P. glabellus (PglLEC) and P. oligospermus (PolLEC1), species belonging respectively to Phaseolus clades 2 and 3 (see Table 1) (Fig. 3, lower part, cluster L1). A second group contained lectins of Phaseolus species from clades 1, 3, 4, 5 and 6 and the third group contained lectins from Phaseolus species of clades 6, 7 and 8 (Fig. 3, lower part, clusters L2 and L3, respectively). In this case the P. augustii lectin (PauLEC) was found closer to the Mesoamerican lectins of P. lunatus (PlLBL5 and PlLBL6) than to the Andean ones (PlLBL 3 and PlLBL 4).
The distribution of wild Phaseolus species lectins in three subgroups suggests that an ancestral lectin, to which PolLEC1 and PglLEC are closer, had undergone two duplication events generating the group of lectins PfiLEC, PpaLEC, PolLEC2, PmiLEC and PleLEC2 and the second group of lectins PmaLEC, PleLEC1 and the lectins of the species of clade 8.
The second main cluster, representing a distinct group, was the one comprising lectin-related sequences. These were subdivided in several clear-cut subclusters (Fig. 3 upper part). The genes of P. coccineus (Pcocα-AI) and P. costaricensis (Pcosα-AI), coding for biologically active α-AI, were grouped together with sequences coding for α-AI of type 1 isolated from cultivated P. vulgaris (Pvα-AI1(I) and Pvα-AI1) (Fig. 3, upper part, cluster LR1). Conversely, the genes of P. acutifolius coding for active αAIs behaved differently: the Paα-AI2 type gene, isolated both by Mirkov et al. (1994) and Yamada et al. (2005), grouped together with the α-AI2 type found in wild Mesoamerican P. vulgaris accessions rich in arcelin, (Fig. 3, upper part, cluster LR1), while the Paα-AI1, a new type of α-amylase inhibitor not activated by proteolitic processing, was quite distant from the other lectin-related genes. Indeed, Yamada et al. (2005) reported that this gene is more related to the lectins.
Sequences named AIL were subdivided in two well defined subclusters. One was that comprising the AIL/ARL genes of P. lunatus (PlAIL1, PlAIL2, PlAIL3, PlARL1, PlARL2) and the AIL of P. augusti (PauAIL) (Fig. 3, upper part, cluster LR2). As observed for the lectin genes of this subgroup, the lectin-related sequences evolved together and the P. augusti sequence was closer to the Andean counterpart of P. lunatus.
The second subcluster grouped AIL sequences of P. maculatus, P. oligospermus, P. microcarpus and P. vulgaris (Fig. 3 upper part, cluster LR3). All the AIL sequences isolated from P. maculatus were grouped together, indicating that they share a common ancestor that underwent successive duplication and diversification processes, while PolAIL(ps) and PmiAIL were quite similar and close to PvAILs. It is interesting that the two subclusters of AIL sequences (LR3 and LR1) are quite distant from each other, suggesting they originated from different duplication events or that in the P. lunatus group a different evolutionary force acted on lectin related genes.
The last and well-defined subgroup of lectin-related genes was the one that grouped all the P. vulgaris arcelin genes together with two lectin-related genes of P. acutifolius.
Discussion
Many seeds contain plant defence proteins. Lectin and lectin-related proteins coded by the APA locus were first identified in common bean (Moreno and Chrispeels 1989) and successively studied in detail only in P. lunatus and, to a lesser extent, in P. acutifolius (Sparvoli et al. 1998; Blanco-Labra et al. 1996; Yamada et al. 2005). The aim of this work was to explore the genetic diversity in the Phaseolus genus, to find possible functional diversification of the APA members.
The data we present here show that lectin genes and proteins are present in all the wild and cultivated Phaseolus species analysed, while lectin-related genes seem to be restricted only to some of them. However, the molecular evolutionary analysis clearly shows that lectin and lectin-related genes originated throughout a paralogous duplication event preceding separation of the Phaseolus genus confirming our previous data based only on APA genes from P. vulgaris and P. lunatus (Sparvoli et al. 2001).
The extent of gene duplication varies depending on the species considered. The method we used for gene identification was based on gene isolation by PCR amplification and subsequent sequencing. This approach may not be considered exhaustive, we nevertheless found for some species a good agreement among the data resulting from Southern, immunoblot and sequencing analyses.
For example, we isolated only one lectin sequence in P. filiformis and P. parvulus, that showed only a few faint cross-reacting polypeptides (likely representing different glycoforms of the same protein) and one hybridising DNA fragment (Figs. 1b, 2b, lanes 9 and 10). On the contrary, in P. maculatus we detected more complex patterns by Southern and western analyses together with many AIL genes (Figs. 1b, 2b, lane 7; Table 1). The high number of AIL genes, together with the high number of putative glycosylation sites along the amino acid sequence and the possibility of different degree of their utilisation, could justify the many cross-reacting polypeptides that are detected in the immunoblot analysis. In other cases, such as P. coccineus, P. costaricensis, P. augusti and P. lunatus, a lower number of lectin and lectin-related sequences was isolated, although Southern and/or western analyses suggested the existence of several duplicated genes. This apparent disagreement may be justified by a low degree of polymorphism among the duplicated lectin and/or lectin-related sequences in these species. Indeed, in P. lunatus AILs and ARLs genes have diverged much less compared to the lectin-related sequences of P. vulgaris (Sparvoli et al. 2001). In addition, in the Southern blot many hybridising fragments may be due to the presence of pseudogenes, not amplifiable by PCR with our primers.
Nucleotide substitutions in homologous lectin sequences have been successfully used for evolutionary and phylogenetic studies (Lioi et al. 2003, 2006; Galasso et al. 2004). So, some consideration on phylogenetic implications are possible. The genus Phaseolus, comprising more than 50 species, has a complex taxonomic and nomenclature history and, still at present, some discrepancies among phylogenetic relationships proposed by different authors exist (Delgado-Salinas et al. 1999, 2004; Freytag and Debouck 2002; Debouck 2004), although in a very recent paper Delgado-Salinas et al. (2006) have given many clarifications.
We isolated several DNA sequences closely related to known lectin and lectin-related genes from nine wild Phaseolus species representative of phylogenetically related clades as described by Delgado-Salinas et al. (1999). In their recent paper, Delgado-Salinas et al. (2006) revised to some extent their previous work and showed that all Phaseolus species analysed are resolved into two primary lineages, defined as clade A and clade B (see Table 1). Phaseolus species ramify into eight of the nine species clades previously determined (Delgado-Salinas et al. 1999), and four independent ones, to which P. microcarpus and P. glabellus belong. In the light of this recent data, the species we chose for the analyses were representative of seven of the eight clades: in fact, P. glabellus, that was chosen to represent the group of P. pedicellatus (former clade 2), turned out to be an independent species together with P. microcarpus. This species uniqueness of P. glabellus and P. microcarpus was indeed also previously proposed by Freytag and Debouck (2002).
The lectin sequence data presented here provide additional evidence that most of the domesticated species of Phaseolus such as P. vulgaris, P. coccineus and P. acutifolius are closely related and all belong to the P. vulgaris group. Similarly, P. costaricensis, a wild species, is confirmed to belong to the same group, in agreement with Delgado-Salinas et al. (1999, 2006), or to the phylum of common bean following the classification of Debouck (2000). In the P. vulgaris group the two lectin genes of P. vulgaris, PvLEC(3) and PvLEC(4), are confirmed to be close to the lectin ancestor gene, while PHA-E and PHA-L genes evolved later.
Our analysis also shows that P. acutifolius is less related to P. vulgaris than are P. coccineus and P. costaricensis as recently reported by Muňoz et al. (2006). The position of both P. augusti sequences (PauLEC and PauAIL) which are found closely related to the corresponding ones of P. lunatus is in agreement with phylogenetic analysis based on ITS sequence variation (Delgado-Salinas et al. 1999, 2006) and AFLP molecular markers (Caceido et al. 1999).
Regarding the other species, the distribution of the genes reflects to some extent the phylogenetic relationships among the species in which they have been identified (according to Delgado-Salinas et al. 2006). In fact, with the exceptions of PfiLEC and PleLEC2 (found in species of lineage B), lectin genes from species of lineage B are clearly separated from those of species belonging to lineage A.
Our results suggest that lectin sequences may be subdivided into at least three putative orthologous groups. This is supported by the finding that in the two wild species P. oligospermus and P. leptostachyus, two distinct distantly related lectin genes have been isolated and these are found in different clusters: indeed, PolLEC1 and PolLEC2 are in clusters L1 and L2, respectively, while PleLEC1 and PleLEC2 are in clusters L3 and L2, respectively. Two hypotheses are possible to explain this finding: (1) during the evolution a duplication event from a common precursor originated a new form that underwent a different evolutionary process, or (2) introgression events could have occurred leading to the coexistence of more than one lectin type. We favour the first hypothesis since these species are quite distant from each other and belong to two different lineages (Delgado-Salinas et al. 2006).
When lectin-related sequences are considered, it appears that different evolutionary dynamics of the APA genes have occurred in the species analysed. First of all, no arcelin type sequences have been identified, also in P. coccineus and P. costaricensis, two species closely related to P. vulgaris. An exception seems to be P. acutifolius, for which different lectin-related genes have been isolated. Although one of them (Pa-αAI1) does not cluster with the related genes of the P. vulgaris group, the genes PaARC and PAARL2 are found with the arcelin group of P. vulgaris close to the PvARL gene. The possibility exists that our primers, although degenerate, did not amplify the arcelin, even if Mirkov et al. (1994) isolated the PaARC with the same couple of primers used here.
Arcelin genes have been often reported as peculiar to a restricted pool of wild Mesoamerican accessions of P. vulgaris, in which they originated from an independent duplication event in respect to AIL. Presence of ARL genes in P. acutifolius but, apparently, not in P. coccineus and P. costaricensis, rises the need to revise the above assumption and opens the question of how this subgroup of lectin-releted sequences evolved in the P. vulgaris group. However, only an extensive molecular analysis on wild and cultivated species representative of the P. vulgaris group could shed light on this point. We suggest to chose the species on the basis of the classification of Freytag and Debouck (2002) that recognises in the P. vulgaris group three sections each grouping well defined species: section A. Acutifolii groups P. acutifolius and P. parvifolius; section B. Phaseoli groups P. vulgaris, P. costaricensis and P. polyanthus and section C. Coccinei to which belong P. coccineus.
Conversely to ARC genes, sequences of the AIL type have been isolated in some species belonging to both clade A and B. The presence of many AIL duplicated sequences appear to be a common feature of Phaseolus species, although, at least in some species, AIL genes could have been lost in the course of evolution. A strong environmental pressure could explain the high number of duplication and diversification events that occurred in AIL genes of P. maculatus leading to a redundancy of AIL forms in this species. Loss or gain in genes are frequent events during genomic evolution. In particular, gene duplication is an important mechanism to create innovation, since paralogs can acquire new functions to respond to changed needs, and this is particularly true of candidate genes responsible of pest/pathogen defence mechanisms (Meyers et al. 2005). Indeed, this is likely what happened with α-AI sequences in the species belonging to the P. vulgaris group.
In this paper we have isolated two new α-AI sequences, one from P. coccineus and the other one from the wild species P. costaricensis. This is the first time that an α-AI gene was found in a non-domesticated species. Also for this aspect, this latter species could be considered a species strictly related to the P. vulgaris–P . coccineus complex. The presence of biologically active α-AI appears to be a unique feature of a limited number of Phaseolus species, restricted to the above cited P. vulgaris–P. coccineus complex, and to P. acutifolius, that is considered as part of the tertiary gene pool of P. vulgaris (Debouck 1991). The main distinctive traits of active α-AIs are considered the presence of three gaps along the nucleotide sequence (Finardi-Filho et al. 1996) and the proteolitic cleavage at the COOH terminal side of an asparagine residue located in the middle of the precursor (Pueyo et al. 1993).
Recently, Yamada et al. (2005) described a new type of bean α-AI, the α-AIPa-1 of tepary bean (here referred as Paα-AI1) that does not require post-translational processing for activation. Another case of an α-amylase inhibitory activity due to an AIL type protein has been reported in Lablab purpureus (Dolichos lablab) (Fakhoury and Woloshuk 2001), a Phaseolinae species. These two reports suggests that, beside the well known α-AIs of the Phaseolus group species, other lectin-related proteins may have acquired the ability to inhibit specific α-amylases. Indeed, when we assayed the α-amylase inhibitory activity in the different species here analysed we detected some inhibition of the human saliva α-amylase in those species (P. maculatus, P. oligospermus and P. microcarpus) whose AIL genes grouped in cluster LR3 (Tables 1, 2, Fig. 3 upper part, cluster LR3). The levels of activity we found were much lower that those measured in P. vulgaris (cv. Taylor). However, it must be pointed out that the inhibitory activity we measured depends on the specificity of the inhibitor for the α-amylase being tested (in our case that from human saliva) as well as on the abundance of the inhibitor in the seed protein extract.
The cv. Taylor of P. vulgaris contains an α-AI type 1 that is specific for mammalian α-amylases. The other Phaseolus species (P. coccineus and P. costaricensis) in which we detected good inhibitory activities also contain type 1 α-AIs (Fig. 3 upper part, cluster LR1). On the contrary, both P. acutifolius α-AIs are specific for weevil α-amylases (Yamada et al. 2001), however, we could detect an inhibitory activity, although much lower that that found in P. vulgaris, against human saliva α-amylase. A similar result was found by Santino et al. (1993) who showed that the inhibitory activity of the α-AI type 2 of P. vulgaris (from accessions G12949 and G12953) against porcine α-amylase is about 100 times less than that of α-AI type 1. This strongly supports the hypothesis that seeds of P. maculatus, P. oligospermus and P. microcarpus contain active α-AIs that may be more specific towards α-amylases other than human saliva α-amylase.
We cannot demonstrate that the AIL genes we isolated code for active α-AIs (for sure this is the case of P. oligospermus where the isolated sequence is probably not active due to the occurrence of a 2 bp deletion that causes a frameshift), however we consider that the finding of a moderate α-amylase inhibitory activity only in species whose AILs cluster together and separately from those of the P. lunatus group (for which no α-amylase inhibitory activity has been detected) is a strong indication that these type of sequences may indeed exert an inhibitory activity of specific α-amylases.
In conclusion, our results confirm that exploring the variability of APA members in wild Phaseolus species may represent a useful tool to detect lectin-related genes coding for proteins that may have evolved insecticidal activities. These findings may be useful for crop improvement either by conventional breeding or gene transfer.
References
Altabella T, Chrispeels MJ (1990) Tobacco plants transformed with the bean α-AI gene express an inhibitor of insect α-amylase in their seeds. Plant Physiol 93:805–810
Bardocz S, Grant G, Ewer SW, Duguit TJ, Brown DS, Englyst K, Pusztai A (1995) Reversible effect of phytohemagglutinin on the growth and metabolism of rat gastrointestinal tract. Gut 37:353–360
Blanco-Labra A, Sandoval-Cardoso L, Mendiola-Olaya E, Valdés-Rodrígues S, López MG (1996) Purification and characterization of a glycoprotein α-amylase inhibitor from tepary bean seeds (Phaseolus acutifolius A. Gray). J Plant Physiol 149:650–656
Bollini R, Chrispeels MJ (1978) Characterization and subcellular localization of vicilin and phytohemagglutinin, the two major reserve proteins of Phaseolus vulgaris L. Planta 42:291–298
Burnette WN (1981) “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112:195–203
Caceido AL, Gaitán MC, Duque C, Toro Chica O, Debouck DG, Tohme J (1999) AFLP fingerprinting of Phaseolus lunatus L. and related wild species from south America. Crop Sci 39:1497–1507
Ceriotti A, Vitale A, Bollini R (1989) Lectin-like protein accumulates as fragmentation products in bean seed protein bodies. FEBS Lett 250:157–160
Chrispeels MJ, Raikhel NV (1991) Lectins, lectin genes and their role in plant defence. The Plant Cell 3:1–9
Chrispeels MJ, Grossi de Sa MF, Higgins TJV (1998) Genetic engineering with α-amylase inhibitors makes seeds resistant to bruchids. Seed Sci Res 8:257–263
De Azevedo Pereira R, Nogueira Batista JA, Mattar da Silva MC, de Oliveira Neto OB, Zangrando Figueira EL, Valencia Jimenez A, Grossi-de-Sa MF (2006) An alpha-amylase inhibitor gene from Phaseolus coccineus encodes a protein with potential for control of coffee berry borer (Hypothenemus hampei). Phytochem 67:2009–2016
Debouck DG (1991) Systematics and morphology. In: van Schoonhoven A, Voysest O (eds) Common beans: research for crop improvement. Commonwealth Agricultural Bureaux International, Wallingford, pp 55–118
Debouck DG (2000) Biodiversity, ecology and genetic resources of Phaseolus beans—seven answered and unanswered questions. In: Proceedings of 7th MAFF international workshop on genetic resources. Part I. Wild legumes. AFFRC and NIAR, Japan
Debouck DG (2004) Phylogeographic migrations of Phaseolus beans in the New World, and consequences for taxonomy, conservation and breeding. Annu Rep Bean Improv Coop 47:29–30
Delgado-Salinas A, Bibler R, Lavin M (2004) Molecular phylogeny of the genus Phaseolus L. (Fabaceae). Annu Rep Bean Improv Coop 47:31–32
Delgado-Salinas A, Turley T, Richman A, Lavin M (1999) Phylogenetic analysis of the cultivated and wild species of Phaseolus (Fabaceae). Syst Bot 24:438–460
Delgado-Salinas A, Bibler R, Lavin M (2006) Phylogeny of the genus Phaseolus (Leguminosae): a recent diversification in an ancient landscape. Syst Bot 31:779–791
Fakhoury AM, Woloshuk CP (2001) Inhibition of growth of Aspergillus flavus and fungal α-amylases by a lectin-like protein from Lablab purpureus. Mol Plant Microbe Interact 14:955–961
Finardi-Filho F, Mirkov TE, Chrispeels MJ (1996) A putative precursor protein in the evolution of the bean α-amylase inhibitor. Phytochemistry 43:57–62
Franco OL, Rigden DJ, Melo FR, Grossi de Sa MF (2002) Plant α-amylase inhibitors and their interaction with insect α-amylases. Structure, function and potential for crop protection. Eur J Biochem 269(2):397–412
Freytag GF, Debouck DG (2002) Taxonomy, distribution, and ecology of the genus Phaseolus (Leguminosae–Papilionoideae) in North America, Mexico and central America. SIDA Bot Misc 23:1–300
Galasso I, Lioi L, Lanave C, Bollini R, Sparvoli F (2004) Identification and isolation of lectin nucleotide sequences and species relationships in the genus Lens (Miller). Theor Appl Genet 108:1098–1102
Galtier N, Gouy M, Gautier C (1996) SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12:543–548
Gepts P (1999) Development of an integrated linkage map. In: Sing SP (ed) Common bean improvement for the twenty-first century. Kluwer, Dordrecht, pp 53–91, pp 389–400
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 7:754–755
Lioi L, Sparvoli F, Galasso I, Lanave C, Bollini R (2003) Lectin-related resistance factors against bruchids evolved through a number of duplication events. Theor Appl Genet 107:814–822
Lioi L, Galasso I, Santantonio M, Lanave C, Bollini R, Sparvoli F (2006) Lectin gene sequences and species relationships among cultivated legumes. Genet Res Crop Evol 53:1615–1623
Meyers BC, Kaushik S, Nandety RS (2005) Evolving disease resistance genes. Curr Opin Plant Biol 2:129–34
Loris R, Hamelryck T, Bouckaert J, Wyns L (1998) Legume lectin structure. Biochim Biophys Acta 1383:9–36
Minney BHP, Gatehouse AMR, Dobie P, Dendy J, Cardona C, Gatehouse JA (1990) Biochemical bases of seed resistance to Zabrotes subfasciatus (Bean weevil) in Phaseolus vulgaris (common bean): a mechanism for arcelin toxicity. J Insect Physiol 36:757–767
Mirkov TE, Wahlstrom JM, Hagiwara K, Finardi-Filho F, Kjemtrup S, Chrispeels MJ (1994) Evolutionary relationships among proteins in the phytohemagglutinin-arcelin-α-amylase inhibitor family of the common bean and its relatives. Plant Mol Biol 26:1103–1113
Moreno J, Chrispeels MJ (1989) A lectin gene encodes the α-amylase inhibitor of the common bean. Proc Natl Acad Sci USA 86:7885–7889
Morgan MR, Manen JF (1981) Lectin variability in Phaseolus coccineus. Phytochem 24:1981–1986
Morton RL, Schroeder HE, Bateman KS, Chrispeels MJ, Armstrong E, Higging TJV (2000) Bean α-amylase inhibitor 1 in transgenic peas (Pisum sativum) provides complete protection from pea weevil (Bruchus pisorum) under field conditions. Proc Natl Acad Sci USA 97:3820–3825
Muňoz LC, Duque MC, Debouck DG, Blair MW (2006) Taxonomy of tepary bean and wild relatives as determined by amplified fragment length polymorphism (AFLP) markers. Crop Sci 46:1744–1754
Paes NS, Gerhardt IR, Coutinho MV, Yokoyama M, Santana E, Harris N, Chrispeels MJ, Grossi de Sa MF (2000) The effect of arcelin-1 on the structure of the midgut of bruchid larvae and immunolocalization of the arcelin protein. J Insect Physiol 46:393–402
Pueyo JJ, Hunt DC, Chrispeels MJ (1993) Activation of bean (Phaseolus vulgaris) α-amylase inhibitor requires proteolytic processing of the proprotein. Plant Physiol 101:1341–1348
Pueyo JJ, Delgado-Salinas A (1997) Presence of α-amylase inhibitor in some members of the subtribe Phaseolinae (Phaseoleae: Fabaceae). Am J Bot 84:79–84
Reynoso-Camacho R, González de Mejía E, Loarca–Piña G (2003) Purification and acute toxicity of a lectin extracted from tepary bean (Phaseolus acutifolius). Food Chem Toxicol 41:21–27
Rougé P, Barre A, Causse H, Chatelain C, Porthé G (1993) Arcelin and α-amylase inhibitor from the seed of common bean (Phaseolus vulgaris) are truncated lectins. Biochem Syst Ecol 21:695–703
Santimone M, Koukiekolo R, Moreau Y, Le Berre V, Rougé P, Marchis-Mouren G, Desseaux V (2004) Porcine pancreatic α-amylase inhibition by the kidney bean (Phaseolus vulgaris) inhibitor (α-AI) and structural changes in the α-amylase inhibitor complex. Biochim Biophys Acta 1696:181–190
Santino A, Daminati MG, Vitale A, Bollini R (1992) The α-amylase inhibitor of bean seed: two-step proteolytic maturation in the protein storage vacuoles of the developing cotyledon. Physiol Plant 85:425–432
Santino A, Orchard J, Daminati MG, Cantoni R, Bollini R (1993) Bean (Phaseolus vulgaris L.) lectins and protection of the stored seed. In: Basu J, Kindu M, Chakrabarti P (eds) Lectins: Biology, Biochemistry, Clinical Biochemistry, vol 9. Wiley Eastern Lt., New Delhi, pp 19–26
Sawada S, Takeda Y, Tashiro M (2002) Primary structures of α- and β-subunits of α-amylase inhibitors from seeds of three cultivars of Phaseolus beans. J Prot Chem 21:9–17
Schmit V, Debouck DG (1991) Observations on the origin of Phaseolus polyanthus Greenman. Econ Bot 45:345–364
Sharon N, Lis H (1990) Legume lectins—a large family of homologous proteins. FASEB J 4:3198–3208
Sparvoli F, Daminati MG, Lioi L, Bollini R (1996) In vivo endoproteolytically cleaved phaseolin is stable and accumulates in developing Phaseolus lunatus L. seeds. Biochim Biophys Acta 1292:15–22
Sparvoli F, Gallo A, Marinelli D, Santucci A, Bollini R (1998) Novel lectin-related proteins are major components in Lima bean (Phaseolus lunatus) seeds. Biochim Biophys Acta 1382:311–323
Sparvoli F, Lanave C, Santucci A, Bollini R, Lioi L (2001) Lectin and lectin-related proteins in Lima bean (Phaseolus lunatus L.) seeds: biochemical and evolutionary studies. Plant Mol Biol 45:587–597
Swofford DL (1998) PAUP*, phylogenetic analysis using parsimony (*and other methods). Version 4 Sinauer Associates, Sunderland
Varner JE, Mense R (1972) Characteristics of the process of enzyme release from secretory plant cells. Plant Physiol 49:187–189
Vitale A, Ceriotti A, Bollini R, Chrispeels MJ (1984) Biosynthesis and processing of phytohemagglutinin in developing bean cotyledons. Eur J Biochem 141:97–104
Yamada T, Hattori K, Ishimoto M (2001) Purification and characterization of two alpha-amylase inhibitors from seeds of tepary bean (Phaseolus acutifolius A. Gray). Phytochem 58:59–66
Yamada T, Moriyama R, Hattori K, Ishimoto M (2005) Isolation of two α-amylase inhibitor genes of tepary bean (Phaseolus acutifolius A. Gray) and their functional characterization in genetically engineered adzuki bean. Plant Sci 169:502–511
Acknowledgments
The authors are grateful to Dr. Marina Torres, CIAT, Cali, Colombia, and Dr. T. Vanderborght, NBGB Phaseolinae Collection, Meise, Belgium for the supply of seed samples. They are also grateful to Dr. Marilina Santantonio for technical assistance. This work was partially supported by “Fondo Integrativo per la Ricerca di Base” of the Italian Ministry of University and Research (RBAUO1JS5C) to RB and by Ministry of Agricultural Food and Forest Politics with funds released by C.I.P.E. (Resolution 17/2003) to FS and LL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. A. Hoisington.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lioi, L., Galasso, I., Lanave, C. et al. Evolutionary analysis of the APA genes in the Phaseolus genus: wild and cultivated bean species as sources of lectin-related resistance factors?. Theor Appl Genet 115, 959–970 (2007). https://doi.org/10.1007/s00122-007-0622-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-007-0622-1