Abstract
Fluorescence in situ hybridization (FISH), using bacterial artificial chromosome (BAC) clone as probe, is a reliable cytological technique for chromosome identification. It has been used in many plants, especially in those containing numerous small chromosomes. We previously developed eight chromosome-specific BAC clones from tetraploid cotton, which were used as excellent cytological markers for chromosomes identification. Here, we isolated the other chromosome-specific BAC clones to make a complete set for the identification of all 26 chromosome-pairs by this technology in tetraploid cotton (Gossypium hirsutum L.). This set of BAC markers was demonstrated to be useful to assign each chromosome to a genetic linkage group unambiguously. In addition, these BAC clones also served as convenient and reliable landmarks for establishing physical linkage with unknown targeted sequences. Moreover, one BAC containing an EST, with high sequence similarity to a G. hirsutum ethylene-responsive element-binding factor was located physically on the long arm of chromosome A7 with the help of a chromosome-A7-specific BAC FISH marker. Comparative analysis of physical marker positions in the chromosomes by BAC-FISH and genetic linkage maps demonstrated that most of the 26 BAC clones were localized close to or at the ends of their respective chromosomes, and indicated that the recombination active regions of cotton chromosomes are primarily located in the distal regions. This technology also enables us to make associations between chromosomes and their genetic linkage groups and re-assign each chromosome according to the corresponding genetic linkage group. This BAC clones and BAC-FISH technology will be useful for us to evaluate grossly the degree to which a linkage map provides adequate coverage for developing a saturated genetic map, and provides a powerful resource for cotton genomic researches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromosome identification is important for karyotyping, cytogenetics, and physical mapping of genetic regions of agricultural importance. Chromosome identification by analyzing chromosome relative lengths, arm ratios, nuclear organization regions (NORs) and chromosome satellite locations in mitotic or meiotic metaphase was applied widely in many plants (Yu et al. 1991; Nandi 1936; Okamoto 1962; Sears 1969; Kohel 1973; Brown 1980; Cheng et al. 1998; Menzel 1954; McClintock 1929; Rick and Barton 1954). However, these procedures have many limitations both genetically and technically. The unambiguous identification of every chromosome is not always possible.
The development of the non-isotopic in situ hybridization techniques in plants is a significant step towards chromosome identification (Rayburn and gill 1985). Although the special DNA as fluorescence in situ hybridization (FISH) probes for chromosome identification is not available in most plants (Jiang and Gill 1994), FISH using special genomic clones as probes presents as an alternative approach. For diploid plants, several research groups used isolated bacterial artificial chromosomes (BACs) as chromosome-specific FISH markers for chromosome identification and physical mapping (Dong et al. 2000; Cheng et al. 2001; Kim et al. 2002). BAC-FISH has shown to be a powerful utility for plant chromosome identification and cytogenetic researches, especially for those species with many small chromosomes.
Cotton is one of the most important natural fiber and edible oil crops in the world (Lee 1984). Tetraploid cotton Gossypium hirsutum L. [n = 2× = 26, (AD)1] has a large genome, with a 1C content of 2,230 Mbp (Arumuganathan and Earle 1991). However, the large genome of cotton is distributed over 26 pairs of small chromosomes without suitable cytogenetic markers such as bands, and it is almost impossible for the routine and unambiguous identification of each chromosomes based only on their morphology. Additionally, polyploid plants are not similar to diploids, as their genomes contain two or more subgenomes that originated from the same ancestor (Wendel 1989, 2000; Levy and Feldman 2002; Wendel and Cronn 2002). Thus, duplicated segments are present in different subgenomes. This situation leads to the difficulties in the isolation of chromosome-specific sequences or, in particular chromosome-specific large chromosomal segments such as yeast artificial chromosomes (YACs) and BACs to distinguish the A and D subgenomic homeologs. Until now, a complete set of chromosome-specific sequences (YACs or BACs) have not been developed in polyploidy plants. We have isolated eight chromosome-specific BACs from G. hirsutum tetraploid cotton and demonstrated that they were excellent cytological markers for chromosome identification in both meiotic and mitotic cells (Wang et al. 2006). Based on those data, all 26 linkage groups in our genetic map were correlated with their respective chromosomes in tetraploid cotton, and new nomenclature based on homeologous chromosomes was proposed (Wang et al. 2006). Here, we report the development of the remaining chromosome-specific BAC clones for tetraploid cotton G. hirsutum, and their uses as FISH markers for the cotton cytogenetic and physical mapping.
Materials and methods
Materials
The BACs used in this study were obtained from two genomic BAC libraries that were constructed by the tetraploid cotton TM-1 line, and a restorer line 0-613-2R for cytoplasmic male sterile lines in the G. harknessii cytoplasm (Yin et al. 2006). Among them, seven chromosome-specific BACs for chromosomes A3, A8, A11, A13, D5, D11 and D8 used for FISH were described and reported previously (Wang et al. 2006). The chromosome-A6-specific BAC 62K03 developed in previously report, was not selected because the BAC 47N15 could generated much more clear signal and was identified as new chromosome-A6-specific BAC clone in this study (Fig. 1; Table 1). The TM-1 BAC library and genetic stocks that were monosomic for G. barbandense chromosomes A1, A2, A3, A6, A7, A9, A10, A12, D7, D13, D10, D9, D6 and D12, and telodisomic for A1sh, A1lo, A2sh, A2lo, A5lo, D2lo, D1lo, D7sh, D7lo, D4lo, D4sh and D6lo for assignment of SSR markers to chromosomes or chromosome arms were kindly provided by the USDA-ARS, Crops Germplasm Research Unit, Texas, USA. TM-1 is a highly inbred line of G. hirsutum L. (2n = 52). The aneuploid hybrids were derived from the cross between the monosomic or monotelodisomic lines of the corresponding chromosome in the TM-1 (G. hirsutum) genetic background as female parent and 3–79 (G. barbadense). New tetraploid cotton nomenclature based on homeologous chromosomes was used, in which the A and D subgenome chromosomes were renamed as A1 through A13, and D1 though D13, respectively (Wang et al. 2006).
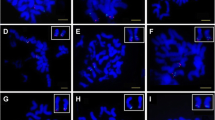
Twenty-six individual cotton mitotic chromosomes with FISH signals derived from the chromosome-specific BACs, and comparisons of linkage and FISH maps. Chr. A1–A13 and Chr.D1–D13 were the A and D subgenome chromosomes of tetraploid cotton G. hirsutum L., respectively. A Linkage maps show three or four loci, including one or two markers used to select BAC clones for FISH and two end markers from each linkage group. B Diagrams of chromosomes in which the colored circles represent signals of BAC clones linked with corresponding markers on A. C FISH images. Another BAC was mapped simultaneously with the corresponding chromosome-specific-BACs for chr. A1, A6, A10, and D12. All bars indicate 50 cM for the linkage maps. The red signals were digoxigenin-labeled probes detected by anti-digoxigenin-rhodamine, and green were biotin-labeled probes detected by avidin-fluorescein
Selection of SSR markers and isolation of chromosome-specific BACs
Molecular markers from a high-density genetic map of tetraploid cotton were used to screen BAC libraries (Han et al. 2006). Markers that mapped to apparently high recombination regions of linkage groups were chosen in this study. For the syntenic analysis of BAC-FISH sites, the markers separated by a substantial genetic distance from a common linkage group were selected. The genetic distance increased the likelihood that syntenic BAC-FISH loci could be distinguishable from each other after hybridization to chromosomes. The selected SSR markers were then used to screen the BAC libraries based on a PCR library screening approach (Wang et al. 2005). All positive clones were picked and individually cultured in a solid LB culture medium to reduce contamination. The selected clones were confirmed with corresponding markers by PCR. Plasmids from all positive BAC clones were used as FISH probes to hybridize to TM-1 mitotic chromosomes. Only the best BAC clone that consistently produced strong and unambiguous signals was selected finally as chromosome-specific BAC clone for each chromosome.
Chromosome preparation and FISH
The cotton TM-1 mitotic metaphase chromosomes were used in the FISH. Methods for root tips, slide preparations and single-color FISH were the same as described previously (Wang et al. 2006). Dual-color FISH was conducted as described by Ji et al. (1997) with some modifications as described below. The purified BAC DNA was labeled with biotin-16-dUTP or digoxigenin-11-dUTP (Roche Diagnostics, Mannheim, Germany) by standard nick translation reactions. Following overnight incubation at 37°C, slides were rinsed at 42°C in 2× SSC, 50% formamide in 2× SSC, and 2× SSC, for 10 min each. The signals from the digoxigenin-labeled and biotin-labeled probes were detected respectively by anti-digoxigenin-rhodamine and avidin-fluorescein. DAPI (4′,6-diamidino-2-phenylindole) in an antifade solution (Vector, USA) was used to counterstain the chromosomes.
Image processing and measurement
The slides were examined under an Olympus BX51 fluorescence microscope. All chromosome and FISH signal images were captured with an Evolution VF CCD camera (Media Cybernetics, USA), and were merged graphically by using an Image-Pro Express software (Media Cybernetics, USA).
Signal position and chromosome length was measured by using the same Image-Pro Express software. The results presented are average lengths of measurements from 20 mitotic chromosomes.
Results
Identification of cotton chromosome-specific BACs
Seven BACs had been identified previously and used as chromosome-specific markers to assign the G. hirsutum chromosomes (A3, A8, A11, A13, D5, D8 and D11) using translocation lines and FISH (Wang et al. 2006). To find more BAC-FISH markers and to develop a reliable system for chromosome identification and genomic researches in cotton, we isolated 19 more BAC clones that hybridized specifically to each of the remaining cotton chromosomes. A total of 72 SSR markers that mapped to regions of highly recombined of these 19 linkage groups on our detailed genetic map (Han et al. 2006) were selected to screen the BAC library. Of these, 49 markers isolated at least one positive clone. Only one positive clone for each marker (so total 49 positive clones) was selected to test as FISH probes for hybridizing to the cotton chromosome. And 31 of the 49 positive clones could present relatively clear signals (little or no background) with the aid of Cot-1 DNA for blocking, and others produced strong background or no main signals. To identify chromosome-specific clones, different clones derived from a common chromosome were further compared in FISH, and the best one that produced more clear and strong signals was selected as chromosome-specific clone. Finally, 19 BAC clones that each could consistently produce one strong and unambiguous signal on one chromosome were selected as chromosome-specific BACs for the remaining 19 chromosomes. The 26 chromosome-specific BAC clones with corresponding SSR markers and their cytological locations are presented in Table 1 and Fig. 1.
Association of BAC-FISH markers to genetic linkage groups
To verify the newly identified BACs as cytogenetic markers in the chromosomes identification, well established monosomic and monotelodisomic genetic stocks in tetraploid cotton were used to test the presence of these markers on the corresponding chromosomes. The aneuploid hybrids were produced by crossing between the monosomic or monotelodisomic lines in the G. hirsutum acc. TM-1 background as the female parent and G. barbadense acc. 3–79, which contains a single chromosome from the G. barbadense 3–79. Thus, if one marker genotype was determined to be similar to that of G. barbadense, and the counterpart marker allele was not observed, we would assign the involved marker locus to corresponding chromosome or chromosome arm. As shown in Fig. 2, the monosomic and monotelodisomic stocks generated 3–79 genotypes with chromosome-specific SSR markers BNL1079 (Fig. 2a) and BNL3971 (Fig. 2b). Therefore, these two loci were located on chromosome D13 and on the short arm of chromosome A2, respectively. Similarly, all the other markers were verified by using corresponding genetic stocks (data not shown) with the exceptions of chromosomes A4, A5, D2, D1 and D3, for which genetic stocks were not available or only monotelodisomic stocks involving one arm were made available. Nevertheless, the association of these BAC-FISH markers with their corresponding genetic linkage groups can be confirmed. Thus, a full set of chromosome specific BAC clones was established and will be an invaluable resource for cotton genomic research.
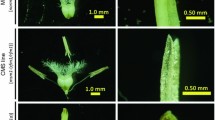
Chromosome-specific SSR markers BNL1079 (a) and BNL3971 (b), and corresponding positive BAC clone of chromosomes D13 and A2 were re-identified by genetic stocks. Lane 1–7 were Gossypium barbadense cv.3–79, Hai7124, F1(TM-1 × Hai7124), (H11 × 3–79)F1 monosomic, TM-1, restorer line 0–613–2R used for BAC library construction by Yin et al. (2006), and positive BAC clone; b Lane 1–8 were 3–79, Hai7124, F1(TM-1 × Hai7124), (Te2sh × 3–79)F1 monotelodisomic, (Te2lo × 3–79)F1 monotelodisomic, TM-1, 0–613–2R, and positive BAC clone. M DNA size marker. Arrows point the different patterns of euploid hybrids according to primer BNL1079 and BNL3971. From the aneuploid test, we can conclude that BNL1079-165 and BNL3971-140 were located on chromosome D13 (a) and the short arm of chromosome A2 (b), respectively
Integration of linkage and physical maps in tetraploid cotton
The relative chromosome positions of the FISH markers can be compared with their genetic positions on the genetic linkage map. Although a few SSR markers (BNL2448, BNL3580, BNL3627, BNL4094, BNL0358, and BNL0169) were defined at interior positions within their corresponding linkage groups, most of the 26 BACs were localized to the distal regions of corresponding chromosomes (Fig. 1; Table 2). Additionally, the total length of cotton chromosome is 71.61 μm calculated by adding up all chromosome lengths in Table 2. According to the estimated total linkage distance and DNA content, 5,200–5,500 cM (Lacape et al. 2003) and 2,230 Mbp (Arumuganathan and Earle 1991), the genetic distance and the DNA content will be 72.61–76.81 cM and 31.14 Mbp per micrometer chromosome length in cotton.
By using a dual-color FISH, we demonstrated that two BAC probes derived from corresponding markers on the same linkage map could be co-localized simultaneously to test the synteny of the BAC signals for four chromosomes. As shown in Fig. 1 (chr. A1, A6, A10 and D12), each pair of BAC clones derived from markers from one linkage group could be detected.
Physical mapping of an EST-containing BAC by chromosome-specific BAC landmarks
To test the utility of the cotton chromosome-specific clone markers, an EST clone was isolated and located onto a chromosome. Sequence-specific primers (Y2232) were designed from an EST, with high sequence similarity to a G. hirsutum ethylene-responsive element-binding factor cloned from a super hybrid Xiangzamian 2 cotton line. The EST has been mapped on one short linkage (Wang, unpublished results in our lab), which contained one marker in common with chromosome A7 in the new cotton genetic map (Guo et al. 2007). A BAC clone, 36D03, was identified by screening 0-613-2R BAC library using Y2232 primer, and co-hybridized with chromosome A7-specific BAC 09N05. The FISH result showed that BAC 36D03 did co-localize with the reference marker, and was placed on the long arm, near the end of chromosome A7 (Fig. 3).
Discussion
In this study, we report the development of all 26 chromosome-specific BACs of tetraploid cotton G. hirsutum towards a simple and reproducible method for chromosome identification by using BAC-FISH cytogenetic markers. And, we also proved this set of cytogenetic markers could be used as excellent landmarks for easy and reliable physical mapping of new BAC clones as well as other sequences that could be visualized by FISH in cotton. Nevertheless, it was a challenge to develop such chromosome-specific BACs for polyploidy plants, because most BACs will contain many repetitive sequences and will not generated locus-specific FISH signals (Zhang et al. 2004). The successful application of this technique in other plants will depend on both the size of the genomic clones analyzed and the percentage of repetitive DNA sequences in the genome (Jiang et al. 1995). The cotton genome contains a relative large percentage (≈60%) of single- or low-copy number DNA (Baker et al. 1995) and this may contribute to the successful development of such chromosome-specific BAC-FISH clones. Additionally, the strategy that using the markers of high recombination regions on linkage groups to screen the library may be helpful for picking BACs with relatively low repetitive sequence content. Combing previous study, we total screened 62 BACs, and found 40 BACs (64%) could generate clear signals with or without the Cot-1 DNA blocking. It indicates that this strategy is helpful for the avoidance of repetitive elements.
The chromosome nomenclature based on chromosomal morphology was not associated with their genetic linkage maps in tetraploid cotton, which could cause confusion in applications. In the current chromosome-specific cytogenetic marker system, the tetraploid cotton chromosomes were identified and named according to their genetic linkage groups. Therefore, chromosome identification based on these chromosome-specific markers is consistent among different varieties and accessions in Gossypium, assuming that there are no different chromosome rearrangements in these species (Dong et al. 2000).
The BAC-FISH technology provided a cytogenetic approach to correlating molecular maps with cytological maps or chromosomes. By evaluating the relative positions of FISH markers on the chromosomes and linkage maps in sorghum, Kim et al. (2002) found that the FISH markers (signals) resided near the ends of chromosomes, although their linkage markers were defined as being interiorly positioned in the respective linkage groups. And they concluded that the recombinationally active regions in sorghum chromosomes are localized primarily in the large distal euchromatic segments. As in sorghum, most of the 26 cotton BACs were localized to or near the chromosomal ends (Fig. 1), which indicated that the recombination-active regions of cotton chromosomes are primarily located in the distal regions. Moreover, the number of markers on the linkage maps is usually less than desired, and the degree of genomic coverage is uncertain. FISH with BAC clones isolated based on markers from the ends of linkage groups was used to evaluate grossly the degree of which a linkage map can provide a good coverage (De Donato et al. 1999; Kim et al. 2002). In this report, two BAC clones, 47N15 and 75F07, were selected based on chromosomal end markers of linkage group A6, and were physically localized to the end of the chromosome (Fig. 1 see Chr A6). These data indicate that the physical coverage of the chromosome is nearly reaching the ends for linkage group A6. It has been estimated that the cotton genome is 5,200–5,500 cM-long, and a total of 5,000 markers are required to develop a saturated linkage group (Lacape et al. 2003). However, for individual chromosomes, it is unknown how many markers are sufficient. Comparisons among linkage and physical maps by BAC-FISH, as described above, can be used to evaluate whether a linkage group or the regions it comprises is completely covered, and then a truly integrated chromosomal map can be constructed. The development of a set of chromosome-specific SSR markers and BAC clones provides an invaluable resource for cotton genome researches, and it will have many applications in physical mapping, gene localization, chromosome identification, QTL tracking, and marker-assisted breeding of the Gossypium species.
References
Arumuganathan K, Earle ED (1991) Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9:208–218
Baker RJ, Longmire JL, Van Den Bussche RA (1995) Organization of repetitive elements in the upland cotton genome (Gossypium hirsutum). J Hered 86:178–185
Brown MS (1980) The identification of the chromosomes of Gossypium hirsutum L. by means of translocations. J Hered 71:266–274
Cheng ZK, Yang XM, Yu HX, Gu MH (1998) A study of the number of SAT-chromosome in rice. Acta Genet Sin 25:225–231
Cheng ZK, Buell CR, Wing RA, Gu M, Jiang J (2001) Toward a cytological characterization of the rice genome. Genome Res 11:2133–2141
De Donato M, Gallagher DS, Davis SK, Ji Y, Burzlaff JD, Stelly DM, Womack JE, Taylor JF (1999) Physical assignment of microsatellite-containing BACs to bovine chromosomes. Cytogenet Cell Genet 87:59–61
Dong F, Song J, Naess SK, Helgeson JP, Gebhardt C, Jiang J (2000) Development and applications of a set of chromosome-specific cytogenetic DNA markers in potato. Theor Appl Genet 101:1001–1007
Guo WZ, Cai CP, Wang CB, Han ZG, Song XL, Wang K, Niu XW, Wang C, Lu KY, Shi B, Zhang TZ (2007) A microsatellite-based, gene-rich linkage map reveals genome structure, function, and evolution in Gossypium. Genetics (in press)
Han ZG, Wang CB, Song XL, Guo WZ, Gou JY, Li CH, Chen XY, Zhang TZ (2006) Characteristics, development and mapping of G.hirsutum derived-EST-SSRs in allotetraploid cotton. Theor Appl Genet 112:430–439
Ji YF, Raska DA, McKnight TD, Islam-Faridi NM, Crane CF, Zwick MS, Hanson RE, Price HJ, Stelly DM (1997) Use of meiotic fluorescence in situ hybridization for identification of a new monosome in Gossypium hirsutum L. Genome 40:34–40
Jiang J, Gill BS (1994) Nonisotopic in situ hybridization and plant genome mapping: the first ten years. Genome 37:717–725
Jiang J, Gill BS, Wang GL, Ronald PC, Ward DC (1995) Metaphase and interphase fluorescence in situ hybridization mapping of the rice genome with bacterial artificial chromosomes. Proc Natl Acad Sci USA 92:4487–4491
Kim JS, Childs KL, Islam-Faridi MN, Menz MA, Klein RR, Klein PE, Price HJ, Mullet JE, Stelly DM (2002) Integrated karyotyping of sorghum by in situ hybridization of landed BACs. Genome 45:402–412
Kohel RJ (1973) Genentic nomenclature in cotton. J Hered 64:291–295
Lacape JM, Nguyen TB, Thibivilliers S, Bojinov B, Courtois B, Cantrell RG, Burr B, Hau B (2003) A combined RFLP–SSR–AFLP map of tetraploid cotton based on a Gossypium hirsutum × Gossypium barbadense backcross population. Genome 46:612–626
Lee JA (1984) Cotton as a world crop. In: Kohel RJ, Lewis CL (eds) Cotton. Agronomy Monograph, No. 24. Crop Science Society of America, Madison, Wisconsin, pp 1–25
Levy AA, Feldman M (2002) The impact of polyploidy on grass genome evolution. Plant Physiol 130:1587–1593
McClintock B (1929) Chromosome morphology in Zea mays. Science 69:629
Menzel MY (1954) A cytological method for genome analysis in Gossypium. Genetics 40:214–223
Nandi HK (1936) The chromosome morphology, second association and origin of cultivated rice. J Genet 33:315–316
Okamoto M (1962) Identification of the chromosomes of common wheat belonging to the A and B genome. Can I Genet Cytol 4:31–37
Rayburn AL, Gill BS (1985) Use of biotin-labeled probes to map specific DNA sequences of wheat chromosomes. J Hered 76:78–81
Rick CM, Barton DW (1954) Cytological and genetical identification of primary trisomics of the tomato. Genetics 39:640–666
Sears ER (1969) Wheat cytogenetics. Ann Rev Genet 3:451–468
Wang K, Song XL, Han ZG, Guo WZ, Yu JZ, Sun J, Pan JJ, Kohel RJ, Zhang TZ (2006) Complete assignment of the chromosomes of Gossypium hirsutum L. by translocation and fluorescence in situ hybridization mapping. Theor Appl Genet 113:73–80
Wang K, Zhang YJ, Zhang TZ (2005) A high throughput approach for cotton BAC-DNA isolation. Cotton Sci 17:125–126
Wendel JF (1989) New world cottons contain Old World cytoplasm. Proc Natl Acad Sci USA 86:4132–4136
Wendel JF (2000) Genome evolution in polyploids. Plant Mol Biol 42:225–249
Wendel JF, Cronn RC (2002) Polyploidy and the evolutionary history of cotton. Adv Agron 78:139–186
Yin JM, Guo WZ, Yang LM, Liu LW, Zhang TZ (2006) Physical mapping of the Rf 1 fertility-restoring gene to a 100 kb region in cotton. Theor Appl Genet 112:1318–1325
Yu HG, Liang H, Kofoid KD (1991) Analysis of C-banding chromosome patterns of sorghum. Crop Sci 31:1524–1527
Zhang P, Li WL, Fellers J, Friebe B, Gill BS (2004) BAC-FISH in wheat identifies chromosome landmarks consisting of different types of transposable elements. Chromosoma 112:288–299
Acknowledgments
This work was financially supported in part by grants from the program of Changjiang Scholars and Innovative Research Team in University of MOE, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. S. Heslop-Harrison.
Rights and permissions
About this article
Cite this article
Wang, K., Guo, W. & Zhang, T. Development of one set of chromosome-specific microsatellite-containing BACs and their physical mapping in Gossypium hirsutum L.. Theor Appl Genet 115, 675–682 (2007). https://doi.org/10.1007/s00122-007-0598-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-007-0598-x