Abstract
Gametophytic selection can drastically reduce the number of selection cycles during crop improvement programs. The objective of the present investigation was to test whether the nature of inheritance of two unlinked disease-resistant loci, h 1 and h 2, against Fusarium wilt in chickpea (Cicer arietinum L.) under gametophytic (pollen) selection was similar to that already observed at sporophytic level. A homozygous dominant (H 1 H 1 H 2 H 2) susceptible genotype JG-62 was crossed to a recessive (h 1 h 1 h 2 h 2) resistant genotype WR-315 to produce 20 F1 hybrid seeds. In the following generation, flower buds of 10 F1 hybrid plants were subjected to toxin stress before anthesis and the remaining ten control F1 plants’ flowers were sprayed with water. Thirty-four selected BC1 plants were generated by test crossing resistant WR-315 individuals with pollen from toxin-stressed F1 individuals. Both control and treated F1 plants were selfed to produce respective F2 generations. Two DNA markers, CS-27700bp and A07C430bp, linked to susceptible alleles H 1 and H 2, respectively, were used to study the inheritance patterns of h 1 and h 2 loci in the F2 and BC1 generations. One hundred and forty-four selected F2, 129 control F2, and 34 selected backcross individuals were tested for the presence or absence of DNA markers. Except for the control F2, observed ratios of selected F2 and BC1 populations exhibited significant chi-square deviations from expected monogenic and digenic ratios. Our results suggest that gametophytic selection is as effective as that realized at the sporophytic level, and that the gametophytic selection can be an effective breeding tool for plant breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The life cycle of higher plants consists of haploid (gametophytic) and diploid (sporophytic) phases. Although, the sporophytic phase predominates between the two forms and the gametophytic generation is often termed as a forgotten generation (Heslop-Harrison 1980), it has been shown time and again that the two phases do share common gene expression profile (Mulcahy et al. 1996; Hormaza and Herraro 1996; Ravikumar et al. 2003). The haploid diploid gene expression implies that selection exerted at the gametophytic level can also lead to detectable changes in allelic frequencies at the sporophytic level (Clegg et al. 1978; Ravikumar et al. 2003). Accordingly, a variety of selection pressures such as temperature (Zamir et al. 1982; Maisonneuve et al. 1986; Clarke et al. 2004; Dominguez et al. 2005), pathotoxin (Laughnan and Gabay 1973; Shobha Rani and Ravikumar 2006), herbicide (Sari Gorla et al. 1989), metal (Searcy and Mulcahy 1985), and water stress (Ravikumar et al. 2003) were enforced during gametophytic generation and were found very effective in increasing the frequency of resistant individuals in the progeny (Hormaza and Herraro 1996; Ravikumar and Patil 2002). Despite these encouraging results—that brought considerable attention to gametophytic selection as a potential new tool to improve crop plants—there were many instances where gametophytic selection failed to improve characters under consideration (Landi et al. 1989) or failed to show any significant response beyond the first generation of selection cycle (Maisonneuve et al. 1986; Dominguez et al. 2005).
In fact, so far only a few investigations have clearly demonstrated the persistence of positive response to gametophytic selection in the succeeding generations. For example Frascaroli and Songstad (2001) following their pollen selection experiments showed a consistent and marked improvement in maize for chlorosulfuron resistance at the end of second sporophytic generation. Similarly, Chikkodi and Ravikumar (2003) have shown that pollen selection was still effective in increasing the proportions of resistant individuals in sunflower for Alternaria leaf and stem blight disease after two generations of selection. Nevertheless, it has been hypothesized that the lack of positive response to gametophytic selection can occur when the traits under selection are quantitative in nature (Ottaviano and Mulcahy 1989; Landi et al. 1989) or have very little genetic variability in the gametic pool (Fernandez-Munoz et al. 1995) or are under transient epigenetic effects (Jorgensen 1993; Jablonka and Lamb 1998; Slack 1998).
Especially with regard to quantitative traits, it has been argued that lack of positive selection probably comes from the nature of quantitative traits, as many genes with small effects control the final phenotype (Pfahler 1983; Landi et al. 1989). In that case, one should see a more reliable genetic evidence for gametophytic selection for those traits that are controlled by one or two genes. Indeed Frascaroli and Songstad (2001) provided such an evidence in maize. A pollen selection was conducted in maize for chlorosulfuron resistance controlled by a single locus with two alleles; the resistant allele dominant over the susceptible one. The alleles followed a simple Mendelian inheritance, and the resistant allele was almost fixed in one generation by means of gametophytic selection.
Molecular genetic evidence could validate the genetic basis of gametic selection eliminating, fully or partially, the involvement of epigenetic changes that were known to transmit meiotically in plants (Fedoroff et al. 1989; Jorgensen 1993; Chandler et al. 2000). In a pilot study involving a very limited sample size and a DNA marker linked to one of the susceptible alleles (H 1) for Fusarium wilt in chickpea (Cicer arietinum L.), Ravikumar and Patil (2004) observed an increase in the frequency of resistant-allele between generations.
Fusarium wilt, caused by Fusarium oxysporum, is a major disease of chickpea in the Indian subcontinent, which accounts for 80% of the global production (Abbo et al. 2005). Although a large number of races of Fusarium have been reported, race 1 is widespread in India. Furthermore, three independent loci were reported to govern resistance to race 1 (Singh et al. 1987), however, an earlier work on Fusarium wilt in one of the authors laboratory (Brinda and Ravikumar 2005) and elsewhere (Upadhyaya et al. 1983a, b) indicated that two major independent loci, h1 and h2, effectively govern resistance to race 1 in chickpea. The dominant alleles at both h1 (H1-) and h2 (H2-) loci result in early wilting, while recessive at either one (h1h1 H2- or H1-h2h2) produce late wilting, whereas recessive at both the loci (h1h1 h2h2) result in resistance (Upadhyaya et al. 1983a, b; Brinda and Ravikumar 2005).
The DNA markers linked to the Fusarium wilt susceptibility loci H 1 (Mayer et al. 1997) and H 2 (Thippeswamy et al. 2005) have already been developed. The primer pair CS-27F/CS-27R, which was developed by Mayer et al. (1997) and was termed as allele-specific associated primer (ASAP), amplifies a fragment of 700 base pair (bp) and is linked to the allele for susceptibility (H 1) to race-1 of Fusarium with an estimated distance of 6 cM. The primer pair A07C, which was developed by Thippeswamy et al. (2005), amplifies DNA marker of 430 bp linked to H 2 locus of wilt susceptibility at an estimated distance of 21.7 cM (R. L. Ravikumar, unpublished). The reliability of both above-mentioned DNA markers in identifying susceptible genotypes was confirmed using different chickpea genotypes and crosses (Brinda and Ravikumar 2005; R. L. Ravikumar unpublished). For example, plants positive for DNA markers linked to both H 1 H 1 H 2 H 2 genotypes were highly susceptible to early wilting. The late wilting genotypes showed DNA marker linked to H 1 H 1 h 2 h 2 or h 1 h 1 H 2 H 2. Resistant plants (h 1 h 1 h 2 h 2) did not show DNA markers linked to either h 1 or h 2 allele in the parental genotypes, or the segregating populations studied.
The main objective of the present investigation was to test whether the nature of inheritance of two unlinked disease resistant loci, h 1 and h 2, against Fusarium wilt in chickpea (Cicer arietinum L.) under gametophytic (pollen) selection was similar to that already observed at the sporophytic level. The investigation was carried out examining the inheritance patterns of DNA markers CS-27700bp and A07C430bp in F2 and BC1 generations. The F2 and BC1 individuals were generated using two types of F1 pollen—one type of pollen was subjected to gametophytic selection, while the other type of pollen without gametophytic selection was used as a control.
Materials and methods
Parents and F1 plants
Two chickpea lines, JG-62 (ICC4951) and WR-315 (ICC8933) that have been well characterized for Fusarium wilt, were selected as parents to generate an F1 population. The genotype JG-62, an early wilting (H 1 H 1 H 2 H 2) type, was crossed to the genotype WR-315, a resistant (h 1 h 1 h 2 h 2) type, to produce F1 (H 1 h 1 H 2 h 2) hybrid seeds. Twenty F1 hybrid plants along with their parents were raised during 2004–2005 chickpea growing season at the Agriculture Experimental Station of the University of Agricultural Sciences, Dharwad, India (15°27′19′′N, 75°0′27′′E).
Gametophytic selection using pathotoxin spray
Two sets of F1, each containing ten plants were raised in the field at 10-m isolation between the two. The plants were closely monitored for flower bud formation and when the flower buds were still young one set of F1 plants was given a once off spray of a fungal pathotoxin, Fusaric acid (Sigma, Cat No. F6513), at a concentration of 1,500 μg/ml. Likewise, another set of F1 plants was sprayed with water as a control. Chickpea is predominantly self-pollinating and generally flowers for 8–10 days.
F2 and backcross generation-1 (BC1) plants
The F1 flowers from both toxin- and water-sprayed plants were selfed to generate F2 seeds. All pods from the 20 F1 plants were allowed to mature in the field. The seeds obtained from the toxin treated and control F1 plants were harvested separately to produce selected and unselected F2 populations, respectively.
Backcross generation-1 seeds were generated by pollinating the emasculated flowers of WR-315 resistant parent plants with the pollen collected from the toxin sprayed F1 plants. Thirty-four selected BC1 progeny seeds were obtained with the pollen formed under the pathotoxin stress. We failed to recover BC1 progeny seeds with the pollen from water treated F1 plants due to loss of plants bearing the control BC1 seeds to heavy wind. However, the absence of control BC1 individuals in our investigation would not severely undermine our data analysis for the following reasons: (1) earlier investigations (see Upadhyaya et al. 1983a, b; Brinda and Ravikumar 2005) reported expected 1:1:1:1 digenic test cross ratios for the h 1 and h 2 loci, therefore we considered the same expected ratio for the control BC1 genotypes for our data analysis; and (2) we did recover adequate number of control F2 genotypes and did not find any significant deviations from expected 9:3:3:1 ratio, validating our assumption that the control populations followed/would have followed the expected Mendelian inheritance for the loci under investigation.
DNA extraction and marker analysis
We raised 144 selected F2, 129 unselected F2, and 34 selected backcross plants in pots in the greenhouse for DNA extraction and to study the segregation pattern of molecular markers associated with susceptibility alleles H 1 or H 2. DNA from the selected F2, control F2 and selected BC1 plants were extracted by following the protocol described by Edwards et al. (1991). The DNA samples were diluted to a working concentration of 20–25 ng/μl and stored at 4°C for further PCR amplification.
DNA from each sampled individual was amplified by PCR using the Allele Specific Associated Primer (ASAP) CS-27F/CS-27R, following the protocol of Mayer et al. (1997) and A07C primer following the protocol of Thippeswamy et al. (2005). The PCR products were separated on 1.1% (W/V) agarose gel stained with ethidium bromide and all the sampled individuals were scored for the presence or absence of specific bands.
Statistical analysis
The segregation of each specific marker band in the selected and control F2 individuals, and in the selected BC1 individuals were analysed for h 1 and h 2 loci separately for the expected monogenic F2 ratio of 3:1 and BC1 test cross ratio of 1:1 using chi-square goodness-of-fit test (Pearson 1900; Lancaster 1969). Likewise, the above-mentioned populations were also analysed for digenic inheritance of h 1 and h 2 loci for the expected F2 ratio of 9:3:3:1 and BC1 test cross ratio of 1:1:1:1 using chi-square goodness-of-fit test.
Results
The toxin treatment induced the symptom of Fusarium wilt in F1 hybrid plants such as drooping of shoot tips and necrotic spots on the leaves, the symptom typically observed for dihybrid F1 individuals. In toxin sprayed plants, the first formed one or two flowers turned yellow and dropped off without setting seeds, whereas the remaining flowers normally developed into pods.
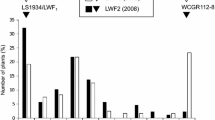
The ASAP and A07C primers amplified the DNA fragments linked to the H 1 (CS-27700bp) and H 2 (A07C430bp) alleles of susceptibility, respectively, in the selected F2 (Fig. 1) and BC1 generations, as well as in JG-62 and F1 hybrids. The resistant parent WR-315 is recessive at both loci (h 1 h 1 h 2 h 2) and did not produce amplicons. When considered separately, the loci h 1 and h 2 segregated for a monogenic ratio (3 susceptible:1 resistant) in the control F2 generation (Table 1). Ninety-two plants of the control F2 showed amplification for ASAP and the remaining 37 were negative for the marker (χ 2 = 1.04, P < 0.31, Table 1). Similarly 97 plants of the control F2 showed amplification for A07C primer and the remaining 32 were negative for the marker (χ 2 = 0.0, P = 1, Table 1). The results obtained in our control F2 population are in accordance with earlier studies that established the Mendelian monogenic inheritance pattern for the two DNA markers (Mayer et al. 1997; Ravikumar and Patil 2004). On the contrary, among the 144 selected F2 plants, 85 were positive for amplification of the ASAP DNA marker and the remaining 59 did not show any amplification for the same. The tests for segregation of the DNA marker showed a significant deviation from the expected monogenic ratio of 3:1 (χ 2 = 19.59, P < 0.0000, Table 1). Similar results were also obtained for the A07C marker, as 82 of the 125 tested plants were positive for amplification of the DNA marker linked to the H 2 allele (χ 2 = 6.17, P < 0.01, Table 1). Likewise, the selected BC1 plants also showed a significant deviation from the expected monogenic backcross ratio of 1:1 for the DNA markers linked to H 1 (χ 2 = 9.53, P < 0.002, Table 1) and H 2 (χ 2 = 4.24, P < 0.04, Table 1) alleles.
Amplifications of DNA markers in F2 individuals: the marker CS-27700bp in the F2 generated a without gametophytic selection, and b with gametophytic selection. The marker A07C430bp in the F2 generated c without gametophytic selection and d with gametophytic selection. L Molecular weight marker, R resistant individual, S susceptible individual
The hybrids (H 1 h 1 H 2 h 2) between the parents JG-62 × WR-315 were known to segregate for a digenic independent ratio (9:3:3:1) in F2 generation (Upadhyaya 1983a, b; Brinda and Ravikumar 2005), therefore it has been expected in our investigation that the two DNA markers, which are linked to susceptible alleles H 1 and H 2, should also segregate in a similar fashion. In the present investigation, the parental genotypes were selected to differ at two loci, h 1 and h 2, in such a way that one of the parents (JG-62) was homozygous dominant for both the loci (H 1 H 1 H 2 H 2) while the other parent (WR-315) carried corresponding recessive alleles for the same two loci (h 1 h 1 h 2 h 2). Accordingly, chickpea plants in the segregating generations of F2 and BC1 were expected to produce four classes of genotypes viz., (a) presence of both DNA markers (H 1 -H 2 -), (b) presence of only the CS-27700 marker (H 1 -h 2 h 2), (c) presence of only the A07C430 marker (h 1 h 1 H 2 -), and (d) absence of both markers (h 1 h 1 h 2 h 2). The chi-square goodness of fit test for the simultaneous inheritance of two genes among the control F2 individuals showed that the segregation pattern of both DNA markers matches that of the expected digenic ratio of 9:3:3:1 (χ 2 = 2.72, P < 0.44, Table 2). On the contrary, frequencies of the resistant-chickpea plants were higher than expected in the selected F2 and BC1 generations. For example, the analysis for the simultaneous segregation of two markers showed amplification for both markers in 56 individuals of the selected F2 as against the expected 70 individuals. In 19 plants of the selected F2 both DNA markers were absent as against expected 8 plants (χ 2 = 19.30, P < 0.0002, Table 2). Similar inheritance pattern was also noticed for the selected BC1 progenies—significantly fewer than expected number of plants were observed with the linked DNA markers, while significantly more number of plants were present without any DNA markers contrary to expectation (χ 2 = 15.65, P < 0.001, Table 2). Overall, pollen selection appears to have disproportionately increased the frequency of resistant-plants in the segregating generations.
Discussion
The deviation from normal Mendelian segregation in the selected F2 and BC1 plants for the DNA markers linked to susceptibility provides a strong, but indirect, evidence that gametophytic selection increases the frequency of advantageous alleles in the progeny.
It is possible that the increased frequency of resistant plants observed in the selected F2 progenies following pollen selection could be due to increased number of pollen with the resistant alleles fertilizing the ovules, or due to selection of zygotes containing the resistant alleles, or both. However, the deviation observed in the selected BC1 progenies is a clear indication of selection occurring at the male gametophytic level, as only pollen that survived the pathotoxin stress were used to generate BC1 individuals.
Although pollen from the F1 plants treated with pathotoxin was expected to undergo gametic selection, it is not clear whether or not the strength of selection was similar for flowers that bloomed at different days after the pathotoxin spray. It is possible that the selection effect could be more in pods formed in the initial stages compared to pods formed at later stages—in which case progenies from many late maturing F1 pods were expected to segregate for normal Mendelian ratios for both h 1 and h 2 loci in segregating generations. However, even if the assumption that there was a temporal reduction in the strength of gametophytic selection between early and late formed flowers was true, the significant increase in the frequency of resistance alleles in the F2 and BC1 generations provides a strong support for the occurrence of selection at the gametic level.
Could this increased frequency of resistant-plants in the F2 and BC1 generations be an artefact of transient epigenetic phenomenon? Probably not, although it is hard to rule out its involvement completely, as the epigenetic regulatory system appears complex and is not very well understood (reviewed in Grant-Downton and Dickinson 2005). Nevertheless, paramutation, one of the well-studied transient epigenetic phenomena in plants has been shown to effect significant deviations in Mendelian ratios (Hollick et al. 1997; Chandler et al. 2000) similar to the ones observed in the selected F2 and BC1 generations. Under the paramutation scenario, the resistant alleles of h 1 and h 2 loci in the pathotoxin treated heterozygote F1 individuals convert the susceptible alleles into resistant alleles. As a consequence, in the selected F2 and BC1 generations, one would observe more than expected frequencies of the DNA markers linked to susceptible alleles. But our observations turned out quite the opposite—significantly less than expected number of chickpea plants in the selected F2 and BC1 generations possessed DNA markers (Tables 1, 2)—ruling out any significant role of paramutation for the observed deviations in Mendelian ratios in the present investigation.
Although practical benefit of pollen selection has already been demonstrated and published previously (Hormaza and Herraro 1996; Chikkodi and Ravikumar 2000; Clarke et al. 2004; Dorminguez et al. 2005), our study tracked the inheritance of a particular trait using linked markers. Furthermore, the present investigation on the gametophytic selection for Fusarium resistance alleles in chickpea demonstrates that haploid expression of the trait (if susceptibility at pollen level was controlled by diploid gene expression all pollen would be susceptible, as were F1 plants) has an extra merit, since it allows selection in favour of recessive alleles at haploid level, with the advantage that they are not masked by dominant alleles. Thus, there is a potential benefit of employing gametophytic selection for recessive alleles in crop improvement.
References
Abbo S, Molina C, Jungmann R, Grusak MA, Berkaitch Z, Reifen R, Kahl G, Winter P, Reifen R (2005) Quantitative trait loci governing carotenoid concentration and weight in seeds of chickpea (Cicer arietinum L.) Theor Appl Genet 111:185–195
Brinda S, Ravikumar RL (2005) Inheritance of wilt resistance in chickpea—a molecular marker analysis. Curr Sci 88:701–702
Chandler VL, Eggleston WB, Dorweiler JE (2000) Paramutation in maize. Plant Mol Biol 43:121–145
Chikkodi SB, Ravikumar RL (2000) Influence of pollen selection for Alternaria helianthi resistance on the progeny performance against leaf blight in sunflower (Helianthus annuus L). Sex Plant Reprod 12:222–226
Chikkodi SB, Ravikumar RL (2003) Effect of pollen selection for Alternaria blight resistance in sunflower on F2 generation. In: Extended summaries, National seminar on stress management in oil seeds for attaining self reliance in vegetable oils. Directorate of Oil Seed Research, Hyderabad, pp 338–339
Clarke HJ, Khan TN, Siddique KHM (2004) Pollen selection for chilling tolerance at hybridization leads to improved chickpea cultivars. Euphytica 139:65–74
Clegg MT, Kahler AL, Allard RW (1978) Estimation of life cycle components of selection in an experimental plant population. Genetics 89:765–792
Dominguez E, Cuartero J, Fernandez-Munoz R (2005) Breeding tomato for pollen tolerance to low temperatures by gametophytic selection. Euphytica 142:253–263
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genome DNA for PCR analysis. Nucleic Acids Res 19:1849
Fedoroff N, Masson P, Banks JA (1989) Mutations, epimutations, and the developmental programming of the maize suppressor-mutator transposable element. BioEssays 10:139–144
Fernandez-Munoz R, Gonzalez-Fernandez JJ, Cuartero J (1995) Variability of pollen tolerance to low temperatures in tomato and related wild species. J Hort Sci 70:41–49
Frascaroli E, Songstad DD (2001) Pollen genotype selection for a simply inherited qualitative factor determining resistance to chlorsulfuron in maize. Theor Appl Genet 102:342–346
Grant-Downton RT, Dickinson HG (2005) Epigenetics and its implications for plant biology. I. The epigenetic network in plants. Ann Bot 96:1143–1164
Heslop-Harrison J (1980) The forgotten generation: some thoughts on the genetics and physiology of angiosperm gametophytes. In: Davies DR, Hopwood DA (eds) The plant genome, 4th John Innes symposium. John Innes Institute, Norwich, pp 1–14
Hollick JB, Dorweiler JE, Chandler VL (1997) Paramutations and related allelic interactions. Trends Genet 13:302–308
Hormaza H, Herraro M (1996) Male gametophytic selection as a plant-breeding tool. Sci Hort 65:321–333
Jablonka E, Lamb MJ (1998) Epigenetic inheritance in evolution. J Evol Biol 11:159–183
Jorgensen R (1993) The germinal inheritance of epigenetic information in plants. Philos Trans R Soc B 339:173–181
Lancaster HO (1969) The chi-squared distribution. John Wiley, New York
Landi P, Frascaroli E, Tuberosa R, Conti S (1989) Comparison between responses to gametophytic and sporophytic recurrent selection in maize (Zea mays L.). Theor Appl Genet 77:761–767
Laughnan JR, Gabay SJ (1973) Reaction of germinating maize pollen to Helminthosporium maydis pathotoxins. Crop Sci 43:681–684
Maisonneuve B, Hogenboom NG, den Nijs APM (1986) Pollen selection in breeding tomato (Lycopersicon esculentum Mill) for adaptation to low temperature. Euphytica 35:983–992
Mayer MS, Tullu A, Simon CJ, Kumar J, Kaiser WJ, Kraft JM, Muehlbauer FJ (1997) Development of a DNA marker for Fusarium wilt resistance in chickpea. Crop Sci 37:1625–1629
Mulcahy DL, Sari-Gorla M, Mulcahy GB (1996) Pollen selection—past, present and future. Sex Plant Reprod 9:353–356
Ottaviano E, Mulcahy DL (1989) Genetics of angiosperm pollen. Adv Genet 26:1–64
Pearson K (1900) On a criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen in random sampling. Philos Mag Ser-5 50:157–175
Pfahler PL (1983) Comparative effectiveness of pollen genotype selection in higher plants. In: Mulcahy DL, Ottaviano E (eds) Pollen: biology and implications for plant breeding. Elsevier Biomedical, New York, pp 361–366
Ravikumar RL, Patil BS (2002) Pollen response as markers (PRM) and pollen selection: novel tools in crop improvement. In: Dris R, Bary-Ryan C (eds) Plant physiology: characteristics, breeding and genetics. Science Publishers, New Hampshire, p 212
Ravikumar RL, Patil BS (2004) Effect of gamete selection on segregation of wilt susceptibility-linked DNA marker in chickpea. Curr Sci 86:642–643
Ravikumar RL, Patil BS, Salimath PM (2003) Drought tolerance in sorghum by pollen selection using osmotic stress. Euphytica 133:371–376
Sari Gorla M, Ottaviano E, Frascaroli E, Landi P (1989). Herbicide-tolerant corn by pollen selection. Sex Plant Reprod 2:65–69
Searcy KB, Mulcahy DL (1985) The parallel expression of metal tolerance in pollen and sporophytics of Silence doica (L.) Clairv, S. alba (Mill) Krause and Mimulus guttatus DC. Theor Appl Genet 69:597–602
Shobha Rani T, Ravikumar RL (2006) Sporophytic and gametophytic selection for improvement of partial resistance to Alternaria leaf blight in sunflower (Helianthus annuus L.) Euphytica 147:421–431
Singh H, Kumar J, Haware MP, Smithson JB (1987) Genetics of resistance to Fusarium wilt in chickpeas. In: Day PR, Jellis GJ (eds) Genetics and plant pathogenesis. Blackwell, Oxford, pp 339–342
Slack JMW (1998) Comments on epigenetic inheritance. J Evol Biol 11:233–237
Thippeswamy S, Ravikumar RL, Salimath PM (2005) Molecular tagging of H 2 locus of wilt (Fusarium oxysporum f. sp. cicers) resistance in chickpea. In: International conference on plant genomics and biotechnology: challenges and opportunities, October 26–28. Indira Gandhi Agricultural University, Raipur, pp 157
Upadhyaya HD, Haware MP, Kumar J, Smithson JB (1983a) Resistance to wilt in chickpea. I. Inheritance of late wilting in response to race 1. Euphytica 32:447–452
Upadhyaya HD, Smithson JB, Kumar J, Haware MP (1983b) Resistance to wilt in chickpea. II. Further evidence of two genes for resistance to race 1. Euphytica 32:749–745
Zamir D, Tanksley SD, Jones RA (1982) Haploid selection for low temperature tolerance of tomato pollen. Genetics 101:129–137
Acknowledgments
We thank two anonymous reviewers for their useful comments on an earlier version of the manuscript, and R. L. Ravikumar thanks the Defense Research and Development Organisation (DRDO), Government of India, for their financial support to carry out the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kearsey.
Rights and permissions
About this article
Cite this article
Ravikumar, R.L., Patil, B.S., Soregaon, C.D. et al. Genetic evidence for gametophytic selection of wilt resistant alleles in chickpea. Theor Appl Genet 114, 619–625 (2007). https://doi.org/10.1007/s00122-006-0462-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-006-0462-4