Abstract
In a previous study, we developed cytoplasmic male sterile lines of Allium fistulosum possessing the cytoplasm of A. galanthum, a wild species, by continuous backcrossing. Furthermore, we reported the presence of a pollen fertility-restoring gene (Rf) for cytoplasmic male sterility (CMS) in A. fistulosum from segregation of pollen fertility of backcross progenies. In the present study, genomic in situ hybridization (GISH), using genomic DNA of A. galanthum as the probe DNA and that of A. fistulosum as the blocking DNA, was applied to F1 hybrids between both species and backcross progenies to determine the chromosomal location of the Rf locus. By means of GISH, eight chromosomes from A. galanthum were clearly discriminated from those of A. fistulosum in the F1 hybrids, and chromosome substitution process through continuous backcrossing was visualized. Interestingly, the chromosome region from A. galanthum, specific to male fertile plants, was detected in one chromosome of BC4 to BC7 generations. Based on the karyotype analysis of the male fertile plants, the chromosome was identified as the 5F chromosome. Our results confirm that the Rf locus is located on the 5F chromosome of the male fertile plants. This is the first report that identified the chromosomal location of the pollen fertility-restoring gene in A. fistulosum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Japanese bunching onion (Allium fistulosum L.), which belongs to section Cepa of genus Allium, is an important vegetable crop in east and southeast Asian countries, especially Japan, China, and Korea (Kumazawa and Katsumata 1965; Inden and Asahira 1990). This species is also one of useful genetic resources for onion (A. cepa L. Common onion group), because it has some beneficial characters, e.g., resistance to Fusarium basal rot, onion leaf blight, and pink root diseases (Emsweller and Jones 1935; van der Meer and van Bennekom 1978; Entwistle et al. 1990). A. fistulosum can be distinguished into two types: one type grown for green leaves, and the other type grown for blanched pseudo-stems. In Japan, A. fistulosum has been consumed long before onion was introduced. At present, many cultivars and strains adapted to different regions exist, and the year-round cultivation is common. Releases of F1 hybrids to a market have been advanced in A. fistulosum; few reports concerning the male sterile line, essential for production of F1 hybrid seeds, have been published. Spontaneous male sterile plants were found in A. fistulosum populations by Nishimura and Shibano (1972), and this male sterility was elucidated to be cytoplasmic male sterility (CMS) by Moue and Uehara (1985). However, the CMS line has not been used for practical F1 seed production because of disease susceptibility of the CMS line (T. Moue, personal communication). In a previous study, we successfully developed alloplasmic male sterile lines of A. fistulosum possessing the cytoplasm of A. galanthum Kar. et Kir., a wild species of section Cepa, by the continuous backcrossing in which A. galanthum was used as a cytoplasm donor and A. fistulosum cultivar ‘Kujyo’ as the recurrent parent (Yamashita et al. 1999a; Yamashita and Tashiro 2004). Backcross progenies were selected in two directions of male sterility and high pollen fertility from the BC2 generation: all plants of BC3, BC4, and BC5 generations selected to the direction of male sterility showed male sterility (Figs. 1, 2), whereas, in the BC3, BC4, and BC5 plants selected to the direction of high pollen fertility, male fertile and male sterile plants segregated at the ratio of 1:1. From these results, the presence of a single dominant pollen fertility-restoring gene (Rf), which originated from the nuclear genome of A. galanthum, was confirmed (Yamashita et al. 1999a). The gene Rf is essential to control systematically male fertility of A. fistulosum possessing the A. galanthum cytoplasm in the breeding of F1 cultivars. Then, we reported that an isozyme gene locus Phosphoglucoisomerase-1 (Pgi-1), a randomly amplified polymorphic DNA (RAPD) fragment in size of 700 bp (OPE03700), and a sequence-characterized amplified region fragment in size of 700 bp (OPJ15700) could be used as markers linked to the Rf locus (Yamashita et al. 1999b, 2002). Chromosomal locations of various isozyme gene loci (Shigyo et al. 1994, 1995a, b, 1996) and RAPDs (Shigyo et al. 1997) of shallot (A. cepa L. Aggregatum group) were determined using alien monosomic addition lines of A. fistulosum with extra chromosomes from shallot. Amplified fragment length polymorphism (AFLP) linkage groups of onion were assigned to the chromosomes of A. cepa via the monosomic addition lines (van Heusden et al. 2000). Although production of monosomic addition lines of A. fistulosum with wild species chromosomes and genetic analyses of them are now in progress (Tashiro et al. 2000), chromosomal location of the markers linked to the Rf locus has not been identified yet. Genomic in situ hybridization (GISH) has been evidenced to be a powerful tool for discriminating different genomes or direct signal detection of chromosome regions introgressed from other species in various Alliums (Keller et al. 1996; Buiteveld et al. 1998; Khrustaleva and Kik 1998, 2000; Puizina et al. 1999; Shigyo et al. 1998, 2003; Yamashita et al. 2000a, 2001; Hou and Peffley, 2000; Peterka et al. 2002). In this study, we determined chromosomal location of the Rf locus by comparing GISH profiles of the male fertile and male sterile plants of advanced backcross generations.
Materials and methods
Plant material
Two plants of F1 hybrids between A. galanthum (seed parent) and A. fistulosum (pollen parent), two plants each from BC1 to BC4, seven plants of BC5, four plants of BC6, and 12 plants of BC7 generations were analyzed by GISH. The pollen fertility of the materials had been investigated beforehand by the smear method with acetocarmine: their percentage fertility was calculated from the morphology and stainability of 500 pollen grains per floret in three florets per plant.
GISH experiment
Root tips of material plants were collected and treated with 0.05% colchicine at 20°C for 2.5 h, after which they were fixed in a mixture of acetic acid and ethyl alcohol (1:3). The procedure of GISH was according to Yamashita et al. (2000a). Total genomic DNA of A. galanthum was extracted from fresh leaves by using the cetyltrimethylammonium bromide method, modified by Yamashita et al. (2000b), labeled with biotin-16-dUTP, using the Nick Translation Kit (Roche Diagnostics) and used as the probe DNA, whereas excess of unlabeled genomic DNA of A. fistulosum was used as the blocking DNA. One microgram probe DNA and 200 μg blocking DNA were contained in 100 μl hybridization mixture (Yamashita et al. 2000a). After hybridization, probe hybridization signals were amplified with fluorescein avidin DN (Vector Laboratories, Burlingame, Calif., USA) and counterstained with propidium iodide. The GISH image was observed on the fluorescence microscope DMRXA (Leica, Germany) by using the software QFISH (Leica).
Results
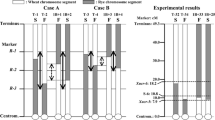
Pollen fertility, number of chromosomes, and chromosome constitution of material plants are shown in Table 1. Both species of A. galanthum and A. fistulosum have 16 chromosomes (2n). All the plants of F1 hybrids and backcross progenies analyzed had also 16 chromosomes (2n), and chromosome deletion or fragment chromosome was not observed. In GISH profiles of the F1 hybrids, eight chromosomes with yellowish green fluorescence were clearly discriminated from the other eight chromosomes with orange fluorescence (Fig. 3a). The former were identified as A. galanthum chromosomes and the latter as A. fistulosum chromosomes. In both BC1 plants, three and five recombinant chromosomes were observed (Fig. 3b; Table 1). With the progress of the backcrossing, chromosome regions of A. galanthum per cell drastically decreased (Fig. 3b–e; Table 1). The chromosomes of a male fertile BC3 plant consisted of 14 chromosomes of A. fistulosum and two recombinant chromosomes, whereas that of a male sterile BC3 plant consisted of 16 chromosomes of A. fistulosum. After the BC4 generation, interestingly, remarkable difference of the amount of A. galanthum chromosome region was detected between male fertile and male sterile plants; all of 14 male fertile plants from BC4 to BC7 generations possessed one chromosome with the A. galanthum chromosome region on its one arm. In contrast, all 11 male sterile plants from BC4 to BC7 generations did not possess the chromosome (Fig. 4a, b; Table 1). Except for the chromosome, all the male fertile plants had no recombinant chromosomes. Karyotype analysis was conducted in the male fertile plants of BC7 generation on the basis of relative chromosome length and position of a centromere according to the nomenclature of Kalkman (1984). Chromosomes 1, 3, 5, and 7 are metacentric, chromosomes 2, 4 and 8 are submetacentric, and the chromosome 6 is subtelocentric in both species. The chromosome with the A. galanthum chromosome region was identified as the chromosome 5F of the male fertile plants (Fig. 4c).
Discussion
In this study, chromosome substitution process between A. galanthum and A. fistulosum through the continuous backcrossing was visualized by means of GISH. The chromosomes of F1 hybrids consisted of each eight chromosomes from the parental species. In a previous study, we reported high frequency of bivalent chromosomes in meiotic metaphase I of pollen mother cells (7.9) in the F1 hybrid (Yamashita et al. 1999a). Theoretically, eight recombinant chromosomes from the F1 hybrid should be transmitted to BC1 plants. However, in both BC1 plants, the number of recombinant chromosomes was five or three, respectively. The present results illustrate that even if homoeologous chromosomes formed bivalent chromosomes at high frequency in meiotic metaphase I of the interspecific F1 hybrid, recombination, detectable by GISH, does not always occur. In the continuous backcrossing, the BC1 plant having such chromosome constitution was spontaneously selected and used as a seed parent for BC2 generation (Yamashita et al. 1999a), which would consequently advance the chromosome substitution. Previously, we reported that GISH was a useful tool for efficient cytoplasmic substitution between A. galanthum and A. cepa, provided plants in which the nucleus substitution had advanced earlier in each backcross generation were selected (Yamashita et al. 2000a). The degree of chromosome substitution varied between plants in each BC1 and BC2 generations regardless of pollen fertility, though the number of plants analyzed was limited. Therefore, GISH technique is effective for efficient cytoplasmic substitution between A. galanthum and A. fistulosum, too.
The chromosome on which the Rf locus is located was successfully determined to be the 5F chromosome of the male fertile plants by GISH. This is the first report that determined chromosomal location of the pollen fertility-restoring gene locus in A. fistulosum. The crossing-over genetically occurs between homoeologous chromosomes from the parental species in meiotic metaphase I of egg mother cells in the F1 hybrids. Therefore, it is inferred that the gene Rf originates from the 5G chromosome of A. galanthum. Shigyo et al. (1995b) reported that an isozyme gene locus Pgi-1 was located on the chromosome 5A of A. cepa by analyzing alien monosomic addition lines of A. fistulosum with extra chromosomes from A. cepa. We previously confirmed that the gene locus Pgi-1 of A. galanthum was linked to the Rf locus (Yamashita et al. 1999b). It is presumed that the locus Pgi-1 of A. galanthum is also located on the chromosome 5G of A. galanthum.
Large amount of blocking DNA was required in contrast to probe DNA (1:200) for discriminating A. galanthum chromosomes from A. fistulosum ones in this study. Less than this ratio, it was difficult to discriminate clearly chromosomes of both species due to cross-hybridization of probe DNA to A. fistulosum chromosomes. The ratios of probe DNA to blocking DNA needed for discriminating chromosomes of A. galanthum from A. cepa, and those of A. cepa from A. fistulosum were 1 to 20 and 1 to 10, respectively (Yamashita et al. 2000; Shigyo et al. 1998). The probe-blocking DNA ratios required in such species combinations would correspond to the degree of their genome homology.
The cytoplasm of A. galanthum was demonstrated to induce CMS in shallot (Yamashita and Tashiro 1999) and onion (Havey 1999), as well as A. fistulosum (Yamashita et al. 1999a). In the three CMS-cultivated species with A. galanthum cytoplasm, the pollen fertility-restoring gene has been found out only in CMS A. fistulosum. Alleles to restore male fertility for onion possessing S cytoplasm showed no male fertility restoration for the CMS onion with A. galanthum cytoplasm (Havey 1999), indicating that the gene Rf regulates the fertility restoration system different from that the Ms of onion regulates. We previously observed microsporogenesis of the CMS A. fistulosum (Yamashita et al. 1999a): after pollen grain mitosis, binucleate pollen grains were produced. Then, protoplasm of the pollen grains drastically degenerated, finally resulting in empty pollen grains in anthers. The cytoplasm of A. galanthum appears to influence the microsporogenesis at the last stage in A. fistulosum. We will embark to verify gene expression of the Rf in microsporogenesis of the male fertile A. fistulosum possessing the A. galanthum cytoplasm.
King et al. (1998) constructed low-density genetic maps of onion consisting of morphological, RAPD, and restriction fragment length polymorphism (RFLP) markers. The Ms locus was affiliated to the linkage group B. A most closely related RFLP marker positioned at 0.9 cM from Ms locus (Gokce et al. 2002). Most recently, Shigyo et al. (2005) reported that the RFLP linkage group including the Ms locus was located on the chromosome 2A of A. cepa. The comparison of genetic backgrounds of the genes, Rf and Ms, such as their functions and constructions will lead to inclusive evaluation of the genes concerning pollen fertility in genus Allium. In A. fistulosum, the development of molecular markers as AFLP, simple sequence repeat, and cleaved amplified polymorphic sequence, and construction of linkage maps have been just started (Ohara et al. 2003; Song et al. 2004; Tsukazaki et al. 2004). After BC4 generations, the chromosome region from A. galanthum possessing the Rf locus was preserved on the 5F chromosome of the male fertile plants. Emsweller and Jones (1935) reported that homologous chromosomes formed plural interstitial chiasmata at diplotene, even if such chromosomes formed localized chiasmata at metaphase I in pollen mother cells of A. fistulosum. In contrast, our GISH result represents that the recombination hardly occurred in the chromosome region of 5F of the male fertile plants. The high stability of the region will make the construction of linkage maps of the Rf locus difficult, though development of the number of molecular markers linked to the Rf locus would be possible. For constructing the linkage maps, it would be necessary to make recombination take place in the chromosome region. Past GISH analyses have visualized various introgression modes of chromosome regions from other species in genus Allium: the chromosome regions of A. galanthum distributed sparsely in interstitial and terminal regions of chromosomes in BC1 progeny, but only in terminal regions in BC3 between A. galanthum and shallot (Yamashita and Tashiro 1999). The recombination points distributed randomly in the first and second generations of the bridge cross for introducing A. fistulosum genome into A. cepa using A. roylei as a bridge species (Khrustaleva and Kik 2000). van Heusden et al. (2000) constructed linkage maps of AFLP markers of A. cepa and A. roylei by analyzing their F2 populations. By the sib crossing between the male sterile and fertile A. fistulosum using A. roylei as a bridge species, inducing of recombination might be possible in the A. galanthum chromosome region of the 5F chromosome. In those cases, it is premised that A. roylei genome does not interfere the gene expression of the Rf. Recently, detection of short DNA sequences as RAPD-generated PCR products and single-copy tDNA sequences became possible by fluorescence in situ hybridization or in situ PCR (Benabdelmouna et al. 1999; Khrustaleva and Kik 2001; Mukai and Appels 1996). Hereafter, we intend to determine chromosomal localization of the Rf locus by applying such techniques to somatic chromosomes of the male fertile A. fistulosum.
References
Benabdelmouna A, Peltier D, Humbert C, Abirached-Darmency M (1999) Southern and fluorescence in situ hybridization detect three RAPD-generated PCR products useful as introgression markers in Petunia. Theor Appl Genet 98:10–17
Buiteveld J, Suo Y, van Lookeren Campagne MM, Creemers-Molenaar J (1998) Production and characterization of somatic hybrid plants between leek (Allium ampeloprasum L.) and onion (Allium cepa L.). Theor Appl Genet 96:65–775
Emsweller SL, Jones HA (1935) An interspecific hybrid in Allium. Hilgardia 9:265–273
Entwistle AR, Rabinowitch HD, Brewster JL (1990) Root diseases. In: Rabinowitch HD, Brewster JL (eds) Onion and allied crops, vol II. CRC, Boca Raton, pp 103–154
Gokce AF, McCallum J, Sato Y, Havey MJ (2002) Molecular tagging of the Ms locus in onion. J Am Soc Hort Sci 127: 576–582
Havey MJ (1999) Seed yield, floral morphology, and lack of male-fertile restoration of male sterile onion (Allium cepa) population possessing the cytoplasm of Allium galanthum. J Am Soc Hort Sci 124:626–629
Heusden AW van, Shigyo M, Tashiro Y, Vrielink-van Ginkel R, Kik C (2000) AFLP linkage group assignment to the chromosomes of Allium cepa L. via monosomic addition lines. Theor Appl Genet 100:480–486
Hou A, Peffley EB (2000) Recombinant chromosomes of advanced backcross plants between Allium cepa L. and A. fistulosum L. revealed by in situ hybridization. Theor Appl Genet 100:1190–1196
Inden H, Asahira T (1990) Japanese bunching onion (Allium fistulosum L.). In: Rabinowitch HD, Brewster JL (eds) Onions and allied crops, vol II. CRC, Boca Raton, pp 159–178
Kalkman ER (1984) Analysis of the C-banded karyotype of Allium cepa L. standard system of nomenclature and polymorphism. Genetica 65: 141–148
Keller ERJ, Schubert I, Fuchs J, Meister A (1996) Interspecific crosses of onion with distant Allium species and characterization of the presumed hybrids by means of flow cytometry, karyotype analysis and genomic in situ hybridization. Theor Appl Genet 92:417–424
Khrustaleva LI, Kik C (1998) Cytogenetical studies in the bridge cross Allium cepa × (A. fistulosum × A. roylei). Theor Appl Genet 96:8–14
Khrustaleva LI, Kik C (2000) Introgression of Allium fistulosum into A. cepa mediated by A. roylei. Theor Appl Genet 100: 17–26
Khrustaleva LI, Kik C (2001) Localization of single-copy T-DNA insertion in transgenic shallots (Allium cepa) by using ultra-sensitive FISH with tyramide signal amplification. Plant J 25:699–707
King JJ, Bradeen JM, Bark O, McCallum JA, Havey MJ (1998) A low density genetic map of onion reveals a role for tandem duplication in the evolution of an extremely large diploid genome. Theor Appl Genet 96: 52–62
Kumazawa S, Katsumata H (1965) Negi (Japanese bunching onion). In: Kumazawa S (ed) Sosai-engei kakuron (vegetable crops). Yokendo, Tokyo, pp 280–289
Meer QP van der, van Bennekom JL (1978) Improving the onion crop (Allium cepa L.) by transfer of characters from Allium fistulosum L. Bull Warzywhiczy 22:87–91
Moue T, Uehara T (1985) Inheritance of cytoplasmic male sterility in Allium fistulosum L. (Welsh onion). J Jpn Soc Hort Sci 53: 432–437
Mukai Y, Appels R (1996) Direct chromosome mapping of plant genes by in situ polymerase chain reaction (in situ PCR). Chromosome Res 4:401–404
Nishimura Y, Shibano M (1972) Male sterility in Allium fistulosum L. Cytological and anatomical studies. Abstr Jpn Soc Hort Sci Spring Meet:180–181
Ohara T, Wako T, Tsukazaki H, Nunome T, Kojima A (2003) Construction of genetic linkage map based on AFLP markers in bunching onion (Allium fistulosum). Abstr Breed Sci 5(Suppl 1):74
Peterka H, Budahn H, Schrader O, Havey MJ (2002) Transfer of a male-sterility-inducing cytoplasm from onion to leek (Allium ampeloprasum). Theor Appl Genet 105:173–181
Puizina J, Javornik B, Bohanec B, Schweizer D, Maluszynska J, Papes D (1999) Random amplified polymorphic DNA analysis, genome size, and genomic in situ hybridization of triploid viviparous onions. Genome 42:1208–1216
Shigyo M, Tashiro Y, Miyazaki S (1994) Chromosomal locations of glutamate oxaloacetate transaminase gene loci in Japanese bunching onion (Allium fistulosum L.) and shallot (A. cepa L. Aggregatum group). Jpn J Genet 69:417–424
Shigyo M, Tashiro Y, Isshiki S, Miyazaki S (1995a) Chromosomal locations of five isozyme gene loci (Lap-1, Got-1, 6-Pgdh-2, Adh-1 and Gdh-1) in shallot (Allium cepa L. Aggregatum group). Jpn J Genet 70:399–407
Shigyo M, Tashiro Y, Isshiki S, Miyazaki S (1995b) Chromosomal locations of isocitrate dehydrogenase and phosphoglucoisomerase gene loci in shallot (Allium cepa L. Aggregatum group). Jpn J Genet 70:627–632
Shigyo M, Tashiro Y, Isshiki S, Miyazaki S (1996) Establishment of a series of alien monosomic addition lines of Japanese bunching onion (Allium fistulosum L.) with extra chromosomes from shallot (A. cepa L. Aggregatum group). Genes Genet Syst 71:363–371
Shigyo M, Miyazaki T, Isshiki S, Tashiro Y (1997) Assignment of randomly amplified polymorphic DNA markers to all chromosomes of shallot (Allium cepa L. Aggregatum group). Genes Genet Syst 72:249–252
Shigyo M, Imamura K, Iino M, Yamashita K, Tashiro Y (1998) Identification of alien chromosomes in a series of Allium fistulosum-A. cepa monosomic addition lines by means of genomic in situ hybridization. Genes Genet Syst 73:311–315
Shigyo M, Wako T, Kojima A, Yamauchi N, Tashiro Y (2003) Transmission of alien chromosomes from selfed progenies of a complete set of Allium monosomic addition lines: the development of a reliable method for the maintenance of a monosomic addition set. Genome 46:1098–1103
Shigyo M, Yamane N, Martin WJ, Yamauchi N, Havey MJ (2005) Assignment of onion RFLP-linkage map to chromosomes by using alien monosomic additions. Abstr J Jpn Soc Hort Sci 74 (Suppl 1):426
Song YS, Suwabe K, Wako T, Tsukazaki H, Ohara T, Nunome T, Kojima A (2004) Development of microsatellite markers in bunching onion (Allium fistulosum L.). Abstracts of 4th International ISHS symposium on edible Alliaceae (ISEA):197
Tashiro Y, Tsutsumi M, Shigyo M (2000) Production and gene analyses of alien monosomic addition lines of Allium fistulosum L. with extra chromosomes from wild species in section Cepa of Allium. Acta Horticult 521:211–217
Tsukazaki H, Ohara T, Wako T, Song YS, Yamashita K, Kojima A (2004) DNA markers linked to seedling growth in fall-sown bunching onion (Allium fistulosum). Abstracts of 4th international ISHS symposium on edible Alliaceae (ISEA):198
Yamashita K, Tashiro Y (1999) Possibility of developing male sterile lines of shallot (Allium cepa L. Aggregatum group) with cytoplasm from A. galanthum Kar. et Kir. J Jpn Soc Hort Sci 68:256–262
Yamashita K, Tashiro Y (2004) Seed productivity test of CMS lines of Japanese bunching onion (Allium fistulosum L.) possessing the cytoplasm of a wild species, A. galanthum Kar. et Kir. Euphytica 136:327–331
Yamashita K, Arita H, Tashiro Y (1999a) Cytoplasm of a wild species, Allium galanthum Kar. et Kir., is useful for developing the male sterile line of A. fistulosum L. J Jpn Soc Hort Sci 68:788–797
Yamashita K, Arita H, Tashiro Y (1999b) Isozyme and RAPD markers linked to fertility restoring gene for cytoplasmic male sterile Allium fistulosum L. with cytoplasm of A. galanthum Kar. et Kir. J Jpn Soc Hort Sci 68:954–959
Yamashita K, Iino M, Shigyo M, Tashiro Y (2000a) Visualization of nucleus substitution between Allium galanthum and shallot (A. cepa) by genomic in situ hybridization. J Jpn Soc Hort Sci 69:189–191
Yamashita K, Noda R, Tashiro Y (2000b) Use of mitochondrial DNA polymorphisms to distinguish cytoplasms of cultivated and wild species in section Cepa of Allium. J Jpn Soc Hort Sci 69:396–402
Yamashita K, Hisatsune Y, Sakamoto T, Ishizuka K, Tashiro Y (2001) Chromosome and cytoplasm analyses of somatic hybrids between onion (Allium cepa L.) and garlic (A. sativum L.). Euphytica 125:163–167
Yamashita K, Takatori Y, Tashiro Y (2002) Development of sequence characterized amplified region (SCAR) markers linked to the fertility restoring gene for cytoplasmic male sterile Allium fistulosum L. possessing the cytoplasm of A. galanthum Kar. et Kir. J Jpn Soc Hort Sci 71:777–779
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.S. Heslop-Harrison
Rights and permissions
About this article
Cite this article
Yamashita, Ki., Takatori, Y. & Tashiro, Y. Chromosomal location of a pollen fertility-restoring gene, Rf, for CMS in Japanese bunching onion (Allium fistulosum L.) possessing the cytoplasm of A. galanthum Kar. et Kir. revealed by genomic in situ hybridization. Theor Appl Genet 111, 15–22 (2005). https://doi.org/10.1007/s00122-005-1941-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-1941-8