Abstract
A gene (temporarily designated Hdic) conferring resistance to the Hessian fly (Hf) [Mayetiola destructor (Say)] was previously identified from an accession of German cultivated emmer wheat [Triticum turgidum ssp. dicoccum (Schrank ex Schübler) Thell] PI 94641, and was transferred to the Hf-resistant wheat germplasm KS99WGRC42. The inheritance of Hdic resistance exhibited incomplete penetrance because phenotypes of some heterozygous progenies are fully resistant and the others are fully susceptible. Five simple sequence repeat (SSR) markers (Xgwm136,Xcfa2153, Xpsp2999,Xgwm33, and Xbarc263) were linked to the Hdic gene on the short arm of wheat chromosome 1A in the same region as the H9, H10, and H11 loci. Flanking markers Xgwm33 and Xcfa2153 were mapped at distances 0.6 cM proximal and 1.4 cM distal, respectively. Marker analysis revealed that a very small intercalary chromosomal segment containing Hdic was transferred from emmer wheat to KS99WGRC42. This is the first emmer-derived Hf-resistance gene that has been mapped and characterized. The Hdic gene confers a high level of antibiosis to biotypes GP and L, as well as to strains vH9 and vH13 of the Hf, which is different from the biotype reaction patterns of the known Hf-resistance genes on chromosome 1A (H5 and H11 susceptible to biotype L, H9 and H10 susceptible to strain vH9). These results suggested that Hdic is either a new gene or a novel allele of a known H gene on chromosome 1A. The broad spectrum of resistance conferred by the Hdic gene makes it valuable for developing Hf resistant wheat cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Hessian fly (Hf), Mayetiola destructor (Say) (Diptera: Cecidomyiidae), is one of the most destructive pests of wheat (Triticum aestivum L.) worldwide, and causes annual losses ranging from 5 to 10% of wheat production to US farmers (Hatchett et al. 1987; Buntin 1999). Historically, the use of wheat resistance genes has been the most effective and cost-efficient approach in protecting wheat from Hf damage and minimizing the use of insecticides (Ratcliffe and Hatchett 1997). However, the success of the plant resistance strategy has been challenged by the ability of the Hessian fly to develop different virulent populations or biotypes that overcome the specific resistance genes in deployed cultivars (Ratcliffe et al. 2000). To counter the development of new biotypes, it is necessary to identify and utilize different resistance genes derived from diverse sources (Ratcliffe and Hatchett 1997).
Todate, 31 major Hf-resistance genes (named H1 through H31) have been identified from wheat and its relatives (Ratcliffe and Hatchett 1997; McIntosh et al. 2003; Harris et al. 2003; Williams et al. 2003). These genes were identified from different sources, including common wheat (H1,H2, H3,H4, H5,H7H8, H12), Aegilops tauschii (H13,H22, H23,H24, H26), T. turgidum ssp. durum (H6,H9, H10,H11, H14,H15, H16,H17, H18,H19, H20,H28, H29,H31), Ae. ventricosa H27), Ae. triuncialis H30), and rye (H21,H25). A Hf-resistance gene, temporarily designated Hdic, was recently identified from an accession of German cultivated emmer wheat [AABB genomes, T. turgidum ssp. dicoccum (Schrank ex Schübler) Thell] PI 94641, and was transferred to the Hf-resistant hard red winter wheat germplasm KS99WGRC42 (PI 635054) (Brown-Guedira et al. 2004).
As a result of changes in virulence of Hessian fly populations in the field in response to the deployment of resistant cultivars, resistance genes such as H3,H5, and H6 no longer provide effective resistance (Patterson et al. 1990; Ratcliffe and Hatchett 1997; Ratcliffe et al. 2000). To ensure the continued success of the plant resistance strategy, different breeding and gene deployment strategies have been suggested (Cox and Hatchett 1986; Smith et al. 1999). Use and rotation (or sequential release) of wheat cultivars containing different resistance genes may reduce new biotype outbreaks. Combining several genes in a single cultivar may also increase the level, duration, and breadth of resistance. However, conventional breeding based on phenotypic selection is time-consuming and sometimes inconclusive, particularly for combining multiple genes. More efficient and accurate techniques are needed to incorporate diverse genes into different cultivars for rotation and to pyramid multiple genes into single cultivars to increase the durability of resistance.
Molecular markers can greatly facilitate the identification of new resistance genes. This is particularly true for Hessian fly resistance because more than 30 resistance genes have been identified, and distinguishing new genes is difficult by traditional phenotypic differentiation with biotypes. Molecular markers can also greatly accelerate breeding for resistance through marker-assisted selection (MAS) (Yencho et al. 2000). Compared with traditional phenotypic selection, MAS can detect desired resistant genotypes in early generations, allowing breeders to avoid carrying numerous unwanted lines. More importantly, MAS makes pyramiding of multiple effective resistance genes a reality.
A variety of molecular markers linked to several Hf-resistance genes have been identified. Ma et al. (1993) identified restriction fragment length polymorphism (RFLP) markers that are linked to the H23 gene on chromosome 6D and H24 on chromosome 3DL. Delaney et al. (1995) tagged the H25 gene on 6BL with RFLP markers. Dweikat et al. (1994, 1997, 2002) developed a series of random amplified polymorphic DNAs (RAPD) and sequence tagged site (STS) markers that were associated with the H3,H5, H6,H9- H14,H16, and H17 genes. Seo et al. (1997, 2001) identified several amplified fragment length polymorphism (AFLP), RAPD, and STS markers linked to the H21 gene. Martin-Sanchez et al. (2003) identified an isozyme marker that was linked with H30, and Williams et al. (2003) mapped a new gene (H31) on chromosome 5BS with AFLP and STS markers. Identification of microsatellite or simple sequence repeat (SSR) markers linked to Hf resistance genes should be useful for MAS because SSRs are abundant, reliable, highly polymorphic, chromosome-specific, and can be easily handled in high-throughput systems (Weber and May 1989; Röder et al. 1998; Pestsova et al. 2000). A SSR marker (Xgwm397) linked to the rye-derived Hf-resistance gene H25 on chromosome 4A was reported (E. D. Souza, http://maswheat.ucdavis.edu/protocols/HF/index.htm). More recently, Liu et al. (2005) mapped H9,H10, and H11 to the distal region of wheat chromosome 1AS with tightly linked SSR markers.
In this study, we characterized the emmer wheat-derived Hf-resistance gene (tentatively named Hdic), and determined its chromosomal location on the short arm of wheat chromosome 1A using SSR markers.
Materials and methods
Hessian fly populations
Biotype GP was from a laboratory colony collected from Ellis County, Kansas (Gagne and Hatchett 1989). The insects were maintained on susceptible wheat seedlings (Karl 92 or Newton). Biotype L culture was supplied by S. E. Cambron, USDA-ARS, West Lafayette, Indiana. The insects were maintained on seedlings of Ike (H3), Magnum (H5), Caldwell (H6) and Seneca (H7H8) sequentially. The H9-virulent strain (vH9) and the H13-virulent strain (vH13) were provided by J. J. Stuart, Purdue Univ., West Lafayette, Indiana. The vH9 and vH13 flies were maintained on seedlings of Iris (H9) and Molly (H13), respectively. Hf pupae together with the infested wheat plants were stored at 4°C until ready for use.
Plant materials and DNA isolation
The original source of the Hf-resistance (PI 94641) was an accession of a German cultivated emmer wheat (T. turgidum ssp. dicoccum) obtained from the USDA/ARS National Small Grains Collection. The resistance gene was transferred to common wheat through the cross Karl 92/PI 94641//Jagger*2/3/Karl 92. A resulting F6–derived line 01-337-1-20 homozygous for resistance to Hessian fly biotypes GP and L was selected for release as Hf-resistant hard red winter wheat germplasm KS99WGRC42 (Brown-Guedira et al. 2004). A mapping population consisting of 121 F5:6lines (equivalent to F2:3families) was derived from eight heterozygous F4plants from the cross Karl 92/PI 94641//Jagger*2 /3/Karl 92. Data from each family were recorded separately and were pooled for analyses. Two additional populations of 134 and 118 F2plants, respectively, were derived from crosses between Hf-susceptible cultivar Tugela- Dn1 and two homozygous resistant F4:5lines, 01-20-12 and 01-186-11. In addition, F2 populations consisting of 188 and 134 plants were developed from reciprocal crosses between PI 94641 and Hf-susceptible emmer accession CI 7685. Wheat genomic DNA was extracted from leaf tissue according to the modified CTAB procedure as described by Gill et al. (1991). DNA concentration was quantified spectrophotometrically.
Seeds of PI 94641 and CI 7685 were provided by the USDA/ARS National Small Grains Research Facility in Aberdeen, Idaho. Seeds of susceptible cultivars Karl 92 and Jagger, were provided by the developer at Kansas State Universtiy. Tugela- Dn1 was provided by the Small Grain Institute, Bethlehem, South Africa. Wheat lines 01-20-12, 01-186-11, and KS99WGRC42 were maintained at USDA/ARS and the Wheat Genetics Resource Center (WGRC) at Manhattan, Kansas. Seeds of wheat genetic stocks used for marker localization including Chinese Spring (CS), CS nullisomic-tetrasomic (NT: N1A-T1D) (Sears 1966), ditelosomic (Dt1AL, Dt1AS) (Sears 1954), and deletion lines del1AS-1 [with fraction length (FL) 0.47], del1AS-2(FL 0.45) and del1AS-3(FL 0.86) (Endo and Gill 1996), were kindly provided by WGRC.
Evaluation of Hf-resistance
Parents (used as checks), F1 plants, and segregating populations were evaluated for phenotypic reaction to Hf infestation in growth chambers at 18±1°C with 14:10 h (L:D) photoperiod or in the greenhouse (20–32°C), as described previously (Hatchett et al. 1981; Maas et al. 1987) with modification. Briefly, approximately 15–20 seeds of each wheat line or family, were planted in uniformly spaced rows (12 rows or 24 half-rows per flat) in flats (52×36×10 cm) containing a mixture (1:1) of soil and vermiculite. Seedlings in each flat at the one-leaf stage were infested by confining ∼200 newly mated Hf females with a cheesecloth tent. Three weeks after infestation, the seedlings were examined to identify susceptible and resistant phenotypes. Susceptible plants were stunted with dark green leaves and harbored live larvae (or pre-pupae). Resistant plants grew normally (unstunted), with light green leaves, and contained dead larvae between the leaf sheaths. Chi-square (χ2) tests were conducted to determine the goodness-of-fit to theoretically expected Mendelian segregation ratios.
Bulked segregant analysis (BSA)
Molecular markers putatively linked to the resistance gene were identified by bulked segregant analysis (Michelmore et al. 1991) of the F5:6population. Resistant and susceptible DNA bulks were assembled, using equal amounts of DNA from five homozygous resistant and five susceptible families, respectively. The DNA samples of resistant and susceptible parents and bulks were amplified and screened for polymorphisms with SSR primers. Once polymorphisms between resistant and susceptible bulks were identified by BSA, genetic linkage between the Hf-resistance gene and the markers was determined in the segregating population.
SSR analyses
The sequences of SSR primers and PCR protocols were obtained from GrainGenes at http://wheat.pw.usda.gov/ggpages/DEM/ggtabledefs.html/. Information for specific primers can be found in the respective references: WMS (or GWM) (Röder et al. 1998), PSP (Stephenson et al. 1998; Devos et al. 1995), GDM (Pestsova et al. 2000), BARC (Ward et al.: http://www.graingenes.org/dbs_images/graingenes/BARC_1A.jpg), WMC (Gupta et al. 2002), and CFA (Sourdille: http://wheat.pw.usda.gov/ggpages/SSRclub/Sourdille/; Sourdille et al. 2004). Because the majority of Hf-resistance genes have been localized on chromosomes 1A and 5A (Ratcliffe and Hatchett 1997), we initially chose markers from these two chromosomes to screen for linkage to resistance. A total of 43 SSR primer pairs specific for wheat chromosome 1A and 32 primer pairs specific for 5A were used for screening.
PCR amplification was essentially performed as described by Röder et al. (1998), with minor modification (Liu et al. 2002). The reaction mixture contained 0.2 mM each of dNTPs, 1.8–2.0 mM MgCl2, 1 U Taq DNA polymerase (Promega, Madison, WI, USA), 1× Thermophilic DNA polymerase buffer (10 mM Tris–HCl, 50 mM KCl and 0.1% Triton X-100), 0.4 μM of each of the forward and reverse primers, and 100 ng of template DNA. PCR amplifications were performed in an MJ Research PTC-200 Thermal Cycler (Watertown, MA, USA) programmed at: 94°C for 3 min, followed by 40 cycles of 94°C for 1 min, 50–60°C (based on primer’s annealing temperature) for 1 min, and 72°C for 2 min, then a final extension step at 72°C for 10 min before cooling to 4°C. PCR amplified fragments were separated on 3% agarose gels (Sigma, St. Louis, MO) through electrophoresis at 5 V/cm in 1× TAE buffer. DNA banding patterns were visualized under UV light with ethidium bromide staining.
Aneuploid and deletion line analyses
To determine the physical location of the SSR markers, DNA from euploids, aneuploids, and deletion lines of CS wheat was amplified by using SSR primers corresponding to loci located on chromosome 1AS. The NT lines for group 1 chromosomes and the Dt lines for the short and long arms of chromosome 1A were used to assign the chromosome and chromosomal arm location of markers. Amplification (or no amplification) of a specific fragment from a deletion stock indicates that the corresponding marker is located proximally (or distally) to the breakpoint of the tested deletion stock. In this manner the markers and linked genes were physically localized into chromosome interval regions (bins) within the chromosome arm.
Linkage analysis and genetic mapping
The linkage map of SSR markers and the resistance gene was constructed by converting Recombination frequencies (RF) to genetic map distance (cM) with the Kosambi mapping function (Kosambi 1944) using MapMaker software version 3.0 (Lander et al. 1987) at LOD>3.0.
Results
Inheritance of resistance
The mechanism of the resistance conferred by the Hdic gene is antibiosis, which results in the death of the first-instar larvae 3–4 days after hatching. All plants of homozygous resistant lines, including the resistant donor PI 94641, the derived homozygous resistant F4:5 lines 01-20-12 and 01-186-11, and the wheat germplasm KS99WGRC42, exhibited resistance (no stunting) to Hf biotypes GP and L as well as strains vH9 and vH13. In all cases, presence of alive larvae within the leaf sheaths and stunting phenotype were observed on all infested plants of Karl 92 and Jagger.
When tested with Hf biotype L, the F2 populations derived from reciprocal crosses between PI 94641 and the Hf-susceptible emmer wheat CI 7685 phenotypically segregated 139-R: 49-S, and 97-R: 37-S. The observed segregation ratios fit a model for a single dominant gene (χ 23:1 =0.114, df=1, P=0.743 > 0.05, and χ 23:1 =0.667, df=1, P=0.438 > 0.05, respectively). In addition, we also analyzed a population of 121 F5:6lines derived from eight heterozygous F4individual plants (from a cross of Karl 92/PI 94641//Jagger*2/3/Karl 92), which were equivalent to F2-derived F3 families at the resistance gene locus. Among the 121 lines, 32 were homozygous resistant, 61 were segregating, and 28 were homozygous susceptible (1RR:2Rr:1rr; χ2 =0.273, df=2, P=0.875 > 0.05). This result also indicated the Hf resistance of PI 94641 is controlled by a single dominant gene.
However, analysis of the 793 individuals within the 61 segregating F5:6 lines indicated that the Hdic gene did not behave in a typical dominant manner. The 793 progeny segregated 374-R: 419-S plants, which is obviously significantly different from 3R:1S ratio. Since the expected number of homozygous resistant and susceptible F4:5 lines was observed, the deviation among F6individuals within the segregating lines must be due to phenotypic variation of the heterozygous plants.
To investigate if the emmer-derived resistance has variable penetrance, F1 progeny from crosses of Tugela- Dn1 and two homozygous resistant lines, 1-20-12 and 01-186-11, were evaluated for reaction to the Hf biotype L. Lines 1-20-12 and 01-186-11 were evaluated for reaction to Hf-biotype L multiple times and no susceptible plants were observed, indicating that the lines were indeed homozygous for the resistance gene. In addition, subsequent marker analysis of progeny from the plants used to make the crosses with Tugela- Dn1 indicated that these plants were homozygous for PI 94641 alleles in the region flanking the resistance gene. However, 22 of the 37 F1 individual plants evaluated (59.4%) were susceptible to Hf. Observed segregation of 72R: 62S and 65R: 53S in the F2 populations derived from resistant F1 plants of these crosses deviated significantly from the 3R:1S ratio expected for a single dominant resistance gene (χ2 =32.328, df=1, P < 0.001, and χ2 =24.960, df=1, P < 0.001). The genotypes of individual F2 plants derived from the two crosses were determined with markers flanking the resistance gene (Xbarc263 and Xcfa2153, or Xgwm33 and Xcfa2153) identified in our marker analysis. These markers were expected to provide selection accuracy greater than 99.97% for the resistance gene (see the Discussion section). In both populations, all plants homozygous for the T. dicoccum-derived alleles for the flanking markers were resistant to Hf. All plants homozygous for the susceptible parent alleles at the marker loci were susceptible. However, 60 of a total of 116 F2plants (51.7%) that were heterozygous at the flanking marker loci were resistant, while 56 heterozygous F2plants (48.3%) were susceptible to Hf. The phenotypes of Hdic heterozygotes were inconsistent (some were R and others were S), whereas homozygotes were consistently resistant. Both the F1 and F2 results demonstrated that the Hdic gene is incompletely penetrant, instead of partially dominant (which implies that all heterozygotes are intermediate to the parents in phenotype).
SSR markers linked to the Hdic gene
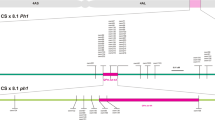
Of the 75 SSR primer pairs tested, five (GWM136, PSP2999, CFA2153, GWM33, and BARC263) from chromosome 1AS gave amplification products of expected sizes that were polymorphic between the resistant and susceptible parents as well as two bulks. Analysis of the F5:6lines indicated that these SSR markers were linked to Hdic. A genetic linkage map was constructed as Fig. 1a based on the F5:6 population evaluation.
Genetic map (a) and physical map (b) of the Hdic gene and linked SSR markers on wheat chromosome arm 1AS. The grey region of the chromosome represents the emmer-derived segment containing the Hdic gene in common wheat germplasm KS99WGRC42, and the white regions represent the genetic background of recipient parents (Karl 92 or Jagger)
The most closely linked proximal marker, Xgwm33, is dominant and 0.6 cM proximal to the Hdic gene (Fig. 1a) in the coupling phase. The GWM33 primer pair amplified a 130-bp DNA fragment from Jagger, PI 94641, homozygous resistant lines 01-20-12, 01-186-11, and KS99WGRC42 (Fig. 2a). No corresponding band was amplified from DNA of susceptible lines Karl 92 or Tugela- Dn1. A co-dominant marker, Xbarc263, 2.0 cM proximal to the resistance gene was also identified. Amplification with the BARC263 primer pair generated a specific 220-bp DNA fragment in samples from the resistant donor parent PI 94641, KS99WGRC42 and 01-20-12. A 200-bp fragment was amplified from the susceptible parents Karl 92, Jagger (Fig. 2b), as well as Tugela- Dn1 and 01-186-11. Both bands were amplified in most heterozygous progeny.
DNA fragments amplified with SSR primers GWM33 (a), BARC263 (b), and CFA2153 (c). DNA samples were prepared from F5:6 lines of “Karl 92/PI 94641//Jagger*2 /3/ Karl 92”, PI 94641(Td), Karl 92(K), Jagger (J), and Tugela- Dn1 (T1), 01-20-12 (12), 01-186-11 (11), and KS99WGRC42 (W). R Hf-resistant progeny, S Hf-susceptible progeny, H heterozygous progeny. L25=25-bp DNA ladder, L100=100-bp ladder
The most closely linked distal marker was Xcfa2153, located 1.4 cM from the Hdic gene (Fig. 1a). The CFA2153 primer pair amplified a 230-bp DNA fragment from the resistant parents PI 94641, 01-20-12, and 01-186-11, as well as from most homozygous resistant F5:6 lines, and amplified a 190-bp DNA fragment from the susceptible parents Karl 92, Jagger and Tugela- Dn1 (Fig. 2c), as well as from KS99WGRC42. Both the 230- and 190-bp fragments were amplified from heterozygous progeny.
Distal markers Xgwm136 and Xpsp2999 are dominant and linked to Hdic in the repulsion phase. The GWM136 primer pair amplified a 320-bp DNA fragment from susceptible parents, Karl 92 and Jagger, and a 280-bp fragment from the susceptible parent, Tugela- Dn1. The 320-bp fragment was also present in KS99WGRC42 amplifications. In contrast, no DNA amplification was achieved from the resistant parents PI 94641, 01-20-12, and 01-186-11, or from most homozygous resistant progeny (data not shown). The PSP2999 primer pair amplified three DNA fragments (with sizes 130, 140 and 145 bp, respectively) from Karl 92 and Jagger, and the susceptible and segregating progeny, as well as from KS99WGRC42. Only the 140-bp fragment was amplified from the resistant parents PI 94641, 01-20-12, and 01-186-11, as well as from most homozygous resistant progeny. Linkage analysis revealed that Xgwm136 and Xpsp2999 are distal to Hdic at distances of 2.4 and 2.0 cM, respectively.
To determine the physical locations of the linked SSR markers, DNA samples of CS, NT, Dt, and deletion lines of chromosome 1A were amplified by using SSR primer pairs GWM136, CFA2153, PSP2999, GWM33, and BARC263. Each primer pair amplified DNA fragments of the expected size(s) from CS and Dt1AS, but no corresponding fragments were amplified from Dt1AL, N1AT1D, del1AS-1 (FL 0.47), del1AS-2 (FL 0.45), and del1AS-3 (FL 0.86). The results demonstrate that all of these markers are located distal to the breakpoint of 1AS-3 in the terminal 14% of the chromosome arm (Fig. 1b).
Size of the T. dicoccum derived chromosomal segments
To determine the size of the introduced chromosome segment in the derived wheat lines and to determine recombination breakpoints of the transfers, allele sizes for the five SSR markers linked to Hf-resistance were determined in resistant lines 01-20-12, 01-186-11, and KS99WGRC42, as well as in the donor PI 94641 and the recipients Karl 92 and Jagger. Two SSR markers (Xwmc24 and Xbarc148) previously mapped proximal to Xbarc263 (http://www.grs.nig.ac.jp/wheat/komugi/maps/markerMap.jsp?chromosome=1) and one SSR marker (Xgdm33) located distal to Xgwm136 (Somers et al. 2004) were also included to determine the chromosomal breakpoints more accurately. In our physical mapping, marker Xwmc24 was located in the same interval (1AS3-0.86-1.00) as the Hdic gene, whereas marker Xbarc148 was located in the more proximal deletion interval 1AS1-0.47-0.86 (Fig. 1b).
Differences detected by these markers among the three homozygous resistant lines indicated that chromosomal segments in different sizes were transferred from PI 94641 in each case. Two recombination events took place during the transfer of Hdic from the donor to germplasm KS99WGRC42 (Fig. 1a). Only two markers, Xgwm33 and Xbarc263, detected polymorphism between KS99WGRC42 and the susceptible recipients (Table 1). Alleles of common wheat parents Jagger and Karl92 were present for the markers distal to Xgwm33 and the markers proximal to Xbarc263. A double crossover, or two separate recombination events, occurred at breakpoints between Xcfa2153 and Hdic, as well as between Xwmc24 and Xbarc263. These crossover events resulted in the transfer of a small intercalary fragment carrying the Hdic gene along with alleles of Xgwm33 and Xbarc263 from the donor parents to KS99WGRC42. In the line 01-20-12, a terminal fragment (distal to Xwmc24) containing Hdic was transferred from the donor into the recipient. Similarly, the terminal segment distal to Xbarc263 in the wheat line 01-186-11 was derived from the donor parent (Table 1)
Discussion
The interaction between wheat and Hessian fly operates in a typical gene-for-gene model (Hatchett and Gallun 1970). Most of the 31 characterized resistance genes are dominant or incompletely (partially) dominant, the exceptions are the H4 gene, which is recessive (Hatchett and Gallun 1970), while H7 and H8 exhibit complementary action (Amri et al. 1990; Ratcliffe and Hatchett 1997). The resistance conditioned by H3,H5, H6,H9, H10,H11, and H31 was reported as incompletely dominant (Cox and Hatchett 1994; El Bouhssini et al. 1999; Williams et al. 2003). In the present study, we determined that inheritance of Hf-resistance in wheat derived from PI 94641 is due to a single major gene (Hdic). The observed inheritance of resistance was dominant in the F2 population from a cross between resistant and susceptible emmer wheat lines. However, in a hexaploid wheat background, the Hdic gene did not exhibit a typical dominant or recessive action. Phenotypic variation (either completely resistant or fully susceptible) was observed for plants that were heterozygous at the resistance locus in both common wheat populations in the study. In each case, about 50% of heterozygotes were resistant and 50% were susceptible to the Hf. This was not a typical incompletely dominant gene action in which heterozygotes have an intermediate phenotype. The variable phenotypes (either R or S) were only observed in heterozygous plants, and not in any homozygous individuals or lines. This indicated that the Hdic gene does not have full penetrance in heterozygotes, and that phenotype variation was not likely caused by the impurity in the tested Hf stocks.
The Hdic gene is the first Hf-resistance gene identified from emmer wheat. Hdic has a different biotype reaction than that reported for Hf-resistance genes H5 (derived from common wheat), H9,H10, and H11 (from durum wheat) which are also located on chromosome 1A (Stebbins et al. 1983; Roberts and Gallun 1984, Liu et al. 2005). The H5 and H11 genes confer resistance to biotype GP, but not to biotype L (Sosa 1978, 1981; Ratcliffe et al. 1996; Ratcliffe and Hatchett 1997). H9 and H10 confer resistance to biotypes GP and L (Ratcliffe and Hatchett 1997), but susceptibility to Hf strain vH9 (Liu et al. unpubl. data). PI 94641 and the derived hexaploid lines in this study are resistant to biotypes GP and L, as well as to strains vH9 and vH13. Accordingly, the Hdic gene may either represent a new locus that is different from all previously named genes or may be a novel allele of an existing H gene. Biotype L is the most virulent population identified so far and has been the predominant biotype in the eastern wheat growing regions in USA (Ratcliffe et al. 2000) The vH13 strain of Hf is virulent to the most stable Hf-resistance gene H13. The broad spectrum of resistance conferred by Hdic makes it a promising gene for use in wheat breeding programs. In addition to biotypes GP and L and strains vH9 and vH13 that were tested, Hdic was also effective to field wild populations collected in 2002 from Idaho, Texas, Oregon, and Washington states (data not shown). In an attempt to isolate a biotype virulent to Hdic, KS99WGRC42 seedlings were infested with every Hessian fly populations we possessed, but no virulent insects were identified in several experiments. This indicated the frequency of virulent insects to Hdic, if any, was very low in the tested Hf stocks.
The markers identified in this study will facilitate the use of the Hdic gene in breeding programs. Since variation in reaction to the Hessian fly was observed in heterozygous progeny, accurate phenotypic selection for this gene needs to be delayed until lines are inbred. However, MAS for the resistance gene could be done on hybrid individuals and/or at early selfing generations. The flanking co-dominant SSR markers can determine the genotype at the resistance locus with a high degree of accuracy. For example, the two co-dominant markers Xcfa2153 and Xbarc263 are linked to the Hdic gene at 1.40 and 2.00 cM, respectively (Fig. 1a). The recombination frequency (RF) between Xcfa2153 and Hdic is 1.38% (with a Kosambi map distance of 1.40 cM) (Kosambi 1944). The RF between Xbarc263 and Hdic is 1.96% (with a map distance of 2.00 cM). These RFs for Xcfa2153 and Xbarc263 translate into selection accuracies of 98.62 and 98.04%, respectively, if they are used separately. The selection accuracy will increase to 99.97% when these two flanking markers are used together based on the product rule of probability. In addition to markers for MAS, the presence of only a small segment transferred from the emmer parent increases the usefulness of the germplasm for breeding programs, since this segment would be less likely to be detrimental to wheat quality and yield. The very small terminal and intercalary transfers and the high degree of polymorphisms between the emmer wheat and common wheat parents also provide effective tools to construct a fine map for the purpose of map-based cloning of the Hdic gene.
References
Amri AT, Cox TS, Hatchett JH, Gill BS (1990) Complementary action of genes for Hessian fly resistance in the wheat cultivar ‘Seneca’. J Hered 81:1224–1227
Brown-Guedira GL, Hatchett JH, Liu XM, Fritz AK, Owuoche JO, Gill BS, Sears RG, Cox TS, Chen MS (2004) Registration of KS99WGRC42 Hessian fly-resistant hard red winter wheat germplasm. Crop Sci (in press)
Buntin GD (1999) Hessian fly (Diptera: Cecidomyiidae) injury and loss of winter wheat grain yield and quality. J Econ Entomol 92:1190–1197
Cox TS, Hatchett JH (1986) Genetic model for wheat Hessian fly (Diptera: Cecidomyiidae) interaction: strategies for deployment of resistance genes in wheat cultivars. Environ Entomol 15:24–31
Cox TS, Hatchett JH (1994) Hessian fly resistance gene H26 transferred from Triticum tauschii to common wheat. Crop Sci 34:958–960
Delaney DE, Friebe BR, Hatchett JH, Gill BS, Hulbert SH (1995) Target mapping of rye chromatin in wheat by representational difference analysis. Genome 38:458–466
Devos KM, Bryan GJ, Collins AJ, Stephenson P, Gale MD (1995) Application of two microsatellite sequences in wheat storage proteins as molecular markers. Theor Appl Genet 90:247–252
Dweikat I, Ohm H, MacKenzie S, Patterson F, Cambron S, Ratcliffe R (1994) Association of a DNA marker with Hessian fly resistance gene H9 in wheat. Theor Appl Genet 89:964–968
Dweikat I, Ohm H, Patterson F, Cambron S (1997) Identification of RAPD markers for 11 Hessian fly resistance genes in wheat. Theor Appl Genet 94:419–423
Dweikat I, Zhang W, Ohm H (2002) Development of STS markers linked to Hessian fly resistance gene H6. Theor Appl Genet 105:766–770
El Bouhssini M, Hatchett JH, Wilde GE (1999) Hessian fly (Diptera: Cecidomyiidae) larval survival as affected by wheat resistance alleles, temperature and larval density. J Agric Urban Entomol 16:245–254
Endo TR, Gill BS (1996) The deletion stocks of common wheat. J Hered 87:295–307
Gagne RJ, Hatchett JH (1989) Instars of the Hessian fly (Diptera: Cecidomyiidae). Ann Entomol Soc Am 82:73–79
Gill KS, Lubbers EL, Gill BS, Raupp WJ, Cox TS (1991) A genetic linkage map of Triticum tauschii (DD) and its relation to the D genome of bread wheat (AABBDD). Genome 34:362–374
Gupta PK, Balyan HS, Edwards KJ, Isaac P, Korzun V, Röder M, Gautier MF, Joudrier P, Schlatter AR, Dubcovsky J, De la Pena RC, Khairallah M, Penner G, Sharp P, Keller B, Wang RCC, Hardouin JP, Jack P, Leroy P (2002) Genetic mapping of 66 new microsatellite (SSR) loci in bread wheat. Theor Appl Genet 105:413–422
Harris MO, Stuart JJ, Mohan M, Nair S, Lamb RJ, Rohfritsch O (2003) Grasses and gall midges: plant defense and insect adaptation. Annu Rev Entomol 48:549–577
Hatchett JH, Gallun RL (1970) Genetics of the ability of the Hessian fly, Mayetiola destructor, to survive on wheats having different genes for resistance. Ann Entomol Soc Am 63:1400–1407
Hatchett JH, Martin TJ, Livers RW (1981) Expression and inheritance of resistance to Hessian fly in hexaploid wheats derived from Triticum tauschii (Coss.) Schmal. Crop Sci 21:731–734
Hatchett JH, Starks KJ, Webster JA (1987) Insect and mite pests of wheat. In: Heyneg EG (ed) Wheat and Wheat Improvement. Agronomy monograph no. 13 (2nd edn). ASA-CSSA-SSSA, Madison, pp 625–675
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Liu XM, Smith CM, Gill BS (2002) Identification of microsatellite markers linked to Russian wheat aphid resistance genes Dn4 and Dn6. Theor Appl Genet 104:1042–1048
Liu XM, Fritz AK, Reese JC, Wilde GE, Gill BS, Chen MS (2005) H9,H10, and H11 compose a cluster of Hessian fly-resistance genes in the distal gene-rich region of wheat chromosome 1AS. Theor Appl Genet 110:1473–1480
Ma ZQ, Gill BS, Sorrells ME, Tanksley SD (1993) RFLP markers linked to Hessian fly-resistance genes in wheat (Triticum aestivum L.) from Triticum tauschii (Coss.) Schmal. Theor Appl Genet 85:750754
Maas FB III, Patterson FL, Foster JE, Hatchett JH (1987) Expression and inheritance of resistance of ’Marquillo’ wheat to Hessian fly biotype D. Crop Sci 27:49–52
Martin-Sanchez JA, Gomez-Colmenarejo M, Del Moral J, Sin E, Montes MJ, Gonzalez-Belinchon C, Lopez-Brana I, Delibes A (2003) A new Hessian fly resistance gene (H30) transferred from the wild grass Aegilops triuncialis to hexaploid wheat. Theor Appl Genet 106:1248–1255
McIntosh RA, Yamazaki Y, Devos KM, Dubcovsky J, Rogers J, Appels R (2003) Catalogue of gene symbols for wheat. MacGene 2003 (http://www.grs.nig.ac.jp/wheat/komugi/genes/download.jsp)
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Patterson FL, Shaner GE, Ohm HW, Foster JE (1990) A historical perspective for the establishment of research goals for wheat improvement. J Prod Agric 3:30–38
Pestsova E, Ganal MW, Röder MS (2000) Isolation and mapping of microsatellite markers specific for the D genome of bread wheat. Genome 43:689–697
Ratcliffe RH, Hatchett JH (1997) Biology and genetics of the Hessian fly and resistance in wheat. In: Bondari K (ed) New Developments in Entomology. Research Signpost, Trivandrum, India, pp 47–56
Ratcliffe RH, Ohm HW, Patterson FL, Cambron SE, Safranski GG (1996) Response of resistance genes H9 H19 in wheat to Hessian fly, (Diptera: Cecidomyiidae) laboratory biotypes and field populations from the Eastern United States. J Econ Entomol 89:1309–1317
Ratcliffe RH, Cambron SE, Flanders KL, Bosque-Perez NA, Clement SL, Ohm HW (2000) Biotype composition of Hessian fly (Diptera: Cecidomyiidae) populations from the southeastern, mid-western, and northwestern United States and virulence to resistance genes in wheat. J Econ Entomol 94:1319–1328
Roberts JJ, Gallun RL (1984) Chromosome location of the H5 gene for resistance to the Hessian fly in wheat. J Heredity 75:147–148
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier M, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Sears ER (1954) The aneuploids of common wheat. Univ Mo Agric Exp Stn Bull 572:1–58
Sears ER (1966) Nullisomic-tetrasomic combinations in hexaploid wheat. In: Rilly R, Lewis KR (eds) Chromosome manipulations and plant genetics. Oliver and Boyd, Edinburgh, pp 29–45
Seo YW, Johnson JW, Jarret RL (1997) A molecular marker associated with the H21 Hessian fly resistance gene in wheat. Mol Breed 3:177–181
Seo YW, Jang CS, Johnson JW (2001) Development of AFLP and STS markers for identifying wheat-rye translocations possessing 2RL. Euphytica 121:279–287
Smith CM, Quisenberry SS, du Toit F (1999) The value of conserved wheat germplasm possessing arthropod resistance. In: Clement SL, Quisenberry SS (eds) Global plant genetic resources for insect-resistant crops. CRC Press, Boca Raton, pp 25–49
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Sosa O (1978) Biotype L, ninth biotype of the Hessian fly. J Econ Entomol 71:458–460
Sosa O (1981) Biotypes J and L of the Hessian fly discovered in an Indiana wheat field. J Econ Entomol 74:180–182
Sourdille P, Singh S, Cadalen T, Brown-Guedira GL, Gay G, Qi L, Gill BS, Dufour P, Murigneux A, Bernard M (2004) Microsatellite-based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.). Funct Integr Genomics 4:12–25
Stebbins NB, Patterson FL, Gallun RL (1983) Inheritance of resistance of PI 94587 wheat to biotypes B and D of Hessian fly. Crop Sci 23:251–253
Stephenson P, Bryan G, Kirby J, Collins A, Devos K, Busso C, Gale M (1998) Fifty new microsatellite loci for the wheat genetic map. Theor Appl Genet 97:946–949
Weber JL, May PE (1989) Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet 44:388–396
Williams CE, Collier CC, Sardesai N, Ohm HW, Cambron SE (2003) Phenotypic assessment and mapped markers for H31, a new wheat gene conferring resistance to Hessian fly (Diptera: Cecidomyiidae). Theor Appl Genet 107:1516–1523
Yencho GC, Cohen MB, Byrne PF (2000) Applications of tagging and mapping insect resistance loci in plants. Annu Rev Entomol 45:393–422
Acknowledgements
The authors thank E. Parker, Xiang Liu, D. L. Wilson, and K. D. Howell for technical assistance and Sue Cambron and J. J. Stuart for the Hessian fly cultures. We appreciate the valuable suggestions and comments on the manuscript from Drs. R. A. McIntosh, B. S. Gill, B. R. Friebe, C. M. Smith, J. C. Reese, and two anonymous referees. This work was supported in part by a grant from the Kansas Wheat Commission and by USDA-IFAFS competitive grant 2001–04462. The experiments comply with the current laws of the USA, where the experiments were performed. Contribution No. 04-405-J from the Kansas Agricultural Experiment Station, Kansas State University, Manhattan, Kansas, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. A. Hoisington
Mention of commercial or proprietary product does not constitute an endorsement by USDA.
Rights and permissions
About this article
Cite this article
Liu, X.M., Brown-Guedira, G.L., Hatchett, J.H. et al. Genetic characterization and molecular mapping of a Hessian fly-resistance gene transferred from T. turgidum ssp. dicoccum to common wheat. Theor Appl Genet 111, 1308–1315 (2005). https://doi.org/10.1007/s00122-005-0059-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-0059-3