Abstract
The application of genetic male sterility in hybrid rice production has great potential to revolutionize hybrid seed production methodology. The two-line breeding system by using thermo-sensitive genic male sterility (TGMS) has been discovered and successfully developed as a breeding strategy in rice. One TGMS gene was investigated by a spontaneous rice mutant line, Sokcho-MS, originated from a Korean japonica variety. It was shown that Sokcho-MS is completely sterile at a temperature higher than 27°C and/or lower than 25°C during the development of spikelets, but fertile at the temperature ranging from 25 to 27°C regardless of the levels of day-length. Genetic analysis and molecular mapping based on SSR, STS and EST markers revealed that a single recessive gene locus involved the control of genic male sterility in Sokcho-MS. By using an F2 mapping population derived from a cross between Sokcho-MS and a fertile indica variety Neda, the new TGMS gene, designated as tms6, was mapped primarily to the long arm of chromosome 5 of Oryza sativa at the interval between markers E60663 (2.0 cM) and RM440 (5.8 cM). Subsequently, tms6 was fine mapped to the interval between markers RM3351 (0.1 cM) and E60663 (1.9 cM). As tms6 appeared to be independent of other mapped TGMS genes in rice, the genetic basis of Sokcho-MS was further discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male sterility in rice, for which a large number of mutants are found, is classified into four major groups: male sterility caused by cytoplasmic male sterility (CMS), photoperiod-sensitive genic male sterility (PGMS), thermo-sensitive genic male sterility (TGMS) and other genic male sterilities (Kurata et al. 2005). Discovery and successful utilization of male sterility in hybrid rice breeding have made a tremendous contribution to food security in the world. CMS, PGMS and TGMS have been used for practical hybrid production in rice (Yuan 1994).
Male sterility expression in TGMS lines is influenced by temperature alteration of environmental condition with temperature shift that can restore fertility in TGMS (Liu et al. 2001; Wang et al. 2003). Thus, TGMS plants can be used not only as male-sterile lines but also as maintainer lines, providing the opportunity to produce hybrid seeds in rice through a simple, less expensive and more efficient two-line seeds production system. Several TGMS genes in rice have been reported from China (Sun et al. 1989), Japan (Maruyama et al. 1991), and the International Rice Research Institute (IRRI) in the Philippines (Virmani and Voc 1991; Virmani et al. 1998). The TGMS trait was identified to be controlled by a single recessive gene (Yang et al. 1992; Borkakati and Virmani 1996; Reddy et al. 2000). Recently, Ku et al. (2003) showed that programmed cell death of premature tapetum is associated with male-sterility of TGMS in rice. Because of a specific temperature requirement, TGMS lines are characterized as two categories: TGMS and the reverse TGMS. Most TGMS lines are sterile at a high temperature (>25°C), but fertile at a lower temperature. Whereas the reverse TGMS lines have an opposite phenotype compared to most TGMS lines, these are sterile at a lower temperature, but fertile at a high temperature (Jia et al. 2001). Owing to the limitation of the temperature requirement, most TGMS rice lines can only be used in a very narrow zone for growing rice, while reverse TGMS lines can be used in a much larger area (Virmani et al. 1998; Lopez and Virmani 2000).
To date, seven TGMS genes, tms1, tms2, tms3, tms4, tms5, rtms1 and ms-h, have been mapped on chromosome 8, 7, 6, 2, 2, 10 and 9, respectively (Wang et al. 1995, 2003; Yamagushi et al. 1997; Subudhi et al. 1997; Dong et al. 2000; Jia et al. 2001; Koh et al. 1999). In the early report, Yang and Wang (1988) designated TGMS gene of 5460S as tms1, Kinoshita (1992) designated the TGMS gene of NorinPL12 as tms2. Wang et al. (1995) mapped tms1 on chromosome 8, whereas the locus of tms2 was estimated to be on chromosome 7 (Yamagushi et al.1997). Later, Subudhi et al. (1997) reported that the TGMS gene in NorinPL12 and IR32364 are non-allelic and identified linked random amplified polymorphic DNA (RAPD) markers for the TGMS trait in IR32364 and designated the TGMS gene in IR32364 as tms3. Using AFLP, RFLP and SSR techniques, Dong et al. (2000) mapped tms4 on chromosome 2 in an indica mutant TGMS-VN1. Similarly, Wang et al. (2003) fine mapped tms5 on chromosome 2 in AnnongS-1, the first indica TGMS rice line to be developed in China. Furthermore, Jia et al. (2001) found that the reverse TGMS gene (rtms1) in J207S was located on chromosome 10. However, none of the TGMS genes of rice have been isolated as yet (Kurata et al. 2005).
In this paper, we report the genetic characterization and molecular mapping of tms6, a new TGMS gene from a Korean japonica rice mutant line, Sokcho-MS. Genetic basis of tms6 were further discussed.
Materials and methods
Plant materials
The spontaneous rice mutant line, Sokcho-MS, was obtained in a farmer’s field from a japonica variety predominately growing at Sokcho in Korea. In the rice growing season during 2001–2004, the field tests at Gyeongsan (35.9°N, 128.6°E, 58 masl.) in Korea demonstrated that spikelets of Sokcho-MS plants were either completely sterile or partially fertile with 40 to 60% of seed setting largely relying on the temperature at spikelet development stage. However, spikelets of Sokcho-MS plants were completely sterile in the field tests undertaken at Kunming (25.0°N, 102.7°E, 1895 masl.) and Yuanjiang (23.6°N, 102.0°E, 398 masl.) in Yunnan of China, where the daily mean temperature is normally lower than 20.0°C and higher than 27.0°C during rice growing season in May to October, respectively.
Identification of fertility behaviors
To determine the basis for sterility of Sokcho-MS, we carried out experiments in growth chambers. Based on preliminary results of the field tests undertaken previously, fertility alternation of spikelets of Sokcho-MS was further investigated under controls of temperature and day-length in growth chambers in the rice growing season at Gyeongsan in Korea. Considering the nature environmental conditions in Korea, Sokcho-MS plants were grown in growth chambers, in which the growth temperature had been set at 23∼20, 25∼20, 25∼23, 27∼25, 30∼27 and 32∼27°C (max∼min of day∼night) under either long day-length (14 h) or short day-length (10 h) treatment during period from panicle initiation stage to early seed ripening. As the standard, each treatment consisted of ten plants of Sokcho-MS with three replicates. Spikelet fertilities of Sokcho-MS plants of each treatment were measured at full ripening stage. Spikelet fertility averaged over ten plants was scored as percentage.
Microscopic analysis
Anther development
Mature flowers of Sokcho-MS and the wild-type at the heading stage (just before anthesis) were prepared for observation of anther development (Itoh et al. 2005). The anthers were socked in glutaraldehyde fixative solution at 4°C as described by Ku et al. (2003). The solution contained 1.4% glutaraldehyde, 2% paraformaldehyde, and 50 mM PIPES (pH 7.2) which were preserved at 4°C overnight. After that, the samples were dehydrated with an ethanol series from 10 to 100% and embedded in paraffin (Paraplast.Plus, Sigma). The paraffin embedded samples were further sliced into 4 μm sections with a microtome (Leica DMR) and stained with 0.5% toluidine blue containing 0.1% sodium carbonate. The tissue sections were observed under a light microscope (Olympus BX51, UV filter set) and finally photographed with digital camera (Nikon Coolpix 4500).
Pollen fertility
Anthers of the wild-type and Sokcho-MS with fertility and sterility conditions were collected from spikelets at flowering stage. Pollens from broken anthers were suspended with a 1% iodine–potassium iodide solution (KI–I2). The stained color and figures of pollens were observed under a light microscope. Plants with less than 5% stainable pollen were considered as completely male sterile, and all others were regarded as fertile.
Genetic analysis
Four combinations were made to analyze the inheritance of the TGMS gene in Sokcho-MS. Sokcho-MS was crossed as female with male parents Jinmibyeo (japonica), Sangjubyeo (japonica), Sangsangbyeo (japonica) and Neda (indica), respectively. Segregation ratio of spikelet fertility and sterility of F2s plants of the crosses were measured at full seed ripening stage. The statistical analysis was performed by the program, statistix for window (Analytical software)
Molecular mapping
Mapping population
An F2 mapping population was derived from a cross between Sokcho-MS and Neda mentioned above. To ensure the accuracy of phenotypic identification, all TGMS plants from the F2 segregation of mapping population were screened under the sterile and fertile conditions within 2 years.
Selection of primers
Based on marker polymorphism survey of genotype of the parents, a total of 41 simple sequence-repeat (SSR) (http://www.gramene.org), 21 sequence tagged site (STS) and 19 cleaved amplified polymorphic sequence (CAPS) and 13 expressed sequence tag (EST) (rgp.dna.affrc.go.jp) markers were applied for mapping of the TGMS gene of Sokcho-MS.
DNA preparation and PCR amplification
Total DNA was extracted from 2 g of fresh leaves at the tillering stage. DNA extraction and PCR amplification were performed as described (Sambrook et al. 1989; Panaud et al. 1996).
Construction of the linkage map
The Kosambi mapping function in the computer program Map Manager QTXb17 (Chmielewicz and Manly 2002) was used to construct the linkage map.
Results
Genetic characterization of Sokcho-MS
Generally, the experiments in growth chambers showed that spikelets of Sokcho-MS were completely sterile at the temperature higher than 27°C and/or lower than 25°C during the development stage of the spikelets, but fertile at the temperature ranging from 25 to 27°C regardless of the levels of day-length (Table 1). On an average, the spikelet fertility reached to 40.8% at temperature ranging from 25°C to 27°C with short day-length treatment, which was significantly higher than that of 7.9% with long day-length treatment (Table 1). Taken together with the field observation on variations in spikelet fertilities of Sokcho-MS under different natural environments in Korea and in China, it was more likely that male sterility expression of Sokcho-MS is mainly controlled by thermo-variation. Unlike the fertility responses to temperature in most TGMS lines and the reverse TGMS line, the TGMS trait of Sokcho-MS is different in phenotype expression (Fig. 1).
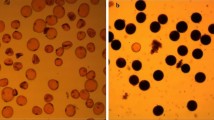
By cytological observation, it was revealed that the formation of anthers and differentiation of pollens in Sokcho-MS were abnormal at sterile condition with the temperature higher than 27°C and/or lower than 25°C during the development of spikelets, but normal at fertile condition with the temperature ranging from 25 to 27°C regardless of the levels of day-length (Fig. 2a, b). Sokcho-MS anthers at fertile condition developed well showing yellow color as that of the wild-type, while the anthers at sterile condition became small showing white color (Fig. 2a). Similarly, Sokcho-MS anthers contained normal pollens at fertile condition but presented complete pollen abortion at sterile condition (Fig. 2b). In comparison to the wild-type, Sokcho-MS at sterile condition displayed defects in the development of anthers and pollens in mature spikelets (Fig. 2c).
a Comparison of floral organs of Sokcho-MS and the wild-type under different temperatures (max∼min of day∼night) with short day-length (10 h) treatment. A1 wild-type anthers showing normal male fertility under controls of temperature and day-length, A2 Sokcho-MS anthers showing male sterile at 25∼23°C, A3 Sokcho-MS anthers showing male fertility at 27∼25°C, A4 Sokcho-MS anthers showing male sterile at 30∼27°C b Comparison of pollen grains of Sokcho-MS and the wild-type at male sterile and male fertile condition. Pollen grains were stained with 1% KI-I2 and normal pollen grains were visible as black. B1 functional pollen grains of the wild-type stained in black, B2 non-functional pollen grains of Sokcho-MS at 23∼20°C, B3 functional pollen grains of Sokcho-MS stained in black at 27∼25°C, B4 non-functional pollen grains of Sokcho-MS at 30∼27°C. Temperature treatments were recorded as max∼min of day∼night c Light-microscopic observation on the development of anthers and pollens in Sokcho-MS and the wild-type at the heading stage under different temperatures (max∼min of day∼night) with short day-length (10 h) treatment. C1 the wild-type, C2 Sokcho-MS under male sterile condition at 23∼20°C, C3 Sokcho-MS under male fertile condition at 27∼25°C, C4 Sokcho-MS under male sterile condition at 30∼27°C
Genetic analysis clearly indicated that inheritance of the TGMS gene in Sokcho-MS is sporophytic and controlled by a single recessive gene in the nucleus, the ratio of fertile and complete sterile plants in 4 tested F2population fits the theoretical 3:1 distribution predicated by Mendelian inheritance (Table 2).
Mapping of the TGMS gene in Sokcho-MS
To determine the chromosome location of the TGMS gene in Sokcho-MS, a total of 72 SSR, STS and CAPS markers were firstly screened on 12 chromosomes with 6 markers on each chromosome of Oryza sativa, using 190 F2 plants of mapping population. To further confirm the map location of the TGMS gene in Sokcho-MS, several SSR, STS, CAPS and EST markers flanking the TGMS gene of Sokcho-MS were selected to fine map the TGMS gene, using 936 F2plants of mapping population.
The phenotypic and genotypic segregation data for the marker (i.e. E60663) showing linkage with the TGMS gene in Sokcho-MS was detected (Fig. 3). As the result, the TGMS gene in Sokcho-MS was mapped primarily to the long arm of chromosome 5 of Oryza sativa at the interval between markers E60663 (2.0 cM) and RM440 (5.8 cM). Because the TGMS gene appeared to be non-allelic to other mapped TGMS genes in rice (Wang et al. 1995, 2003; Yamagushi et al. 1997; Subudhi et al. 1997; Dong et al. 2000; Jia et al. 2001; Koh et al. 1999), we therefore designated tms6 as the TGMS gene in Sokcho-MS. Subsequently, tms6 was fine mapped to the interval between markers RM3351 (0.1 cM) and E60663 (1.9 cM) (Fig. 4). The alignment and genetic distances between tms6 gene and flanking markers were constructed. These molecular markers spanned approximately 132.9 cM, with all markers clustered and located on chromosome 5 (Fig. 4).
Phenotype of parents and F2 mapping population as revealed by PCR of E60663. M 100 bp size marker, T Sokcho-MS, N Neda, 1∼30 individual plants of F2 population derived from cross Sokcho-MS/Neda. In the E60663 marker and phenotype, A homozygous Sokcho-MS genotype, B homozygous Neda genotype, H heterozygote genotype, C donor genotype when that phenotype is the dominant genotype either heterozygous or homozygous donor
Molecular linkage map of the rice chromosome 5 showing the location of the tms6 gene. One of STS marker, E60663 and one of SSR marker, RM440, showed close linkage with tms6 gene on the long arm of chromosome 5. The linkage distance between tms6 gene and the linkage markers E60663, RM3351, was 1.9, 0.1 cM, respectively. The genetic distances between loci were derived by Kosambi mapping function and are shown in centiMorgans (cM)
Discussion
The TGMS trait of Sokcho-MS was found to be controlled by a single recessive gene in nucleus, this result is consistent with other TGMS lines identified (Yang et al. 1992; Borkakati and Virmani 1996; Reddy et al. 2000). However, the mapping results of this study clearly indicated that tms6 is located on rice chromosome 5. Therefore, tms6 is a new TGMS gene which appears to be independent of the above discussed seven rice TGMS genes (tms1, tms2, tms3, tms4, tms5, rtms1 and ms-h).
Interestingly, characteristics of tms6 appeared to be different from the TGMS lines of various sources. Under controls of temperature and day-length in growth chambers, we found that male sterility expression of Sokcho-MS is mainly controlled by thermo-variation. However, it was identified that spikelets of Sokcho-MS plants were fertile only within a narrow range of temperature from 25 to 27°C during the development of rice spikelets. This finding may help to simply explain the cause of Sokcho-MS plants being completely sterile or partially fertile at different locations in Korea and China. The field tests in Korea and China also revealed that the fertile condition of Sokcho-MS was strictly limited in a geographic zone with a narrow critical temperature threshold for the development of rice spikelets. Regardless of the day-length, it was observed that seed setting of Sokcho-MS at Gyeongsan in Korea reached to 40∼60% under the favorable temperature condition during the development of spikelets. By contrast, it was shown that Sokcho-MS plants tested at Kunming and Yuanjiang in China were completely sterile mainly because the daily mean temperature for rice growing is usually either lower than 25°C or higher than 27°C at the experimental sites. This meant that there is a possibility to practically utilize tms6 for two-line hybrid rice seed production. Overall, the experimental results offered an insight in potential utilization of tms6 in Korea and China.
Considering genetic classification of TGMS, we designated tms6 as a new TGMS gene in Sokcho-MS. Although spikelet fertility of Sokcho-MS was significantly increased under favorable temperature with short day-length, it should be not surprised for the interaction between temperature and day-length attributing to fertility alternation of Sokcho-MS. In practice, it has been already noticed that the fertility expression of PGMS lines were controlled simultaneously by day-length and temperature (Virmani et al. 1998). The basis for male sterility of Sokcho-MS might be somewhat similar to most PGMS lines which performed as fertile under short day-length. In this regard, we would suggest that fertility behavior of tms6 is not identical to that of most TGMS lines and the reverse TGMS line. The expression of male sterility of Sokcho-MS corresponding to the temperature was simply depicted (Fig. 1), but detailed analysis of the critical temperature and day-length thresholds of fertility alternation remain further study.
Thermo-sensitive genic male sterility lines from various sources differ in their stage of sensitivity to temperature during the development of spikelets. By cytological observation, the distinct variations were presented between fertile and sterile anthers and pollens of Sokcho-MS in the mature flowers. It implied that expression of tms6 would have occurred at the early stage of spikelet development. Ku et al. (2003) reported that programmed cell death of premature tapetum is associated with male-sterility of TGMS in rice. The functions of tms6 in regulation of the formation of anthers and the differentiation of pollens need to be carried out.
Even though the PGMS and TGMS systems are being widely applied in hybrid rice breeding, little information is available regarding on the molecular basis of the TGMS trait in rice. Neither PGMS gene nor TGMS gene has been isolated as yet (Kurata et al. 2005). Genetic characterization and fine mapping of tms6 provided a foundation for isolation of the TGMS gene of rice. With recent advances in map-based cloning techniques and the sequenced genome information, we are now approaching isolation of the candidate gene of tms6 in Sokcho-MS, which may contribute significantly to characterization of TGMS at the molecular level and reveal its functions in rice.
References
Borkakati RP, Virmani SS (1996) Genetics of thermosensitive genic male sterility in rice. Euphytica 88:1–7
Chmielewicz KM, Manly KF (2002) Tutorial for QTX, software for genetic mapping of Mendelian markers and quantitative trait loci. Roswell Park Cancer Institute
Dong NV, Subudhi PK, Luong PN, Quang VD, Quy TD, Zheng HG, Wang B, Nguyen HT (2000) Molecular mapping of a rice gene conditioning thermosensitive genic male sterility using AFLP, RFLP and SSR techniques. Theor Appl Genet 100:727–734
Itoh JI, Nonomura KI, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y (2005) Rice plant development: from zygote to spikelet. Plant Cell Physiol 46(1):23–47
Jia JH, Zhang DS, Li CY, Qu XP, Wang SW, Chamarerk V, Nguyen HT, Wang B (2001) Molecular mapping of the reverse thermo-sensitive genic male-sterile gene (rtms1) in rice. Theor Appl Genet 103:607–612
Kinoshita T (1992) Report of the committee on gene symbolization nomenclature and linkage groups. Rice Genet Newsl 9:2–4
Koh HJ, Son YH, Heu MH, Lee HS, McCouch SR (1999) Molecular mapping of a new genic male-sterility gene causing chalky endosperm in rice (Oryza sativa L.). Euphytica 106:57–62
Ku SJ, Yoon HJ, Suh HS, Chung YY (2003) Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 217:559–565
Kurata N, Miyoshi KI, Nonomura KI, Yamazaki Y, Ito Y (2005) Rice mutants and genes related to organ development, morphogenesis and physiological traits. Plant Cell Physiol 46(1):48–62
Liu N, Shan Y, Wang FP, Xu CG, Peng KM, Li XH, Zhang Q (2001) Mol Genet Genomics 266:271–275
Lopez MT, Virmani SS (2000) Development of TGMS lines for developing two-line rice hybrids for the tropics. Euphytica 114:211–215
Maruyama K, Araki H, Kato H (1991) Thermosensitive genic male sterility induced by irradiation. Rice Genetics II. International Rice Research Institute, Manila, pp 227–235
Panaud O, Chen X, McCouch SR (1996) Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet 252:597–607
Reddy OUK, Siddiq EA, Sarma NP, Ali J, Hussain AJ, Nimmakayala P, Ramasamy P, Pammi S, Reddy AS (2000) Genetic analysis of temperature-sensitive male sterility in rice. Theor Appl Genet 100:794–801
Sambrook L, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Subudhi PK, Borkakati RK, Virmani SS, Huang N (1997) Molecular mapping of a thermosensitive genetic male-sterility gene in rice using bulked segregant analysis. Genome 40:188–194
Sun ZX, Min SK, Xiong ZM (1989) A temperature-sensitive male sterile line found in rice. Rice Genet Newslett 6:116–117
Virmani SS, Voc PC (1991) Induction of photo- and thermo-sensitivegenic male sterility in indica rice. Agron Abst 119
Virmani SS, Saddiq EA, Muralidharan K (1998) Advances in hybrid rice technology. International Rice Research Institute, Manila
Wang B, Xu WW, Wang JZ, Wu W, Zheng HG, Yang ZY, Ray JD, Nguyen HT (1995) Tagging and mapping the thermosensitive genic male-sterile gene in rice (Oryza sativa) with molecular markers. Theor Appl Genet 91:1111–1114
Wang YG, Xing QH, Deng QY, Liang FS, Yuan LP, Weng ML, Wang B (2003) Fine mapping of the rice thermo-sensitive genic male-sterile gene tms5. Theor Appl Genet 107:917–921
Yamagushi Y, Ikeda R, Hirasawa H, Minami M, Ujihara P (1997) Linkage analysis of the thermo-sensitive genic male sterility gene tms2 in rice (Oryza sativa L.). Breed Sci 47:371–377
Yang RC, Wang NY (1988) 5460S Indica photosensitive genic male-sterile rice. Int Rice Res Newsl 13:6–7
Yang RC, Liang KJ, Wang NY, Chen SH (1992) A recessive gene in indica rice 5460 s for thermosensitive genic male sterility. Rice Genet Newslett 9:56–67
Yuan LP (1994) Increasing yield potential in rice by exploitation of heterosis. In: Virmanni SS (ed) Hybrid rice technology. New developments and future prospects. IRRI, Manila, pp 1–6
Acknowledgements
We thank Prof. Rita Taylor, Yeungnam University, for proof reading of the manuscript. This work was supported, in part, by grants from Biogreen 21 project of Rural Development Administration, Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Sasaki
Rights and permissions
About this article
Cite this article
Lee, D.S., Chen, L.J. & Suh, H.S. Genetic characterization and fine mapping of a novel thermo-sensitive genic male-sterile gene tms6 in rice (Oryza sativa L.). Theor Appl Genet 111, 1271–1277 (2005). https://doi.org/10.1007/s00122-005-0044-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-0044-x