Abstract
Wheat scab (Fusarium Head Blight, FHB) is a destructive disease in the warm and humid wheat-growing areas of the world. Finding diverse sources of FHB resistance is critical for genetic diversity of resistance for wheat breeding programs. Leymus racemosus is a wild perennial relative of wheat and is highly resistant to FHB. Three wheat- L. racemosus disomic addition (DA) lines DA5Lr#1, DA7Lr#1 and DALr.7 resistant to FHB were used to develop wheat- L.racemosus translocation lines through irradiation and gametocidal gene-induced chromosome breakage. A total of nine wheat-alien translocation lines with wheat scab resistance were identified by chromosome C-banding, GISH, telosomic pairing and RFLP analyses. In line NAU614, the long arm of 5Lr#1 was translocated to wheat chromosome 6B. Four lines, NAU601, NAU615, NAU617, and NAU635, had a part of the short arm of 7Lr#1 transferred to different wheat chromosomes. Four other lines, NAU611, NAU634, NAU633, and NAU618, contained translocations involving Leymus chromosome Lr.7 and different wheat chromosomes. The resistance level of the translocation lines with a single alien chromosome segment was higher than the susceptible wheat parent Chinese Spring but lower than the alien resistant parent L. racemosus. At least three resistance genes in L. racemosus were identified. One was located on chromosome Lr.7, and two could be assigned to the long arm of 5Lr#1 and the short arm of 7Lr#1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat scab (Fusarium Head Blight, FHB) caused by Fusarium graminearum Schw. is a destructive disease in the warm and humid wheat-growing areas of the world, such as China, Japan, and Brazil. However, since the late 1980s, scab epidemics have also become more frequent in North America and Europe because of global weather changes, maize–wheat or rice–wheat crop rotations and minimum tillage. FHB not only causes yield loss but also decreases grain quality due to the presence of a mycotoxin. Development and utilization of resistant varieties is the most efficient and economic strategy for controlling FHB disease.
Scab resistance in wheat is controlled by two or three major genes and several minor genes. Recombination and convergence of different resistant components have been used successfully for scab-resistance breeding. A pedigree analysis of recently released varieties indicated that genetic resources for scab resistance were limited to only a few varieties, Sumai 3 and Frontana and their derivatives. To broaden the genetic diversity, we evaluated wild relatives of wheat for FHB resistance. Leymus racemosus Lam. (Elymus gigantus L.) is known to have high levels of wheat scab resistance (Mujeeb-kazi et al. 1983; Wang et al. 1986). Two other grass species, Roegneria kamoji C. Koch and Roegneria ciliaris (Trin.) Nevski, which grow in the warm and humid environment of southern China, also were identified with high levels of resistance to scab (Weng and Liu 1989). Since 1985, the Cytogenetics Institute of Nanjing Agricultural University began the transfer of scab-resistance genes from alien species into common wheat. Hybrids between Triticum aestivum L. cv. Chinese Spring and L. racemosus (2n=4x=28, JJNN) were obtained by embryo rescue, and eight chromosome addition lines with different L. racemosus chromosomes were identified. Multi-year evaluations identified three wheat- L. racemosus disomic addition lines, DALr.2, DALr.7, and DALr.14, with high scab resistance (Chen et al. 1993, 1995). RFLP analysis revealed that the L. racemosus chromosomes in lines DALr.2 and DALr.14 belonged to homoeologous group 7 and 5, and, thus, were designated as 7Lr#1 and 5Lr#1, respectively (Qi et al. 1997). The homoeology of the L. racemosus chromosome in line DALr.7 remains to be established. To reduce unnecessary alien chromatin, irradiation and gametocidal gene-induced chromosome breakage were used to develop wheat- L. racemosus intergenomic translocation lines (Chen et al. 1998; Liu et al. 1999, 2000a, b; Wang et al. 2001; Yang et al. 2002; Liu 2002; Yuan et al. 2003). In the present paper we describe the development and characterization of wheat- L. racemosus translocation lines and their potential for improving scab resistance in wheat.
Materials and methods
Materials
Wheat- L. racemosus addition lines with scab resistance, DA5Lr#1, DA7Lr#1, and DALr.7, were developed by the Cytogenetics Institute, Nanjing Agricultural University (CINAU). The wheat- Aegilops cylindrica Hose disomic chromosome addition line with the gametocidal chromosome 2C (DA2C) was kindly provided by Dr. T. R. Endo, Kyoto University, Japan. The common wheat varieties Chinese Spring, Yangmai 5 and Mianyang 11 are maintained at CINAU.
Irradiation treatment
Plants of the wheat- L.racemosus monosomic addition lines with scab resistance were irradiated at meiosis at boot stage by 60Co γ-ray 500R-1125R (75–100R/min) or the mature pollen was irradiated at early flowering stage by 60Co γ-ray 1000R (100R/min). The irradiated plants were allowed to self-pollinate or used as pollen donors in crosses with susceptible varieties. The progenies were analysed by C-banding and genomic in situ hybridisation (GISH) to identify intergenomic translocations involving wheat and Leymus chromosomes. Irradiation treatments were carried out at the Jiangsu Academy of Agricultural Sciences.
Gametocidal gene-induced translocations
Wheat- L. racemosus addition lines with scab resistance were also crossed with DA2C (Endo 1988). The F1 hybrids were backcrossed with common wheat cv. Chinese Spring. The BC1F1 plants without chromosome 2C were identified by C-banding and self-pollinated. Their progenies were used to identify chromosome translocations and deletions by C-banding and GISH.
Molecular-cytogenetic analysis
C-banding and chromosome identification were according to Gill et al. (1991), and GISH followed the protocol of Jiang and Gill (1994) and Zhang et al. (2002). Meiotic metaphase I pairing was analysed in C-banded pollen mother cells (PMCs) in testcrosses with double ditelosomic stocks of Chinese Spring wheat. RFLP analysis was according to Sharp et al. (1989) and was used to determine the homoeologous relationships and breakpoint positions of the translocation chromosomes. RFLP probes were kindly provided by Dr. M. E. Sorrells, Ithaca, NY, USA (designated BCD, CDO, and WG), Dr. M. D. Gale, Norwich, UK (designated PSR). KSU probes were developed by Gill K.S. et al (1991).
Screening for Fusarium head blight resistance
Scab resistance was evaluated under natural or severe artificial epidemic conditions. The single-floret inoculation was used for further verification of scab resistance using a mixed conidiospore suspension of four highly aggressiveness F. graminearum isolates, F4, F15, F17, and F34 (kindly provided by the Plant Protection Institute, Jiangsu Academy of Agricultural Sciences) (Wang et al. 1982). Sumai 3 was used as the resistant check, and Chinese Spring and Mianyang 85–45 were used as susceptible check varieties. Plants were scored relative to the resistant (R) and susceptible checks (S) as MR (moderately resistant), MS (moderately susceptible) and intermediate (MS-MR).
Results
Translocation involving 5Lr#1
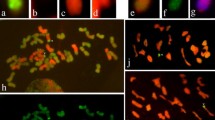
Line NAU614 was selected from the M3 of Mianyang11/DA5Lr#1 (spikes irradiated before anthesis by γ-ray) and has 2n=42 chromosomes. The translocation chromosome consists of approximately half of the long arm of 5Lr#1, approximately 50% of the short arm of 6B and the entire long arm of 6B (Fig. 1). This translocation line was designated as T6BL·6BS-5Lr#1L.
Translocations involving 7Lr#1
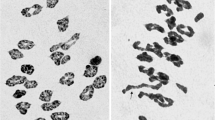
Line NAU601 was selected from the M3 of Yangmai5/MA7Lr#1 (spikes irradiated by γ-ray before anthesis) and has 2n=42 chromosomes. The wheat chromosome involved in the translocation chromosome was 4B. GISH identified the breakpoint at about 40% in the long arm (Fig. 1). To further verify the chromosome constitution, line NAU601 was crossed with Chinese Spring ditelosomic 4BS. After chromosome C-banding of PMCs at MI, a heteromorphic rod bivalent consisting of 4BS and the translocation chromosome was observed in 79% PMCs in the F1 (Fig. 2a), confirming that translocation chromosome consists of the complete short arm of 4B, a part of the long arm of 4B and a distal segment derived from 7Lr#1S.
Meiotic metaphase I pairing analysis of translocation chromosome with wheat telosomic chromosomes. a C-banded chromosome configuration of PMC at MI of meiosis in F1 of Chinese Spring ditelosomic 4B and translocation line NAU 601. 4BS and a translocation chromosome paired as a heteromorphic bivalent. b C-banded chromosome configuration of PMC at MI of meiosis in F1 of Chinese Spring double ditelosomic 1A and a disomic addition translocation line NAU 618. 1AS paired with a translocation chromosome (arrowed) and 1AL paired with a normal 1A (arrowed) as heteromorphic bivalents, respectively
To further characterize the 7Lr#1 segment involved in the translocation, 13 group-7 probes and 15 group-4 probes were selected for RFLP analysis. The results showed that this line had specific bands for 7Lr#1 when using BCD93, CDO676, MWG705, BCD385, PSR152, PSR103, CDO595, BCD349, and MWG808 as probes. These probes have been mapped previously to the short arm of homoeologous group 7. However, no specific bands for 7Lr#1 were observed when using ABG476.1, WG669, PSR690, and PSR311. This data indicated that the short arm of 7Lr#1 was involved in the translocation chromosome with the breakpoint between the MW808 and ABG476.1 (Table 1). The RFLP results also showed that specific bands of PSR139, PSR110 for 4BS and bands of BCD1262, PSR163, CDO1387, BCD110, CDO189, CDO1395, CDO938, and CDO541 for 4BL (close to the centromere) were present, but that the bands of PSR164, KSUC2, CDO1312, KSUG10 and WG114 for 4BL (distal) were absent (Table 2). This result confirmed cytological data and thus the translocation chromosome in NAU601 can be designated as T4BS·4BL-7Lr#1S with the breakpoint between CDO541 and PSR164 in the 4B long arm.
Line NAU615 also was selected from the M3 of Yangmai5/MA7Lr#1 (spikes irradiated by γ-ray before anthesis) and has 2n=42 chromosomes. The C-banding and GISH patterns of the translocation chromosome in this line were very similar to that in the line NAU601 (T4BS·4BL-7Lr#1S). However, RFLP analysis using the same probes for NAU601 revealed that the specific bands of probes BCD349 and MWG 808, which were present in NAU601, were absent in NAU615. This suggested that the segment of 7Lr#1S in the translocation chromosome of NAU615 was shorter than that of NAU601. The breakpoint of 7Lr#1S in NAU615 was located between CDO595 and BCD349 (Table 1). The translocation line NAU615 was designated as T4BS·4BL-7Lr#1S-1.
Line NAU617 was selected from the Gc-induced progenies of DA7Lr#1 /DA2C//CS and has 2n=42 chromosomes. C-banding analysis showed that the translocation chromosome consists of the short arm of 7Lr#1 and the long arm of 6A. GISH identified the breakpoint close to the centromere of 6AS (Fig. 1).
To further detect the segment and breakpoint of the translocation, 13 group-7 probes and seven group-6 probes were used for RFLP analysis. The RFLP patterns of group-7 probes in this line were same as that in line NAU601 (Table 1), indicating a similar translocated segment of 7Lr#1S in both lines. However, specific 6AS fragments detected by BCD342, PSR627 and CDO1158 were absent in line NAU617, whereas the 6AL fragments detected by BCD276, BCD340, CDO497 and WG286 were present in NAU617 (Table 2). This confirmed the cytological data and identified the translocation chromosome as T6AL·7Lr#1S.
Line NAU635 was selected from the Gc-induced progenies of DA7Lr#1/DA2C//CS and has 2n=42 chromosomes. C-banding analysis showed the translocation chromosome consisting of the short arm of 7Lr#1 and the long arm of 1B. GISH identified the breakpoint near the centromere (Fig. 1).
RFLP analysis revealed that the translocated segment of 7Lr#1S in line NAU635 was similar to those in lines NAU601 and NAU617 (Table 1). However, the specific 1BS fragments of BCD1434, BCD371 and BCD1072 were absent in this line, whereas the specific 1BL fragments detected by probes of KSU127, PSR162, WG241, CDO105, and BCD386 were present (Table 2). These results confirmed the data of C-banding and GISH. The translocation chromosome in line NAU635 was designated as T1BL·7Lr#1S.
Translocations involving Lr.7
Line NAU611 was selected from the progenies of MALr.7 (plants irradiated by γ-ray at meiosis) and has 2n=42 chromosomes. C-banding analysis showed a pair of translocation chromosomes consisting of the short arm of Lr.7 and the long arm of 4A. GISH identified the breakpoint close to the centromere (Fig. 1). This translocation chromosome line was described as T4AL·Lr7S.
Line NAU 618 was selected from the progenies of MALr.7 plants irradiated by γ-ray at meiosis and has 2n=44 chromosomes. C-banding identified the added translocation chromosome pair consisting of the long arm and part of the short arm of Lr.7 and distal segment of 1AS. GISH identified the breakpoint at approximately 30% of the short arm (Fig. 1).
To verify the chromosome constitution, line NAU618 was crossed with Chinese Spring double ditelosomic 1A, and meiotic metaphase I pairing was analysed in C-banded PMCs. Chromosome 1AS paired with the translocation chromosome as a heteromorphic rod bivalent in 70% of the PMCs, whereas 1AL paired with the normal 1A and formed another heteromorphic rod bivalent (Fig. 2b). This result confirmed that the added translocation chromosome in NAU618 was T1AS-Lr.7S·Lr.7L.
Line NAU633 was selected from the BC1F2 of DALr.7/DA2C//CS and has 2n=44 chromosomes. C-banding analysis showed that this line has an added translocation chromosome pair consisting of long arm of Lr.7 and the short arm of wheat chromosome 1D. GISH identified the breakpoint near the centromere (Fig. 1). This translocation chromosome was designated as T1DS·Lr.7L.
Line NAU634 was selected from the BC1F2 of DALr.7/DA2C//CS and has 2n=42 chromosomes. The C-banding identified the wheat chromosome 4A involved in the translocation. GISH showed the breakpoint at approximately 30% of the short arm of 4A (Fig. 1). The translocation chromosome was described as T4AL·4AS-Lr.7S.
Scab resistance evaluation
Most of the translocation lines showed higher resistance to FHB than the susceptible wheat parent Chinese Spring. Some of them were similar to the resistant check Sumai 3 but had a lower level of resistance than the alien parent L. racemosus (Table 3; Fig. 3). Different levels of scab resistance were observed in the progenies involving the same wheat- L. racemosus translocation chromosome. Lines with a higher level of scab resistance were obtained after successive selection under natural and artificial epidemic conditions.
The spikes of some translocation lines inoculated by Fusarium graminearum. From left to right: Chinese Spring (susceptible wheat parent), NAU601, NAU611, NAU615, NAU618, NAU614, Sumai 3 (resistant check) and Mianyang 8545 (susceptible check). Disease symptoms only occurred on one or two spikelets in the translocation lines, whereas the symptom occurred on half spike in susceptible parent Chinese Spring and susceptible check Mianyang 8545
Discussion
Irradiation (Sears 1956), induced-homeoelogous pairing (Riley et al. 1968) and Gc-induced chromosome breakage (Endo 1988, 1994) are effective methods for intergenomic transfer of genes in wheat. These methods involve manipulation of cytologically detectable chromosomal segments and have been described as ‘chromosome engineering’ (Sears 1972) in contrast to ‘genetic engineering’ which involves asexual transfer of a single or a few genes by transformation (via Agrobacterium or biolostics). For genetic engineering, the cloning of the target gene is the first important step, nearly an impossible task especially for alien species. Therefore, we have initiated transfers of scab resistance from perennial Triticeae species using all three methods of chromosome engineering. Of the three methods, induced homoeologous pairing is the method of choice, because it involves exchanges between homoeologous segments and, thus, genetic transfers are compensating and more likely to be agronomically desirable. Both irradiation and Gc-induced chromosome breakage and reunion events are random and are more likely to be non-compensating and agronomically undesirable. However, if the size of the transferred segment is small then such transfers may be agronomically useful. Moreover, these methods allow transfer of a target gene on the alien segment to a wheat chromosome, which can be a starting material for further manipulation by induced homoeologous pairing. So far, we have no results of any transfers using induced-pairing, but we have obtained successful transfers of alien segments carrying at least three different scab resistance genes into wheat using irradiation and Gc gene. These translocation lines will provide much needed new sources of scab resistance for wheat breeding.
For irradiation, both dry seeds and spikes at meiosis or pollen stage can be treated (Sears 1993). In the present research, the frequency of translocations through irradiation of adult plants at meiosis or in spikes before pollination was much higher than that of irradiation of dry seeds (Liu et al. 1999, 2000a). Although an irradiation treatment at meiosis has the advantage that chromosome translocations can occur in both male and female gametes, treating spikes just before anthesis is much easier. Monosomic addition lines were irradiated and used as male parents in crosses with susceptible varieties. The plants of the M1 progeny with 2n=42 chromosomes and good scab resistance were selected for further screening of translocations by chromosome C-banding and GISH. Because a monosomic alien chromosome will tend to be lost during meiosis and a male gametophyte (n+1) with a complete alien chromosome is at a disadvantage, meiosis and pollen competition favour preferential transmission of gametes with translocations.
Gametocidal chromosomes can induce chromosome breakage and rejoining in gametes without the Gc gene during first division of pollen mitosis (Nasuda et al. 1998). The breakage occurs at random in both the alien and the wheat chromosomes resulting in non-compensating translocation and deletions (Endo 1988, 1994). Similar results were also observed in the present study and all of the translocations obtained were non-compensating. Seed set is an important index for genetic compensation and cytological stability and has been used to identify several lines with good fertility.
Scab resistance in wheat is controlled by several major and some minor genes and is highly affected by environment conditions. A similar situation also was observed in the transfer of scab resistance from alien species to common wheat. At least three chromosomes were involved in the scab resistance of L.racemosus (Chen et al. 1993, 1995). Furthermore, we can now map these resistance genes to smaller chromosomal segments. The resistance level of the addition, substitution and translocation lines with only a single chromosome or chromosome segment was generally lower than that of alien parent. Translocation lines involving 5Lr#1(Lr.14), 7Lr#1(Lr.2) or Lr.7 were more resistant than susceptible parents but less resistant than their alien parent L. racemosus. Different levels of scab resistance also were observed frequently in the progenies that involved the same alien chromosome translocation as well as those involving the same alien chromosome addition, possibly related to the other components of resistance in the genetic background. In order to develop lines with a high level of scab resistance, intercrosses between the translocation lines involving different alien chromosomes carrying scab resistance, and crosses between different alien translocation lines and wheat varieties with resistant components were adopted. Successive screening and molecular-marker assisted selection have been used to pyramid scab resistance genes and have shown good potential.
Wild species have both good and poor characteristics for wheat improvement, and undesirable alien genes are also introduced along with the resistance gene. To reduce the amount of alien chromatin, two approaches should be followed: First emphasis should be on developing resistant translocations with a minimum alien chromatin. Next translocations with the smallest alien segment should be used for additional rounds of transfer by homoeologous pairing. Molecular markers should be used to screen recombinants that have been already identified for the chromosomes. Second, conduct a vigorous backcrossing program using elite cultivars or strains as recurrent parents under high selection pressure. Anecdotal evidence suggests that spontaneous recombination can occur especially as most resistant genes are located at telomeric ends and such rare recombinants can be selected under high selection pressure.
References
Chen PD, Wang ZT, Wang SL, Huang L, Wang YZ, Liu DJ (1993) Transfer of scab resistance from Elymus giganteus into common wheat. In: Li ZS, Xin ZY (eds) Proceedings of the 8th international wheat genetics symposium. China Agricultural Scientech Press, Beijing 1:153–157
Chen PD, Wang ZT, Wang SL, Huang L, Wang YZ, Liu DJ (1995) Transfer of useful germplasm from Leymus racemosus Lam. to common wheat. III. Development of addition lines with wheat scab resistance (in Chinese with English abstract). Acta Genet Sin 22:206–210
Chen PD, Sun WX, Liu WX, Yuan JH, Liu ZH, Wang SL, Liu DJ (1998) Development of wheat- Leymus racemosus translocation lines with scab resistance. In: Slinkard AE (ed) Proceedings of the 9th international wheat genetics symposium. University Extension Press, Saskatoon, Canada 2:32–34
Endo TR (1988) Induction of chromosomal structural changes by a chromosome of Aegilops cylindrica in common wheat. J Hered 79:366–370
Endo TR (1994) Structural changes of rye chromosome 1R induced by gametocidal chromosome. Jpn J Genet 69:11–19
Gill BS, Friebe B, Endo TR (1991) Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat. Genome 34:830–839
Jiang JM, Gill BS (1994) Different species-specific chromosome translocations in Triticum timopheevii and T. turgidum support the diphyletic origin of polyploid wheat. Chromosome Res 2:59–64
Liu DJ (2002) Genome analysis in wheat breeding for disease resistance. Acta Bot Sin 44:1096–1104
Liu WX, Chen PD, Liu DJ (1999) Development of Triticum aestivum Leymus racemosustranslocation lines by irradiating adult plants at meiosis (in Chinese with English abstract). Acta Bot Sin 41(5):463–467
Liu WX, Chen PD, Liu DJ (2000a) Studies of the development of Triticum aestivum Leymus racemosustranslocation lines by pollen irradiation (in Chinese with English abstract). Acta Genet Sin 27:40–49
Liu WX, Chen PD, Liu DJ (2000b) Selection, breeding and identification of T01- T.aestivum-L.racemosus translocation line (in Chinese with English abstract). Acta Agron Sin 26:305–309
Mujeeb-Kazi A, Bernard M, Bekele GT, Mirand JL (1983) Incorporation of alien genetic information from Elymus giganteus into Triticum aestivum. In: Sakamoto S (ed) Proceedings of the 6th international wheat genetics symposium. Plant germ-plasm Institute, Kyoto, Japan pp 223–231
Nasuda S, Friebe B, Gill BS (1998) Gametocidal genes induce chromosome breakage in the interphase prior to the first mitotic cell division of the male gametophyte in wheat. Genetics 149:1115–1124
Qi LL, Wang SL, Chen PD, Liu DJ, Friebe B, Gill BS (1997) Molecular cytogenetic analysis of Leymus racemosus chromosomes added to wheat. Theor Appl Genet 95:1084–1091
Riley R, Chapman V, Johnson R (1968) Introduction of yellow rust resistance of Aegilops comosa into wheat by genetically induced homoeologous recombination. Nature 217:383–384
Sears ER (1956) The transfer of leaf rust resistance from Aegilops umbellulata to wheat. Brookhaven Symp Biol 9:1–21
Sears ER (1972) Chromosome engineering in wheat. Stadler Symp 4:23–38
Sears ER (1993) Use of radiation to transfer alien segments to wheat. Crop Sci 33:897–901
Sharp PJ, Chao S, Desai S and Gale MD (1989) The isolation, characterization and application in the Triticeae of a set of wheat RFLP probes identifying each homoeologous chromosome arm. Theor Appl Genet 78:342–348
Wang XE, Chen PD, Zhou B, Yuan JH, Liu WX, Gill BS, Liu DJ (2001) RFLP analysis of wheat- L. racemosus translocation lines (in Chinese with English abstract). Acta Genet Sin 28:1142–1150
Wang YN, Chen PD, Liu DJ (1986) Transfer of useful germplasm from Elymus giganteus L. to common wheat. I. Production of (T.aestivum cv. Chinese Spring- Elymus giganteus) F1 (in Chinese with English abstract). J Nanjing Agric Univ 9:10–14
Wang YZ, Yang XN, Xiao CP (1982) The improvement of identification technique for wheat scab resistance and development of resistant sources (in Chinese). Sci Agric Sin 5:67–77
Wen YQ, Liu DJ (1989) Morphology, scab resistance and cytogenetics of intergeneric hybrids of Triticum aestivum L. with Roegneria C. Koch (Agropyron) species (in Chinese with English abstract). Scentia Agric Sin 22:1–7
Yang BJ, Dou QW, Liu WX, Zhou B, Chen PD (2002) Development of Triticum aestivum-Leymus racemosus translocation lines NAU601 and NAU618 and their test-cross analysis with double ditelosomic (in Chinese with English abstract). Acta Genet Sin 29:350–354
Yuan JH, Chen PD, Liu DJ (2003) Development of Triticum aestivum Leymus racemosus translocation lines using gametocidal chromosomes. Science in China 46:522–532
Zhang P, Friebe B, Gill BS (2002) Variation in the distribution of a genome-specific DNA sequence on the chromosomes reveals evolutionary relationships in the Triticum and Aegilops complex. Plant Syst Evol 235:169–179
Acknowledgements
We thank Dr. Endo, Kyoto University, Japan, for providing seeds of wheat- Ae.cylindrica addition line; the Jiangsu Academy of Agricultural Sciences for irradiation treatment and supplying the pathogen isolates of Fusarium graminearum; Jianyang Institute of Agricultural Sciences, Fujian province for identification of the scab resistance; and John Raupp and Duane Wilson for research assistance. The project was supported by the National Natural Sciences of China, the Jiangsu Natural Science Foundation, Education Ministry of China and McKnight Foundation (USA) CCRP grant to Nanjing Agricultural University and Kansas State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Möllers
Rights and permissions
About this article
Cite this article
Chen, P., Liu, W., Yuan, J. et al. Development and characterization of wheat- Leymus racemosus translocation lines with resistance to Fusarium Head Blight. Theor Appl Genet 111, 941–948 (2005). https://doi.org/10.1007/s00122-005-0026-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-0026-z