Abstract
In the pathosystem of turnip mosaic virus (TuMV) and Arabidopsis thaliana, two distinct symptoms (mosaic symptom and veinal necrosis) were observed that were dependent upon the combination of the TuMV isolate and the Arabidopsis ecotype. The Col-0 ecotype developed mosaic symptoms after infection with the TuMV isolate Azu while the Ler ecotype developed veinal necrosis after infection with the same TuMV isolate. The Ler phenotype is controlled by a single dominant gene TuNI (TuMV necrosis inducer) which is located on chromosome 1. The TuNI gene was precisely mapped to the ~105 kb interval between the two markers of mXF41 and mRF28 by using several types of DNA polymorphism markers. Within this region, which included largely duplicated sequences, a total of 19 putative genes were predicted and 15 of these were classified into five gene families. The genes belonging to the gene families At1g58480 and At1g58602 may function in response to infection by pathogens. The gene family At1g58480 encodes lipase-like proteins, which might be involved in the induction of defence responses that are mediated by salicylic acid. The gene family At1g58602 encodes the CC-NBS-LRR (CNL) proteins, which are known to function as one of the plant resistance (R) proteins against pathogens. In the present study, the possibility that TuNI might function as an R gene was discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Turnip mosaic virus (TuMV), a member of the genus Potyvirus, is one of the most important viruses in the world that infect field-grown vegetables (Tomlinson 1987). The host range of TuMV covers at least 318 species found within over 43 dicot families, including Brassicaceae, Asteraceae, Chenopodiaceae, Fabaceae and Caryophyllaceae (Walsh and Jenner 2002). TuMV particularly causes damage in horticultural and arable Brassica crops in Asia, North America and Europe (Walsh and Jenner 2002). Infection of the plants by TuMV results in various symptoms such as the typical leaf mosaic with stunting (mosaic) and the occurrence of necrosis along leaf veins (veinal necrosis). In most cases, veinal necrosis results in more severe damage than the mosaic symptoms. Although genetic improvement of susceptible crops is the most effective method to control TuMV symptoms, the mechanism for symptom induction in the pathosystem of TuMV and the Brassica genus is not fully understood.
In the pathosystems of some members of Potyvirus and their host plants, the helper component-proteinase (HC-Pro) and the P3 protein have been identified as the symptom determinants (Gal-On 2000; Saenz et al. 2000; Redondo et al. 2001; Saenz et al. 2001; Jenner et al. 2003; Suehiro et al. 2004). These viral factors are usually associated with the severity of the symptoms displayed by host plants. On the other hand, the NIb replicase in potato virus Y (PVY) was reported to be the factor that elicited veinal necrosis in Nicotiana tabacum (Fellers et al. 2002). A single dominant gene, which may be identical to a root knot nematode (RKN) resistance gene, Rk, controls the type of symptoms displayed by hosts (either veinal necrosis or mosaic symptoms) (Rufty et al. 1983a). In the pathosystem of PVY and N. tabacum, the type of symptoms is determined by the gene-for-gene relationships between the virus and the host plant (Fellers et al. 2002).
Arabidopsis thaliana, a weed-like member of the family Brassicaceae, is a dicot model plant that possesses many advantages for genetic and host-pathogen interaction studies (Dangl 1993). Since Arabidopsis reveals conserved chromosomal synteny to other Brassica species (Lan et al. 2000), we consider the pathosystem of TuMV and Arabidopsis to serve as a model system for studying the interactions between TuMV and Brassica species. Martin-Martin et al. (1999) classified 106 Arabidopsis ecotypes into three groups according to the response to the isolate UK1 of TuMV. Four ecotypes classified as group III showed extreme resistance to the isolate UK1. The remaining 102 ecotypes classified as either group I or II were susceptible to the isolate and these ecotypes developed similar symptoms including plant stunting, developmental arrest, leaf mosaic, sawed and curly leaves, and aborted flowers. No susceptible ecotypes showed necrotic lesions. On the other hand, we found that TuMV-infected Arabidopsis developed two distinct symptom patterns (mosaic and veinal necrosis) depending on the particular combinations of Arabidopsis ecotypes and TuMV isolates. Veinal necrosis was systemically displayed in the infected plants but not concurrent with mosaic symptoms. In order to understand the genetic basis for the development of symptoms in this pathosystem, we identified a 105 kb interval where the genetic locus controlling the type of symptoms induced by TuMV infection resides.
Materials and methods
Plant materials
Seeds from nine Arabidopsis ecotypes were purchased from Lehle Seeds (Tucson, Ariz., USA) (Table 1). Bayreuth (Bay-0) seeds were obtained from the Sendai Arabidopsis Seed Stock Center (SASSC) (Miyagi University of Education, Japan). For genetic analysis, F1, F2 and B1F1 plants that were derived from a cross between Columbia (Col-0) and Landsberg erecta (Ler) ecotypes, were produced. Col-0 was used as a recurrent parent in the backcrossing to produce the B1F1 plants. One hundred recombinant inbred lines (RILs) derived from the cross between Col-0 and Ler (Lister and Dean 1993) were obtained from the Nottingham Arabidopsis Stock Center (NASC) and were used as a mapping population. For the precise mapping of TuNI, an additional set of 476 F2 plants resulting from the cross between C24 and Ler was produced and used to generate additional RILs.
TuMV isolates and inoculation tests
Four TuMV isolates (Azu, TuR1, TuC and C42J) were used for the inoculation tests. TuMV-Azu was isolated from Chinese cabbage (Brassica rapa spp. pekinensis) in Tochigi prefecture, Japan and has been maintained at the Hokkaido University, Japan for more than 10 years. TuMV-TuR1 and -TuC were derived from the cDNA clones of the original isolates Tu-2R1 and Tu-3, respectively (Suehiro et al. 2004). TuMV isolate Tu-2R1 was isolated from Japanese radish (Raphanus sativus) and Tu-3 was from cabbage (B. oleracea var. capitata) in Tochigi prefecture (Suehiro et al. 2004). TuMV-C42J isolated from turnip (B. rapa spp. rapifera) in Saga prefecture, Japan, was a kind gift donated by Dr. K. Oshima (Saga University, Japan). Plants were grown in growth rooms under constant light (3,000 lx) and constant temperature (21°C). TuMV isolates were maintained in the susceptible hosts Col-0 or a turnip cultivar Yuki-Hime. Leaves from approximately 2-week-old plants were dusted with carborundum and rub-inoculated with the leaf sap. Infected plants were scored for virus symptoms 2 weeks after inoculation.
Hammer blotting
Hammer blots were prepared as previously described by Takahashi et al. (2001). Two weeks after inoculation the entire plant was pressed onto a filter paper (Advantec No. 2) with a hammer. The tissue print blots were incubated with an anti-TuMV primary antibody and subsequently incubated with a goat anti-rabbit immunoglobulin alkaline phosphatase conjugate (Bio-Rad, Cailif., USA). Color reactions were developed in 15 ml of the substrate solution (100 mM Tris-Cl pH 9.5, 100 mM NaCl and 5 mM MgCl2) containing 100 μl of 50 mg of nitro blue tetrazolium (NBT) per ml of 70% dimethylformamide and 50 μl of 25 mg of 5-bromo-4-chloro-3-indolyl phosphate (BCIP) per ml of dimethylformamide.
DNA polymorphism markers
Total DNA was extracted from green leaves by the conventional cetyltrimetylammonium bromide (CTAB) method. For precise mapping of TuNI, cleaved amplified polymorphic sequence (CAPS), derived cleaved amplified polymorphic sequence (dCAPS), simple sequence length polymorphism (SSLP), single nucleotide polymorphism (SNP) and insertion/deletion (indel) markers were developed. These sequences were based on the available genome sequences of Col-0 (GenBank accession NC_003070). Table 2 lists the primer sequences, the amplification conditions and the restriction enzymes that were used for the development of CAPS and dCAPS markers. The amplified segments, with or without cleavage by restriction enzymes, were analyzed on a 4% agarose gel. The amplified segment for the SNP marker was directly sequenced using an ABI PRISM 310 genetic analyzer that was operated under standard conditions.
Results
When infected with TuMV-Azu, two Arabidopsis ecotypes, Col-0 and Ler, showed different symptoms (Fig. 1a). The Col-0 plants infected with TuMV-Azu showed developmental arrest, leaf mosaic and aborted flowers. However, no systemic necrosis was observed in these plants. On the other hand, systemic veinal necrosis, which eventually led to plant death, was observed in the infected Ler plants. Virus distribution was also different in the two ecotypes (Fig. 1b). In the Col-0 plants, TuMV was detected throughout the entire plant, however in the Ler plants, it was only detected in the vascular tissues (Fig. 1b). These two symptom patterns induced by TuMV-Azu were reproducible and distinct. Moreover, it was evident that the symptoms of all ten ecotypes used in this study were classified as either veinal necrosis (N) or mosaic symptoms (M) when these plants were inoculated with four different TuMV isolates (Azu, TuR1, TuC and C42J) (Table 1). The symptom types appeared to be specifically determined, and dependent upon the combination of the Arabidopsis ecotype and the TuMV isolate, thereby suggesting that symptom determinants exist in both the host plant and the virus. The ten Arabidopsis ecotypes were classified into three groups (Col-0, Ler and Bay-0) according to their respective response patterns to the four viral isolates.
a Symptoms induced by TuMV isolate Azu in Arabidopsis thaliana ecotypes Col-0 and Ler 2 weeks after inoculation. A typical mosaic symptom was observed in Col-0, while Ler showed veinal necrosis. b Distribution of TuMV in the TuMV-Azu infected plants of Col-0 and Ler 2 weeks after inoculation. TuMV spread into the entire Col-0 plant. However, TuMV was only detected in the vascular tissues in the Ler plant.
In order to investigate the inheritance of the symptom determinant, F1, F2 and B1F1 plants derived from a cross between Col-0 and Ler were inoculated with TuMV-Azu. Since all 17 F1 plants showed the same N type for Ler, the N type was likely dominant over the M type. In the F2 and B1F1 populations, the observed ratios of N type to M type fitted the theoretical ratios of 3:1 and 1:1, respectively. These data indicated that a single dominant gene conditioned the development of the N type symptom (Table 3). The locus that regulates symptom type was named TuNI (TuMV Necrosis Inducer).
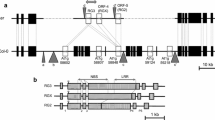
To determine the chromosomal location of TuNI, the mapping population, which consisted of 100 recombinant inbreds that were derived from a cross between Col-0 and Ler, was inoculated with TuMV Azu. This mapping population was obtained from the NASC. In this study eight new DNA polymorphism markers were developed for more precise mapping of TuNI. The results of the linkage analysis indicated that TuNI was located between the two markers mT8L23 and mF23H11 on chromosome 1 (Fig. 2a). For more precise mapping of TuNI, 476 F2 plants from a cross between Col-0 and Ler were developed, and the F2 plants possessing a recombinant chromosome segment in the interval between the two markers were selected from this population. The selected F2 plants were subsequently advanced to the F4 generation and four individual F4 plants (RIL85, RIL168, RIL224, RIL335) were finally selected for precise mapping of TuNI. Comparison of these recombinant genotypes with the six markers in the interval between mT8L23 and mF23H11 showed that the TuNI locus was located in the ~105 kb genomic region from mXF41 to mRF28 (Fig. 2b). According to the annotation data of Arabidopsis chromosome 1 in GenBank (accession no. NC_003070) and a more detailed report by Meyers et al. (2003), a total of 19 genes are predicted to reside in this genomic region. Most of this region consists of two nearly identical repeats of an approximately 36 kb genomic segment and a 10 kb imperfect repeat. As a result, 15 of the predicted genes actually exist as multiple copies in this region (Kato et al. 1999; Meyers et al. 2003). The 15 putative genes were classified into five gene families (Table 4). The gene families At1g58480, At1g58602 and At1g58684 were predicted to encode a GDSL-motif lipase, the disease resistance protein (CNL) and the 40S ribosomal protein S2, respectively. The two gene families At1g58643 and At1g58766 were predicted to encode hypothetical proteins with unknown functions. The single genes At1g58450, At1g58470, At1g58520 and At1g58460 were predicted to encode a peptidyl-prolyl cis-trans isomerase FKBP-type family protein, an RNA binding protein, an early-response to dehydration (ERD) protein and an expressed protein with an unknown function, respectively.
Genetic and physical mapping of the TuNI locus. a Linkage relationships between TuNI and four DNA polymorphism markers on chromosome 1. The number of recombinants between the markers is indicated by parentheses. b Graphical genotypes of key recombinants defining the genomic region containing TuNI for the DNA polymorphism marker loci. Additional DNA polymorphism markers between mT8L23 and mF23H11 are located on the physical map. The phenotype of each recombinant is indicated on the right side of the figure.
Discussion
In this study, we have shown that the type of symptoms in Arabidopsis plants infected by TuMV were determined by the specific interaction between the host plant ecotype and the virus strain. Systemic veinal necrosis was controlled by a single dominant gene, TuNI, that was mapped to a 105 kb interval defined by the markers mXF41 and mRF28. This region has been previously described to include the CNL protein gene clusters (Meyers et al. 2003). The CNL protein contains a nucleotide-binding site (NBS), leucine-rich-repeat (LRR) domains and an N-terminal coiled-coil (CC) motif and is thought to function in pathogen recognition (Dangl and Jones 2001). According to the sequence analysis by Meyers et al. (2003), this region consists of some large duplicated sequences. Specifically, 15 out of 19 potential candidate genes were actually found to exist as multiple copies and have been classified into five putative gene families; including CNL protein gene clusters. In Arabidopsis, at least 51 genes belonging to this CNL group have been identified and were classified into four subgroups based upon the position of introns and sequence conservation (Meyers et al. 2003). All five CNL R genes cosegregating with TuNI were classified into the CNL-D subgroup. At the present time, we do not have enough evidence to show the relationships between the R genes and the necrotic symptoms shown by TuMV-infected Arabidopsis. However, transgenic tobacco plants which contain the mutant alleles of N, a well known R gene against tobacco mosaic virus (TMV) showed incomplete resistance, with systemic necrosis when inoculated with TMV (Dinesh-Kumar and Baker 2000; Dinesh-Kumar et al. 2000). Considering its ability to induce systemic necrosis, it is conceivable that TuNI is a candidate for the R gene. It is also possible that lipase-like protein genes in the TuNI region may be a putative necrosis inducer; lipase-like proteins were found to be required for the induction of defence responses mediated by salicylic acid (SA) (Falk et al. 1999; Jirage et al. 1999; Kumar and Klessig 2003). The PRLIP genes encoding lipase-like proteins are tandemly clustered on chromosome 5 in Arabidopsis (Jakab et al. 2003), and it has been shown that the expression of some members in this gene family is induced by the resistance-inducer β-aminobutylic acid, SA, methyl jasmonate, ethylene or pathogens (Jakab et al. 2003).
The systemic lethal necrosis induced by TuNI apparently causes more severe damage than mosaic symptoms. Although the induction of lethal necrosis by TuNI may decrease the fitness of Arabidopsis populations, why has such a gene evolved? TuNI is actually widely distributed in natural populations of Arabidopsis and has even differentiated, giving rise to specificity for TuMV isolates among the different ecotypes. One explanation is that restriction of the virus in veinal tissues followed by plant death could reduce the viral spread through the population more efficiently than mosaic symptoms alone. Since rapid plant death following viral infection could minimize the chance of transmission of the virus by aphids, lethal necrosis could have a positive impact on the host population fitness through a negative impact on the virus. This is likely especially when the TuNI allele is dominant in the host population, and the inoculum level is consistently low. Alternatively, it is possible that TuNI may possess a pleiotropic effect that is dependent upon different isolates and pathogens. For instance, a systemic veinal necrosis has been reported in tobacco plants that were infected with PVY MSNR . This systemic response was found to be controlled by a single gene which was tightly linked to a RKN resistance gene, Rk, in tobacco (Rufty et al. 1983a). Since no recombinants were detected between these two traits in a genetic analysis which used more than 15,000 F2 progeny (Rufty et al. 1983a), it was suggested that these two phenotypes resulted from pleiotropic effects of a single gene. In addition, RKN resistance and development of necrosis by PVY MSNR infection showed the same temperature sensitivity and plant age specificity (Rufty et al. 1983b). Another example has been described for soybean Rsv genes. In soybean, at least three Rsv loci conditioning resistance to soybean mosaic virus (SMV) have been identified and multiple alleles were subsequently differentiated at the Rsv1 locus (Ma et al. 2003). At this locus, the most dominant alleles were reported to induce either resistance or systemic necrosis to the seven SMV strains while a recessive allele at the Rsv1 locus showed mosaic symptoms in response to infection with the same SMV strains (Ma et al. 2003). In this pathosystem, both the responses of resistance and systemic necrosis are thought to be triggered by the same gene (Chen et al. 1994). In the TuMV/Arabidopsis pathosystem it has been reported that several ecotypes including Bay-0 showed extreme resistance to the isolate UK-1 (Martin-Martin et al. 1999). The Bay-0 resistance was found to be due to an interference with viral cell-to-cell movement (Martin-Martin et al. 1999). Because the inheritance of this resistance in Bay-0 was not known, the resistance gene(s) must be identified to discuss the relationship with TuNI.
In the pathosystems of TuMV and the Brassica genus, some resistance genes have been reported but they have not yet been isolated (Walsh and Jenner 2002). The viral avirulence (avr) genes to TuRB01 and TuRB05 is the CI gene (Jenner et al. 2000, 2002), while TuRB03 and TuRB04 seem to interact with the P3 gene (Jenner et al. 2002, 2003). When B. napus was infected by some virulent TuMV isolates, it is important to note that systemic necrosis, as observed in TuMV-infected Arabidopsis, was induced in the lines possessing TuRB01 or TuRB03 (Jenner et al. 2000, 2003). Interestingly, we also found that the viral pathogenicity determinant responsible for the Arabidopsis symptoms (mosaic or veinal necrosis) occurred within the region containing the P3 and CI genes of TuMV using chimeric constructs between TuMV-TuR1 and -TuC (data not shown). With our current data, we cannot confirm whether the putative avr gene to TuNI is the P3 or CI gene or both. Future comparative studies of TuNI and TuRB genes may enable us to understand the relationships between the resistance response and the systemic necrosis against TuMV infection.
In this study, we demonstrated that TuNI was located within a region containing the cluster of R genes in the Arabidopsis genome. However, the multiple repeated sequences within this region complicate our efforts to more precisely map the TuNI gene within this region. Complementation tests for TuNI which use BAC clones to cover the entire locus are now underway and this will enable us to determine which gene(s) are involved in the induction of systemic veinal necrosis in Arabidopsis.
References
Chen P, Buss GR, Roane CW, Tolin SA (1994) Inheritance in soybean of resistant and necrotic reactions to soybean mosaic virus strains. Crop Sci 34:414–422
Dangl JL (1993) Applications of Arabidopsis thaliana to outstanding issues in plant–pathogen interactions. Int Rev Cytol 144:53–83
Dangl JL, Jones JDG (2001) Plant pathogens and integrated defense responses to infection. Nature 411:826–833
Dinesh-Kumar SP, Baker BJ (2000) Alternatively spliced N resistance gene transcripts: their possible role in tobacco mosaic virus resistance. Proc Natl Acad Sci USA 97:1908–1913
Dinesh-Kumar SP, Tham WH, Baker BJ (2000) Structure-function analysis of the tobacco mosaic virus resistance gene N. Proc Natl Acad Sci USA 97:14789–14794
Falk A, Feys BJ, Frost LN, Jones JDG, Daniels MJ, Parker JE (1999) EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci USA 96:3292–3297
Fellers JP, Tremblay D, Handest MF, Lommel SA (2002) The potato virus Y MSNR NIb-replicase is the elicitor of a veinal necrosis-hypersensitive response in root knot nematode resistant tobacco. Mol Plant Pathol 3:145–152
Gal-On A (2000) A point mutation in the FRNK motif of the Potyvirus helper component-protease gene alters symptom expression in cucurbits and elicits protection against the severe homologous virus. Phytopathology 90:467–473
Jakab G, Manrique A, Zimmerli L, Metraux JP, Mauch-Mani B (2003) Molecular characterization of a novel lipase-like pathogen-inducible gene family of Arabidopsis. Plant Physiol 132:2230–2239
Jenner CE, Sanchez F, Nettleship SB, Foster GD, Ponz F, Walsh JA (2000) The cylindrical inclusion gene of turnip mosaic virus encodes a pathogenic determinant to the Brassica resistance gene TuRB01. Mol Plant Microbe Interact 13:1102–1108
Jenner CE, Tomimura K, Ohshima K, Hughes SL, Walsh JA (2002) Mutations in turnip mosaic virus P3 and cylindrical inclusion proteins are separately required to overcome two Brassica napus resistance genes. Virology 300:50–59
Jenner CE, Wang X, Tomimura K, Ohshima K, Ponz F, Walsh JA (2003) The dual role of the Potyvirus P3 protein of turnip mosaic virus as a symptom and avirulence determinant in Brassicas. Mol Plant-Microbe Interact 16:777–784
Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM, Glazebrook J (1999) Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc Natl Acad Sci USA 96:13583–13588
Kato A, Suzuki M, Kuwahara A, Ooe H, Higano-Inaba K, Komeda Y (1999) Isolation and analysis of cDNA within a 300 kb Arabidopsis thaliana genomic region located around the 100 map unit of chromosome 1. Gene 239:309–316
Kumar D, Klessig DF (2003) High-affinity salicylic acid-binding protein 2 is required for plant innate immunity and has salicylic acid-stimulated lipase activity. Proc Natl Acad Sci USA 26:16101–16106
Lan TH, DelMonte TA, Reischmann KP, Hyman J, Kowalski SP, McFerson J, Kresovich S, Paterson AH (2000) An EST-enriched comparative map of Brassica oleracea and Arabidopsis thaliana. Genome Res 10:776–788
Lister C, Dean C (1993) Recombinant inbred lines for mapping RFLP and phenotypic markers in Arabidopsis thaliana. Plant J 4:745–750
Ma G, Chen P, Buss GR, Tolin SA (2003) Genetic study of a lethal necrosis to soybean mosaic virus in PI 507389 soybean. J Hered 94:205–211
Martin-Martin A, Cabrera y Poch HL, Martinez-Herrera D, Ponz F (1999) Resistance to turnip mosaic Potyvirus in Arabidopsis thaliana. Mol Plant Microbe Interact 12:1016–1021
Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15:809–834
Redondo E, Krause-Sakate R, Yang SJ, Lot H, Gall OL, Candresse T (2001) Lettuce mosaic virus pathogenicity determinants in susceptible and tolerant lettuce cultivars map to different regions of the viral genome. Mol Plant Microbe Interact 14:804–810
Rufty RC, Wernsman EA, Powell NT (1983a) A genetic analysis of the association between resistance to Meloidogyne incognita and a necrotic response to infection by a strain of potato virus Y in tobacco. Phytopathology 73:1413–1418
Rufty RC, Powell NT, Gooding GV Jr (1983b) Relationship between resistance to Meloidogyne incognita and a necrotic response to infection by a strain of potato virus Y in tobacco. Phytopathology 73:1418–1423
Saenz P, Cervera MT, Dallot S, Quiot L, Quiot JB, Riechmann JL, Garcia JA (2000) Identification of a pathogenicity determinant of plum pox virus in the sequence encoding the C-terminal region of protein P3+6K1. J Gen Virol 81:557–566
Saenz P, Quiot L, Quiot JB, Candresse T, Garcia JA (2001) Pathogenicity determinants in the complex virus population of a plum pox virus isolate. Mol Plant Microbe Interact 14:278–287
Suehiro N, Natsuaki T, Watanabe T, Okuda S (2004) An important determinant of the ability of turnip mosaic virus to infect Brassica spp. and/or Raphanus sativus is in its P3 protein. J Gen Virol 85:2087–2098
Takahashi H, Suzuki M, Natsuaki K, Shigyo T, Hino K, Teraoka T, Hosokawa D, Ehara Y (2001) Mapping the virus and host genes involved in the resistance response in cucumber mosaic virus-infected Arabidopsis thaliana. Plant Cell Physiol 42:340–347
Tomlinson JA (1987) Epidemiology and control of virus diseases of vegetables. Ann Appl Biol 110:661–681
Walsh JA, Jenner CE (2002) Turnip mosaic virus and the quest for durable resistance. Mol Plant Pathol 3:289–300
Acknowledgements
We thank Dr. Kazusato Oshima (Saga University, Japan) for providing us with a TuMV isolate and Dr. Hideki Takahashi (Tohoku University, Japan) for helping us handle Arabidopsis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Charcosset
Y.K. and T.I. contributed equally to this study
Rights and permissions
About this article
Cite this article
Kaneko, Yh., Inukai, T., Suehiro, N. et al. Fine genetic mapping of the TuNI locus causing systemic veinal necrosis by turnip mosaic virus infection in Arabidopsis thaliana. Theor Appl Genet 110, 33–40 (2004). https://doi.org/10.1007/s00122-004-1824-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-004-1824-4