Abstract
Predators are traditionally classified as generalists and specialists based on the presence of adaptations that increase efficiency of prey capture and consumption and selection of particular prey types. Nevertheless, empirical evidence comparing foraging efficiency between generalist and specialist carnivores is scarce. We compared the prey-capture and feeding efficiency in a generalist and a specialist (araneophagous) spider predator. By using two related species, the generalist Harpactea rubicunda (Dysderidae) and the specialist Nops cf. variabilis (Caponiidae), we evaluated their fundamental trophic niche by studying the acceptance of different prey. Then, we compared their predatory behavior, efficiency in capturing prey of varying sizes, feeding efficiency, and nutrient extraction. Nops accepted only spiders as prey, while Harpactea accepted all offered prey, confirming that Nops is stenophagous, while Harpactea is euryphagous. Further, Nops displayed more specialized (stereotyped) capture behavior than Harpactea, suggesting that Nops is a specialist, while Harpactea is a generalist. The specialist immobilized prey faster, overcame much larger prey, and gained more mass (due to feeding on larger prey) than the generalist. Both the specialist and the generalist spider extracted more proteins than lipids, but the extraction of macronutrients in the specialist was achieved mainly by consuming the prosoma of the focal prey. We show that the specialist has more efficient foraging strategy than the generalist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food acquisition is fundamental for animals as it directly influences their fitness (Stephens and Krebs 1986; Breed and Moore 2015). Predictions about food choice in predators have long been based on the optimal diet theory (ODT) (e.g., Pyke et al. 1977). Most studies related to ODT have focused on generalist or facultative specialist predators; thus, evidence on the optimal prey of obligatory specialist predators is lacking.
As a consequence of possessing specialized adaptation, capture and feeding efficiency on the focal prey should be higher in obligatory specialist predators when compared to generalist predators (Futuyma and Moreno 1988). Prey-capture strategies in obligatory specialist predators have been shown to be highly stereotyped, yet very efficient towards focal prey (Lauder 1983; Pekár 2004; Řezáč et al. 2008). Predatory specialization is also reflected in adaptations used by predators in prey handling and nutrient extraction (Pekár and Toft 2015). Thus, energy and nutrient optimization are likely achieved differently in specialists and generalists (Futuyma and Moreno 1988).
Energy intake is optimized by selecting and feeding on a prey of a certain size (e.g., Elner and Hughes 1978; Molles and Pietruszka 1987). Specialist arthropod predators were found to catch relatively larger prey (e.g., Bulbert et al. 2014; Pekár et al. 2014). A comparative study on crabs showed that specialist crabs captured larger mussels when compared to generalist (Yamada and Boulding 1998). This suggests that optimal prey is larger for specialists than for generalists. If a single prey is considered as a patch, then specialists are expected to remain in the patch for a longer time (i.e., exploit it more) because the search for a new patch takes longer than in generalists (Heller 1980). Indeed, a specialist spider fed for a longer time than a generalist (Michálek et al. 2017).
Nutrient extraction strategies also seem to vary between specialists and generalists. For example, the specialist herbivore selected food of a lower protein content and retained nitrogen more efficiently than generalists (Raubenheimer and Simpson 2003). Predators, however, are not so limited in proteins as herbivores (Mayntz et al. 2009); therefore, they should balance intake of all macronutrients. While generalist predators can balance nutrient intake by consuming different kinds of nutritionally complementary prey (Kohl et al. 2015), specialists are expected to feed on a prey that closely approximates the required balance of macronutrients (Raubenheimer and Simpson 2003) due to limited ability to compensate for nutrient imbalance (Lee et al. 2003). The nutrient balancing in specialists might be achieved by consuming more efficiently different body parts of the focal prey (Pekár et al. 2010).
Spiders are considered the most diverse group of terrestrial true predators (Pekár et al. 2017). The huge diversity of this group is reflected not only in the high number of species but also in the wide variety of prey-capture strategies used as well as in the variety of trophic specializations exhibited (Cardoso et al. 2011). Pekár and Toft (2015) showed that spiders specialize in at least six arthropod groups, namely ants, dipterans, isopods, moths, spiders, and termites. Among these, myrmecophagous, termitophagous, and araneophagous spiders have specialized on dangerous prey and have evolved a variety of specialized adaptations. Spiders are also considered as dangerous prey since they possess venom and silk which might turn the possible predator into prey (Jackson and Hallas 1986; Whitehouse 1987; Wignall and Taylor 2009); therefore, an araneophagous specialist should possess highly specialized adaptations for feeding on spiders (Jackson and Hallas 1986; Cerveira and Jackson 2005; Harland and Jackson 2006; Pekár et al. 2011).
Our aim in this study was to compare energy and nutrient extraction in a stenophagous specialist araneophagous spider and a related euryphagous generalist species. We used two spider species for which anecdotal observations suggested to be specialist and a generalist. Therefore, we first investigated their trophic niche, and predatory behavior, to reveal whether they are stenophagous/euryphagous and specialist/generalist. We hypothesized that the specialist would use highly stereotyped strategy, capture larger prey, and exploit it more than the generalist as predicted by the theory. With respect to nutrient extraction, we expected that the specialist would exploit prey thoroughly in order to obtain specific nutrients.
Material and methods
Study species
As a putative specialist spider, we used Nops cf. variabilis (Caponiidae), further named Nops. This Neotropical genus includes medium-sized wandering spiders that live in litter and search for prey during the night (Sánchez-Ruiz 2004). Our preliminary field observations revealed Nops to prey on other spiders from the families Lycosidae, Dipluridae, and Oecobiidae (García, unpublished). Ideally, a generalist from the same family should be used for a comparison. However, very little is known about the trophic ecology of these very rare spiders. Therefore, we used a putative generalist spider, Harpactea rubicunda (C. L. Koch) further named Harpactea, from a phylogenetically closely related family Dysderidae (Wheeler et al. 2017). Although a few other spider families are more related to Caponiids, these either include specialists or use a very different prey-capture strategy (a web) than Nops. Harpactea is a species widely distributed across Europe (World Spider Catalog 2016). It is a wandering species living in litter as is Nops. The diet of this species has not yet been rigorously studied, but unpublished observations suggest it captures various arthropods (M. Řezáč, pers. comm.).
Juvenile and subadult individuals of Nops were collected in the city of Neiva, Colombia (2° 59′ 55″ N, 75° 18′ 16″ W), and juvenile and adult individuals of Harpactea were collected in Brno, Czech Republic (2° 59′ 55″ N, 75° 18′ 16″ W). The spiders were housed individually in tubes (60 mm long, 10 mm in diameter). The experiments were performed in the Czech Republic.
Prey acceptance
One week before starting the experiments, the spiders were fed to satiation using different prey. In the case of Nops, we used small spiders (Araneidae, Araniella sp.) as prey, while in the case of Harpactea, we used small crickets (Acheta domestica). We used different prey for each species because many Harpactea individuals rejected spiders as prey and no Nops accepted other prey than spiders. All individuals were fed with the selected prey to satiation. Offered prey represented approximately the same length as a spider prosoma. Afterwards, spiders were deprived of food during 1 week after satiation in order to standardize hunger.
After starvation period, we performed prey acceptance experiments with both species in order to determine the breadth of their fundamental trophic niche. We selected six prey types related to those found sympatrically with both species (Table 1). The prey was offered to 29 individuals of Nops (mean prosoma size ± SE = 1.54 ± 0.08 mm) and to 20 individuals of Harpactea (3.48 ± 0.21 mm). The body size of each spider was obtained by measuring its prosoma length under a stereomicroscope with an ocular ruler. Spiders were placed individually into Petri dishes (diameter 6.5 cm) 1 day before the start of the experiment to acclimatize. A prey was released into the dish and attack, capture, and consumption were recorded. If after 30 min the spider had not captured the prey, it was replaced by another one until one was accepted. If a prey was accepted, the spider was left to consume it, and another trial took place 5 days later. This procedure was repeated until all prey types were offered to all spider individuals in a complete block design. Prey were offered randomly to avoid any effect of order offering and only once to each spider. Acceptance was measured as binary scoring (consumption or rejection).
The breadth of the fundamental trophic niche for each species was based on the proportion of each prey type captured. It was estimated by using the standardized Levin’s index (B A ), using the following formula:
where pj is the proportion of individuals which consume the jth prey, and n is the total number of prey types offered. Values of B A vary between 0, when the niche breadth is minimal, and 1, when the species does not discriminate among prey types (Krebs 1999).
Predatory behavior
To reveal whether the species used similar behaviors to catch prey, we analyzed the predatory behavior of Nops and Harpactea using spiders as prey. Another 24 juvenile and subadult individuals of Nops and another 22 adult and juvenile individuals of Harpactea were starved for a period of 1 week after being fed to satiation following the same procedure as in the previous section. Afterwards, spiders were fed with juvenile individuals of Pardosa cf. agrestis (the relative prey/predator body size was 0.54–2.16 in Harpactea, and 1.37–2.62 in Nops). We selected this prey type as it was accepted by both species.
Spider predators were placed singly into a Petri dish (diameter 4.5 cm) and prey was released 5 min later. In the case of Harpactea, the hunting sequence was recorded with a Canon Legria HFG10 camera. In the case of Nops, due to its extremely fast attack behavior, prey capture was recorded using a MotionXtra N3 high-speed camera, with a speed of 500 fps. The complete predatory sequence from the first capture attempt until the prey was grasped was recorded.
We identified behaviors (Table 2) used in the prey capture which were common to both species. Afterwards, we generated predatory sequences from the observed behaviors and transition probabilities by means of the TramineR package (Gabadinho et al. 2011). From the predatory sequence for each species, we estimated each species’ entropy index (H′) (Lehner 1996), which evaluates the sequence complexity by measuring the diversity of behavioral events (Gabadinho et al. 2011), using the following formula:
where a is the total number of behaviors and p i is the proportion of occurrences of the ith behavior in the considered sequence. To compare the entropy index between both species, 95% confidence intervals were estimated by means of bootstrap with 1000 replications.
Capture efficiency
During observations of capture behavior, we recorded the paralysis latency as the interval between the first bite and the complete immobilization of prey. We also recorded the size of prey. Paralysis latency was compared between species by generalized linear model (GLM) with Gamma distribution (GLM-g) and logarithmic link due to heteroscedasticity (Pekár and Brabec 2016), with prey/predator size ratio as a covariate.
To compare efficiency in the capture of size-varying prey, we offered spiders of the genus Pardosa of various body sizes to another 39 individuals of Nops and another 25 individuals of Harpactea (so that the relative size was 1.50–9.50 in Harpactea, and 1.20–16.84 in Nops). The body size of prey was estimated as follows: a picture of each prey was taken using a Canon Legria HFG10 camera and the size was measured using ImageJ 1.46r software (Schneider et al. 2012).
The experimental procedure was similar to that used in the acceptance experiments (see above). Spiders were starved for 1 week, then placed in a Petri dish (diameter 4.5 cm), and the prey was released. We compared the relationship between capture (recorded in a binary way) and the predator–prey size ratio (the ratio of the predator’s prosoma length and the prey’s total body size) between Nops and Harpactea. We used only prosoma and not the whole body of the predator because this is a constant measure unlike the length of abdomen, which is changing with the state of satiation (Anderson 1974). We used a generalized linear model with a binomial distribution (GLM-b) to compare capture efficiency between the two predator species, with prey/predator size ratio as a covariate.
Prey consumption
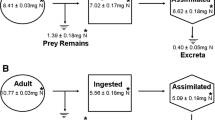
We compared the feeding time and the mode of feeding between Nops and Harpactea as follows. Before the experiments, another 20 individuals of Nops and another 16 individuals of Harpactea were starved for 1 week after satiation. Predators were released singly into the Petri dish, and wolf spiders (Pardosa cf. agrestis) of different sizes (the relative prey predator size was 0.48–1.31 for Harpactea, and 1.43–3.68 for Nops) were offered as prey. Once prey were captured, we recorded every 15 min which body part the spider was feeding on, i.e., the prosoma (including legs) or abdomen. Afterwards all observations on one individual were summed per individual to avoid repeated measurements.
We compared the total feeding time and the percentage of the total time feeding spent on each body part of the prey. Feeding time was compared by using a GLM with Gamma distribution (GLM-g) and logarithmic link. Percentages of feeding time on different body parts were compared using linear models (two-way ANOVA) following arcsine transformation to approach a normal distribution and homogenize variance.
We also calculated the mass gained after feeding as the difference between the initial and final weights of each spider, i.e., the weights before and after feeding. The mass was measured using a KERN 770 balance with a precision of 0.01 mg. Feeding efficiency was expressed as the mass gain percent divided by the total feeding time and scaled by the body size ratio. Feeding efficiency was compared between both species by using GLM-g and logarithmic link due to heteroscedasticity.
Macronutrient extraction
We analyzed the macronutrient (lipid and protein) composition of each juvenile Pardosa individual separately for abdomen and prosoma using 10 freshly killed individuals. To do this, body parts were dried at 60 °C, and the dry mass was measured to the nearest gram 10−5. Afterwards, samples were submerged in chloroform, centrifuged for 5 min at 500 rpm at room temperature, and dried again for 6 h. This procedure was repeated twice. The lipid content was then estimated as the difference in dry mass measured by KERN balance before and after extraction (Hawley et al. 2014). For protein extraction, which followed afterwards, we used the protocol for PRO-PREP™ (Intron Biotechnology), a protein extraction detergent. In this method, the dry sample was mixed and homogenized with the detergent by centrifuging it every 2 min for 20 min (seven times), at 500 rpm and room temperature. Afterwards, the sample was centrifuged at 12,000 rpm for 5 min. The mixture supernatant was removed and the tissue was dried for 6 h and weighed again. The mass difference between the initial and final weightings reflected the protein content. Cuticle remains were excluded from the analyses since these do not dissolve and are not consumed by the spiders.
To compare the amounts of nutrients extracted from each Pardosa individual between spider species, we followed the procedure for analyzing body parts as above, comparing the remains of prey consumed by Nops and Harpactea. Nops consumed prey by sucking liquefied tissue so that intact prey carcass was left, whereas Harpactea crushed prey and a bundle of remains was left. Comparison of the nutrients (proteins and lipids), in the prosoma and the abdomen as well as of nutrient extraction for both species, was made using a linear model on arcsine-transformed data (two-way ANOVA).
All statistical analyses were made within the R environment (R Core Team, 2016).
Results
Prey acceptance
We observed the acceptance of all prey types by Harpactea, with the greatest frequency of consumption for termites and flies. In contrast, Nops captured only spiders and never attempted to attack any other prey type offered (Fig. 1). In all recorded cases, the attacked prey was also consumed.
The standardized Levin’s index was much wider for Harpactea (B A = 0.93) than for Nops (B A = 0). These results indicate that Harpactea is euryphagous, while Nops is stenophagous consuming only one prey type.
Predatory behavior
We recognized five behaviors during prey capture of Pardosa (Table 2). The main strategy in Nops consisted of approaching the prey and afterwards pouncing on it, followed by grasping and biting (Fig. 2, Video S1). All spiders bit the prey’s prosoma during capture. Although the same pattern was recorded in Harpactea, the sequence had more transitions between “approach,” “wait,” and “grasp,” since the prey could escape and Harpactea needed more than one attempt to capture it. When comparing predatory sequences, these were significantly more complex in Harpactea than in Nops (Fig. 3): the entropy for Nops was 0.65 (CI95: 0.16, 1.24), while for Harpactea it was 3.92 (CI95: 2.86, 6.27). The results suggest that Nops is a specialist, while Harpactea is a generalist.
Capture efficiency
With respect to paralysis latency, we found significant interaction between spider species and the prey/predator ratio (GLM-g, F1,41 = 19.18, P < 0.0001, Fig. 4). In Harpactea, the latency increased with the prey/predator size ratio (t = 6.02, P < 0.0001), while in Nops the size had no effect (t = − 0.16, P = 0.87).
We found a significant difference between the two species in the capture efficiency with differently sized Pardosa prey (GLM-b, X12 = 8.69, P = 0.0029). In both species, the capture efficiency decreased with increasing prey/predator size ratio but at different rates: in Harpactea, 50% acceptance probability was observed for prey with a body length about 1.5 times the size of the spider’s prosoma, while in Nops 50% acceptance probability was observed for prey with a body length about three times the size of the spider’s prosoma (Fig. 5).
Prey consumption
Total feeding times on Pardosa were significantly different between species (GLM-g, F1,34 = 94.30, P < 0.0001): Nops fed for a longer time when compared to Harpactea (Fig. 6). However, when taking into account the size of prey, the feeding efficiency expressed as mass percent gain per minute was not significantly different between both species (GLM-g, F1,34 = 0.002, P = 0.98), with an average of 0.75%/min.
In terms of the proportion of feeding time, the interaction between the body part and the species was significant (ANOVA, F1,68 = 52.7, P < 0.0001, Fig. 7): Nops fed on the prosoma for a significantly higher proportion of the time than on the abdomen (contrast, t = 9.56, P < 0.0001), whereas Harpactea fed evenly on the two body parts (contrast, t = − 0.93, P = 0.35).
Macronutrient extraction
The body parts of Pardosa differed significantly in their nutritional composition (ANOVA, F1,48 = 16.48, P < 0.0001, Fig. 8). The percentage of proteins was significantly higher when compared to lipids in both the prosoma (contrast, t = − 19.59, P < 0.0001) and abdomen (contrast, t = 13.85, P < 0.0001). The percentage of lipids was significantly higher in the abdomen than in prosoma (contrast, t = − 2.87, P = 0.006).
The trends of nutrients extracted showed no significant difference between species (ANOVA, F1,36 = 1.51, P = 0.22, Fig. 9), suggesting both species extract nutrients similarly. When comparing nutrient extraction for both species simultaneously, we found that extraction for proteins was higher than that for lipids in both species (ANOVA, F1,36 = 124.81, P < 0.0001).
Discussion
Results on prey acceptance indicate that Nops has a more limited diet than Harpactea and is consistent with our hypothesis that Nops is a stenophagous predator and Harpactea is a euryphagous predator. Unlike other araneophagous spiders that feed also on other prey than spiders (Harland and Jackson 2006; Pekár et al. 2011), Nops captured only spiders and thus had a very narrow diet breadth. Although all prey offered to Nops was novel, it readily accepted only spiders. Thus, a novelty of prey should be of limited effect on their acceptance. All prey types were palatable and accepted by the euryphagous Harpactea; thus, these prey types should be accepted by Nops if considered as a suitable prey. The absence of even attacks on these prey types suggests that Nops did not recognize them as prey—presumably its senses are fine-tuned to a specific prey similarly to other highly specialized predators (e.g., Pekár 2004; Petráková et al. 2015).
The results of predatory behavior experiments confirmed Nops as a specialist and Harpactea as a generalist. We found that the predatory sequence observed in Nops was highly stereotyped, compared to that of Harpactea. Although some specialist predators show versatile predatory strategies (e.g., Harland and Jackson 2006), stereotyped strategies are most likely the result of a strict specialization: Nops is an obligatory araneophage. Similar stereotyped prey-capture strategies have been recorded in other highly specialized spiders that prey exclusively on a single prey type (Pekár 2004; Petráková et al. 2015). In specialized predatory fishes, display of stereotyped predatory strategies is explained as a consequence of morphological constraints which limits the use of alternative predatory strategies (Lauder 1983; Sanderson 1991).
We found that the specialist spider captured markedly larger prey than the generalist. A similar trend was observed in crabs as a consequence of specialized claw morphology (Yamada and Boulding 1998) and in an araneophagous gnaphosid spider, which possesses adhesive hairs for holding the prey and a thickened cuticle to minimize injury by prey (Michálek et al. 2017). The higher efficiency of Nops in the capture of spiders was also reflected in shorter paralysis latency. While this parameter increased with prey size in Harpactea, suggesting that larger spiders are more difficult to paralyze and may require more venom to be injected (Wigger et al. 2002), it was almost constant for Nops suggesting it has a more powerful venom to paralyze its prey or alternatively that it is able to adjust the dose of venom (according to the size of prey) more efficiently than Harpactea. Similar results for paralysis time were observed for termite specialists (Petráková et al. 2015) or ant-eating spiders (e.g., Pekár et al. 2014). Short immobilization times have been reported in animals which specialize on capturing spiders, suggesting the presence of specific venom to paralyze the prey (Pekár et al. 2014; Pekár and Toft 2015; Konno et al. 2016). Since spiders are dangerous prey, we expect there to be a selection for quick paralysis to prevent counter-attack (Mukherjee and Heithaus 2013).
The feeding efficiency measured as extraction rate was found to be similar for both species. But in absolute terms, i.e., the total feeding time, the specialist fed for a longer time than generalist simply because the prey of the former was larger. To our knowledge, empirical evidence supporting the hypothesis of a higher feeding efficiency in specialist predators is scarce. It has been observed in slug-specialist snakes in which assimilation and energetic efficiencies were higher when compared to generalist counterparts (Britt et al. 2006).
We hypothesized that the capture of a large prey in the specialist might be linked to the exploitation of specific macronutrients from certain body parts, as has been shown in some specialist spiders (Pekár et al. 2010). Indeed, Nops exploited more prosoma than abdomen, while Harpactea fed similarly on both parts. Both Nops and Harpactea extracted two macronutrients (proteins and lipids) in similar proportions; the difference must have been due to content of other nutrients (e.g., micronutrients) which we did not measure. An alternative hypothesis would be that Nops would be able to extract nutrients stored in the abdomen via pedicel and prosoma. Since both the prosoma and abdomen showed similar macronutrient compositions, the marked consumption of the former structure in Nops could be related to the presence of specific substances in this body part (Toft et al. 2010), or it could be an adaptation to avoid consumption of some noxious substances, which are typically stored in the abdomen (Michálek et al. 2017). As we observed and measured the consumption of nutrients only once, we do not know how the nutritional target is achieved in this specialist. This remains to be investigated in the future.
A study comparing nutrient extraction in specialist and generalist herbivores showed that the generalist selected more proteins than carbohydrates when compared to the specialist (Raubenheimer and Simpson 2003). The specialist herbivore selected food of lower protein content, which is in direct contrast to our results. Overall, the generalist herbivore showed greater behavioral and physiological flexibility than the specialist in response to nutrient imbalance.
An obvious cost of specialization is decreasing ability (or incapability) to capture alternative prey (Pekár and Toft 2015). This may have serious consequences on the fitness of specialists if the focal prey becomes rare or even absent. It should also be explored if Nops prefers certain spider guilds or families, as it has been shown in other specialist spiders (e.g., Harland and Jackson 2006; Haddad et al. 2016).
In this study, we show how various parameters of energy and nutrient extraction differ between one generalist and one specialist predators. In a similar and parallel study with two other spider species (Michálek et al. 2017), very similar results were obtained. Specifically, stereotypy, higher capture efficiency, shorter paralysis latency, and longer feeding time were observed in the specialist. But both species had similar feeding efficiency. Thus, it seems that observed differences between specialist and generalist are universal. Yet, to confirm this, it is necessary to conduct a similar study using more pairs, and predators specialized on other prey types, such as ants, termites, or woodlice.
Our results confirm that specialist’s efficiency in capturing its focal prey is greater than that of the generalist; the specialist achieved greater level of efficiency by displaying stereotyped prey-capture behavior, and probably using more potent venom.
References
Anderson JF (1974) Responses to starvation in the spiders Lycosa lenta Hentz and Filistata hibernalis (Hentz). Ecology 55:576–585
Breed MD, Moore J (2015) Animal behavior. Academic Press, San Diego
Britt EJ, Hicks JW, Bennett AF (2006) The energetic consequences of dietary specialization in populations of the garter snake, Thamnophis elegans. J Exp Biol 209:3164–3169
Bulbert MW, Herberstein ME, Cassis G (2014) Assassin bug requires dangerous ant prey to bite first. Curr Biol 24:R220–R221
Cardoso P, Pekár S, Jocqué R, Coddington JA (2011) Global patterns of guild composition and functional diversity of spiders. PLoS One 6:e21710
Cerveira A, Jackson RR (2005) Specialised predation by Palpimanus sp. (Araneae: Palpimanidae) on jumping spiders (Araneae: Salticidae). J East African Nat Hist 94(2):303–317
Elner RW, Hughes RN (1978) Energy maximization in the diet of the shore crab, Carcinus maenas. J Anim Ecol 47:103–116
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Gabadinho A, Ritschard G, Müller NS, Studer M (2011) Analyzing and visualizing state sequences in R with TraMineR. J Stat Softw 40(4):1–37
Haddad CR, Brabec M, Pekár S, Fourie R (2016) Seasonal population dynamics of a specialized termite-eating spider (Araneae: Ammoxenidae) and its prey (Isoptera: Hodotermitidae). Pedobiologia 59:105–110
Harland DP, Jackson RR (2006) A knife in the back: use of prey-specific attack tactics by araneophagic jumping spiders (Araneae: Salticidae). J Zool 269:285–290
Hawley J, Simpson SJ, Wilder SM (2014) Effects of prey macronutrient content on body composition and nutrient intake in a web-building spider. PLoS One 9:e99165
Heller R (1980) On optimal diet in a patchy environment. Theor Popul Biol 17:201–214
Jackson RR, Hallas SEA (1986) Comparative biology of Portia africana, P. albimana, P. fimbriata, P. labiata, and P. schultzi, araneophagic, web-building jumping spiders ( Araneae : Salticidae ): utilisation of webs, predatory versatility, and intraspecific interactions. N Z J Zool 13:423–489
Kohl KD, Coogan SCP, Raubenheimer D (2015) Do wild carnivores forage for prey or for nutrients? Evidence for nutrient-specific foraging in vertebrate predators. BioEssays 37:701–709
Konno K, Kazuma K, Nihei K (2016) Peptide toxins in solitary wasp venoms. Toxins 8:114
Krebs CJ (1999) Ecological methodology, 2nd edn. Addison-Wesley Educational Publishers, Menlo Park
Lauder GV (1983) Functional and morphological bases of trophic specialization in sunfishes (Teleostei, Centrarchidae). J Morphol 178:1–21
Lee KP, Raubenheimer D, Behmer ST, Simpson SJ (2003) A correlation between macronutrient balancing and insect host-plant range: evidence from the specialist caterpillar Spodoptera exempta (Walker). J Insect Physiol 49:1161–1171
Lehner P (1996) Handbook of ethological methods, 2nd edn. Cambridge University Press, Cambridge
Mayntz D, Nielsen VH, Raubenheimer D, Hejlesen C (2009) Balancing of protein and lipid intake by a mammalian carnivore, the mink, Mustela vison. Anim Behav 77:349–355
Michálek O, Petráková L, Pekár S (2017) Capture efficiency and trophic adaptations of a specialist and generalist predator: a comparison. Ecol Evol 7(8):2756–2766
Molles MC Jr, Pietruszka RD (1987) Prey selection by a stonefly: the influence of hunger and prey size. Oecologia 72:473–478
Mukherjee S, Heithaus MR (2013) Dangerous prey and daring predators: a review. Biol Rev 88:550–563
Pekár S (2004) Predatory behavior of two European ant-eating spiders (Araneae, Zodariidae). J Arachnol 32:31–34
Pekár S, Brabec M (2016) Modern analysis of biological data: generalized linear models in R. Masaryk University Press, Brno
Pekár S, Toft S (2015) Trophic specialisation in a predatory group: the case of prey-specialised spiders (Araneae). Biol Rev 90:744–761
Pekár S, Mayntz D, Ribeiro T, Herberstein ME (2010) Specialist ant-eating spiders selectively feed on different body parts to balance nutrient intake. Anim Behav 79:1301–1306
Pekár S, Šobotník J, Lubin Y (2011) Armoured spiderman: morphological and behavioural adaptations of a specialised araneophagous predator (Araneae: Palpimanidae). Naturwissenschaften 98:593–603
Pekár S, Šedo O, Líznarová E, Korenko S, Zdráhal Z (2014) David and Goliath: potent venom of an ant-eating spider (Araneae) enables capture of a giant prey. Naturwissenschaften 101:533–540
Pekár S, García LF, Viera C (2017) Trophic niches and trophic adaptations of prey-specialized spiders from the Neotropics: a guide. In: Viera C, Gonzaga MO (eds) Behaviour and ecology of spiders: contributions from the Neotropical region. Springer, Cham, pp 247–274
Petráková L, Líznarová E, Pekár S, Haddad CR, Sentenská L, Symondson WOC (2015) Discovery of a monophagous true predator, a specialist termite-eating spider (Araneae: Ammoxenidae). Sci Rep 5:14013
Pyke GH, Pulliam HR, Charnov E (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52:137–154
Raubenheimer D, Simpson SJ (2003) Nutrient balancing in grasshoppers: behavioural and physiological correlates of dietary breadth. J Exp Biol 206:1669–1681
Řezáč M, Pekár S, Lubin Y (2008) How oniscophagous spiders overcome woodlouse armour. J Zool 275:64–71
Sánchez-Ruiz A (2004) Current taxonomic status of the family Caponiidae (Arachnida, Araneae) in Cuba with the description of two new species. Rev Iber Aracnol 9:95–102
Sanderson SL (1991) Functional stereotypy and feeding performance correlated in a trophic specialist. Funct Ecol 5:795–803
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton
Toft S, Daiquin L, Mayntz D (2010) A specialized araneophagic predator’s short-term nutrient utilization depends on the macronutrient content of prey rather than on prey taxonomic affiliation. Physiol Entomol 35:317–327
Wheeler WC, Coddington JA, Crowley LM et al (2017) The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics 33:574–616
Whitehouse MEA (1987) “Spider eat spider”: the predatory behavior of Rhomphaea sp. from New Zealand. J Arachnol 15:357–364
Wigger E, Kuhn-Nentwig L, Nentwig W (2002) The venom optimisation hypothesis: a spider injects large venom quantities only into difficult prey types. Toxicon 40(6):749–752
Wignall A, Taylor P (2009) Alternative predatory tactics of an araneophagic assassin bug (Stenolemus bituberus). Acta Ethol 12:23–27
Yamada S, Boulding E (1998) Claw morphology, prey size selection and foraging efficiency in generalist and specialist shell-breaking crabs. J Exp Mar Bio Ecol 220:191–211
World Spider Catalog (2016) v17.5. http://wsc.nmbe.ch. Accessed 26 October 2016
Acknowledgments
We thank Juan Valenzuela, Julio González and Martín Santana for their help with specimen collection. We are also grateful to Milan Řezáč for information on the trophic niche of Harpactea and Ondřej Michálek and Radek Michalko for their collaboration during the development of the project.
Funding
The study was supported by PEDECIBA, grant 8880 of the Uruguayan Agency for Research and Innovation (ANII), and by the Czech Science Foundation (GA15-14762S).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by: Matjaž Gregorič
Electronic supplementary material
Video S1
Prey capture sequence of Nops catching Pardosa. The video was taken by high-speed camera with the frame-rate 500fps. (MP4 8685 kb)
Rights and permissions
About this article
Cite this article
García, L.F., Viera, C. & Pekár, S. Comparison of the capture efficiency, prey processing, and nutrient extraction in a generalist and a specialist spider predator. Sci Nat 105, 30 (2018). https://doi.org/10.1007/s00114-018-1555-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-018-1555-z