Abstract
Males should be more selective when they have a high investment in reproduction, especially in species with biparental or paternal care. In this context, male mate choice can promote size-assortative mating (SAM) when (1) large males win intrasexual disputes, (2) large females are more fecund, and (3) males prefer larger females to smaller ones. In the spider Manogea porracea, males exhibit high reproductive investment by building their webs above those of females and exhibiting extended care of offspring in the absence of females. Under these circumstances, we expect the occurrence of SAM and male preference for large females. Herein, we performed observations and experiments in the field to evaluate the hypotheses that (1) M. porracea mates assortatively by size and (2) SAM is influenced by male mate choice. Furthermore, we measured variables that could affect mating patterns, the sex ratios, and densities of both sexes. Pairing in M. porracea was positively size-assortative in 2012, but not in 2013. Large males won most disputes for mates and preferred larger females, which produced more eggs. The inconsistency in detection of SAM was due to population dynamics, namely variations in sex ratio and population density across the breeding season. Furthermore, we found that the significance of male mate choice on sexual selection of body size in M. porracea strongly depends on the competition intensity for mating opportunities. The traditional sexual selection hypothesis of SAM needs to be reviewed and must include measures of competition intensity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assortative mating is a pattern of pairings based on phenotypic (or genotypic) similarity or dissimilarity between males and females (Burley 1983; Ridley 1983). It can be homotypic, when there is a positive correlation between one or more traits of males and females, or heterotypic, when this correlation is negative (Burley 1983). These patterns have been documented in several taxa, including invertebrate (e.g., annelids: Michiels et al. 2001; arthropods: Harari et al. 1999; Masumoto 1999; mollusks: Erlandsson and Rolán-Alvarez 1998) and vertebrate taxa (e.g., amphibians: Gutiérrez and Lüddecke 2002; birds: Harris and Siefferman 2014; fishes: Taborsky et al. 2014), among others (see references in Jiang et al. 2013). Assortative mating may depend on various traits, such as color (Bortolotti et al. 2008, Gade et al. 2016), parasite load (Thomas et al. 1999), age (Cèzilly et al. 1997), behavior (Kralj-Fišer et al. 2013), and size (Bel-Venner et al. 2008). Most documented cases of assortative mating in animals (47% of 360 species reviewed by Jiang et al. 2013) are those characterized by correlations between body sizes of mating pairs (size-assortative mating (SAM)).
Assortative mating is often used to explain patterns of nonrandom mating and sympatric speciation for premating isolation (Johannesson et al. 1995; Seehausen et al. 1997; Jiang et al. 2013). Indeed, SAM may promote speciation associated with ecological or behavioral constraints (Kirkpatrick and Ravigne 2002; Ritchie 2007), such as the ecological specialization in sympatric morphs of threespine sticklebacks (Gasterosteus aculeatus) with different body sizes (Ólafsdóttir et al. 2006) or paternal care in pregnant male seahorses (Jones et al. 2003). Most studies have concluded that species mate assortatively by size considering a single measure of SAM during a breeding season (Jiang et al. 2013 and references therein). However, to be an effective driver of speciation, assortative mating needs to be consistent during and between breeding seasons (see Kondrashov and Shpak 1998). It is important to include data collected in distinct breeding seasons in the analysis because some studies have already detected spatial and temporal changes in SAM and suggested that variation in population parameters may be the cause (Dodson and Marshall 1984; Crespi 1989b). Other empirical and theoretical studies indicated, for example, that sex ratio and density might affect SAM (McLain 1982; McLain and Boromisa 1987a, b; Crespi 1989a, b; Kokko and Rankin 2006).
Other hypotheses may explain the occurrence of SAM in animals (see Crespi 1989a; Härdling and Kokko 2005), such as (i) mating availability—when male and female sizes are correlated in space (e.g., Johannesson et al. 1995) and time (e.g., Miyashita 1994); (ii) mating constraints—when there are physical or physiological constraints during the breeding courtship or copulation caused by mate body size (e.g., Robertson 1990; Brown 1993); (iii) mate choice—when larger individuals are more successful in intrasexual competition for larger partners and/or where one or both sexes prefer to copulate with larger partners, because they likely acquire advantages (e.g., Masumoto 1999; Hoefler 2007; Bel-Venner et al. 2008); and (iv) prudent habitat choice—when competition for high-quality sites in heterogeneous environments is costly and large individuals have competitive advantages over smaller ones, larger individuals monopolize high-quality sites and subdue smaller individuals to low-quality sites (Taborsky et al. 2014). The mate choice hypothesis is usually tested when reproductive costs are unbalanced between sexes, there is no apparent difference in mate availability in space or time, no mating constraints, and habitat is apparently homogeneous.
Reproductive investment of each sex is often positively correlated with the increase in choosiness (Kokko and Johnstone 2002; Parker 2006; Hoefler 2007; Edward and Chapman 2011). Therefore, the sex that invests more in reproduction may have an important role in mate choice and affect SAM (e.g., Hoefler 2007). Females are usually the selective sex because of their high investment in each egg, the production of a limited number of eggs, and parental investment (Anderson 1994; Clutton-Brock 2007). However, males also have high costs with production of millions of spermatozoa, the ejaculation of secretions produced in accessory glands, production of nutritive substances donated to females during or prior to copulation, intrasexual and/or intersexual competition, and in some cases, parental investment (Johnstone et al. 1996; Kvarnemo and Simmons 1998; Kokko and Johnstone 2002; Wong and Jennions 2003; Parker 2006; Hoefler 2007; Edward and Chapman 2011). Therefore, males would also be selective and promote SAM when (i) the advantage of large males in intrasexual competition is associated with preference for large females and (ii) the size of females is positively correlated with their fecundity (Ridley 1983; Crespi 1989a; Hoefler 2007; Edward and Chapman 2011). In arthropods, female fecundity is often correlated with body size (Honěk 1993). In addition, large males usually have the advantage in competition (Rittschof 2010; Vieira and Peixoto 2013) and, consequently, they may have preferential access to large females. Furthermore, small males may avoid the costs of intrasexual competition by choosing small, but defendable females (Venner et al. 2010 and references therein). This scenario favors SAM by male mate choice.
Female spiders can exert pre- and postcopulatory mate choice (see Eberhard 1996; Prenter et al. 2006). However, female choice is constrained by the number of males that are attracted to her web (Christenson 1984). Therefore, male mate choice may have a significant role in mate assortment of spiders. Thus, as we discussed above, males would be choosier than females when the reproductive investment is high. In the spider Manogea porracea (C. L. Koch, 1838) (Araneidae), males have two additional costs rarely found in spiders: (1) they build webs above those of females, where they remain at least until copulation (Moura et al. 2017) and (2) they invest in extended parental care in the absence of females (i.e., amphisexual care), reducing humidity of eggs sacs, protecting them against predation and rebuilding damaged portions of the web (Moura et al. 2017). In support to the significance of male mate choice in M. porracea, females did not reject mating with randomly selected males in laboratory conditions (R. R. Moura, personal observation). Therefore, these features make M. porracea an appropriate model to test the male mate choice hypothesis as a possible cause of SAM and to investigate the effects of sexual selection on mating behaviors.
Eight studies have investigated assortative mating in spiders (Rubenstein 1987; Miyashita 1994; Masumoto 1999; Hoefler 2007; Bel-Venner et al. 2008; Schulte et al. 2010; Kralj-Fišer et al. 2013; Zimmer et al. 2014). Of these, six studies detected SAM and proposed possible causes, such as aggressive behavior of females in aggregations (Rubenstein 1987), intense competition among males (Bel-Venner et al. 2008), female choice (Masumoto 1999) and male choice (Hoefler 2007; Schulte et al. 2010). The causes of SAM appeared to be variable among spider species. Therefore, further studies are necessary to identify whether a pattern exists. Here, we test the hypotheses that (1) M. porracea mates assortatively by size and (2) male mate choice promotes SAM. We predicted that (1) body size of mating pairs will be positively correlated; (2a) larger males will win more disputes for females; (2b) female fecundity will be positively correlated with body mass; and (2c) larger females will attract males faster than smaller females. Furthermore, we also measured sex ratios and densities to describe the breeding season of M. porracea and evaluate their relationship with possible variation in SAM.
Materials and methods
Study area and species
We performed this study between August and October in years 2012 and 2013 in an Eucalyptus plantation in Estrela do Sul, Minas Gerais, Brazil (18° 49′ 27″ S, 47° 51′ 47″ W). The plantation is surrounded by native Cerrado vegetation. The spiders have two breeding cohorts: the longest occurs between August and December and the shorter occurs between January and April (Moura et al. 2017).
Distribution of M. porracea extends from Panama to Argentina (Levi 1997; World Spider Catalog 2017). The webs are horizontally oriented, non-viscid orb-webs under which the spider remains during most of the time. This orb structure is modified into a dome and is suspended by a three-dimensional structure of threads that confers a conical shape to the entire web (Levi 1997; Fig. 1). In Fazenda Nova Monte Carmelo, the spiders build their dome-shaped webs close to the ground, using branches of Eucalyptus sp. as support. Most males reach maturity before females (i.e., protandry). During this stage, they abandon their own webs to locate females (Moura et al. 2017). When they choose a mate, males build a dome-shaped web using threads of the female webs, above the position occupied by them (Fig. 1). This position may assure to males the opportunity to court and copulate. We did not observe furtive copulations of satellite males (Moura et al. 2017). More than one male may eventually build a dome-shaped web on the web of the same female, but at different heights. Only the males located close to the resting position of females enter in their webs to copulate. After copulation and egg laying, most females die and some males stay on their webs taking care of the offspring (Moura et al. 2017). They may remove humidity of egg sacs after raining, repair webs when they are damaged and protect offspring against predation (Moura et al. 2017).
Size-assortative mating
Jiang et al. (2013) separated structural- and size-assortative mating based in measures of body parts and the whole body, respectively. This classification may be applied to SAM in spiders because carapace width is less affected by immediate nutritional condition than other variables, such as total length, whereas body mass can also be important in SAM, because males must choose mates based on their actual condition (Foellmer and Moya-Larano 2007). However, most studies used linear dimensions of some structures of spider bodies to describe the size of individuals (Rubenstein 1987; Miyashita 1994; Masumoto 1999; Hoefler 2007; Bel-Venner et al. 2008; Schulte et al. 2010); only Zimmer et al. (2014) presented both measures for SAM, body mass and first leg length. Therefore, we measured both, body length and mass, to evaluate whether or not they have different meanings for assortative mating in M. porracea. Thus, we collected the first 46 adult couples (a couple was defined as a female and a male which constructed its web attached to hers, just above female resting position) during 3 days of August 2012 and 34 couples during 3 days of October 2013. These spiders were weighed alive in laboratory. After this procedure, they were kept in ethanol 70% and then measured. We measured carapace width and all other linear measures described hereafter using a stereomicroscope Leica 250C equipped with a Leica DFC 290 camera and a LAS Application Suite assembling Interactive Measurement Module. Our definition of couples was determined considering that (1) a male have to construct its web above the web of a female to have mating opportunities and (2) it may stay on the web after mating for a long time, because some males care for offspring until spiderlings dispersion, even after females’ death (Moura et al. 2017).
To test SAM, we evaluated the relationship between male and female size (body mass and carapace width) in both years using Pearson’s correlations. We log transformed body mass and carapace width of individuals to improve homogeneity of variances (except for carapace width of mating pairs in 2012). We also compared male and female size in both breeding seasons using a factorial analysis of variance.
Male mate choice hypothesis
To evaluate size advantages of males, we first used a natural variation in outcome of intrasexual competition. During collection of 2012, we eventually observed that two males build dome-shaped webs on the same female web. One of them assumes a dominant position, located closer to female’s dome-shaped web. This dominant position is possibly determined by disputes between males and may have consequences on mating opportunities. Therefore, we predicted that males located closest to females would be larger than those situated at peripheral positions because of intrasexual competition involving antagonistic interactions. To test this prediction, we collected and weighed 36 males, 18 from dominant and 18 from peripheral positions. The mass of males from different positions was compared using a paired t test.
To test the influence of male size on the outcomes of aggressive interactions, we collected 50 males in the field and weighed them in the laboratory. To establish a wide range of variation in mass disparity among males, we chose to pair the largest male with the smallest one, the second largest with the second smallest, and so on. Then, we marked the larger and smaller males with two different colors of Humbrol synthetic enamel paint. In the field, we selected first 25 webs occupied by one male and one female and carefully removed the males without damaging the female’s web. Following this procedure, we placed the marked males at equidistant positions from the dome-shaped web of the removed male. We recorded the males’ behaviors for 1 h thereafter. We considered the winner males the one that took over the dome-shaped web after this period. Finally, we conducted a logistic regression between the probability of the larger male winning the contest, as response variable, and body mass difference between contestants, as explanatory variable. Then, we used a chi-squared test to evaluate which males (small or large) were more successful in intrasexual disputes. We also described aggressive interactions between males during the experiments.
To test the effect of female size on fecundity parameters, we measured the carapace area of 20 females and the number and diameter of their eggs. We estimated the carapace area as an ellipse with diameters corresponding to carapace width and length. We randomly selected ten eggs from each egg sac to estimate their average diameter. To evaluate if female carapace area was correlated with the number of eggs and average egg diameter, we used linear regressions.
To determine whether larger females were preferentially selected by males, we first marked 52 webs of mating pairs and removed all the males. We collected these males and weighed them in the laboratory to compare their average body mass with that of males that arrived after the removal, occupying their original position. After that, we released all removed males in the field, approximately 20 km distant from the experimental area. During the following 3 days, we observed the webs daily and collected those females and males that were observed paired to weigh in the laboratory. At the end of the third day, we collected the remaining females and weighed them. We evaluated the probability of the female pairing with a new mate as a function of female mass using a logistic regression. We also compared the difference in body mass between the first and the second males paired with the same female using a paired t test. Secondly, we collected and weighted paired and unpaired females during the middle of the breeding season (between September and October), when all males and females were adults. To determine whether female body mass influenced male selection, we compared the body mass of 27 paired and 10 unpaired females using a t test. We conducted all experiments described in this section between August and October 2013, except the experiment of male preference, conducted during the breeding seasons of 2012 and 2013.
Sex ratios and population density
Because M. porracea males may select subadult females, we estimated some parameters with and without considering subadult females. This is important because males may prefer subadult females to ensure access to the first copulation instead of searching for adult ones and risk to confronting spermatic competition. Considering these conditions, we counted all encountered spiders (males and females; adult and subadult individuals) in an area of approximately 1000 m2. Next, we estimated (1) operational sex ratio (OSR), as the number of adult males to adult females (Emlen and Oring 1977); (2) total sex ratio, similar to OSR, but including subadult females; and (3) female pairing ratio, as the number of paired females to unpaired ones (also including subadult females). We used the last measure to estimate more precisely female availability. Even when OSR and total sex ratio are unbiased, female availability would be low if most females are already paired. Then, we measured male, female and population densities as the number of males, females and individuals encountered per m2, respectively. We included subadult females in the estimation of female density, only adult males in male density, and all individuals in population density. We evaluated all these measures between August and October in 2012. We also estimated the OSR in October 2013.
Results
Size-assortative mating in M. porracea
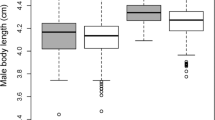
Males were smaller and lighter than females, and both sexes were larger and heavier during the reproductive season in 2012 than during 2013, except for males, which had similar body mass (Tables 1 and 2). Carapace width and body mass were correlated in males (2012: r = 0.746, df = 18, p < 0.001; 2013: r = 0.803, df = 18, p < 0.001) and females (2012: r = 0.476, df = 18, p = 0.034; 2013: r = 0.487, df = 18, p = 0.030). We found a positive SAM in 2012 (log of body mass: r = 0.386, df = 44, p = 0.008, Fig. 2a; carapace width: r = 0.538, df = 18, p = 0.014, Fig. 2b), but we did not observe the same pattern in 2013 (log of body mass: r = 0.069, df = 32, p = 0.697, Fig. 2c; log of carapace width: r = −0.054, df = 18, p = 0.820, Fig. 2d).
Male mate choice hypothesis
During disputes between males, we observed two markedly different interactions that we called of “evaluation” and “aggression.” Initially, one of the males took over the dome-shaped web of the removed male. In evaluation, the dispute began with the male who was out of the dome-shaped web pulling the silk until it decided to abandon the web or began the aggression phase. In aggression, the male entered the dome-shaped web and began aggressive interactions with the male who had initially occupied the web. That interaction involved physical contact and injuries, such as the loss of a leg (Fig. 3a). In the end of observations, the winner of the contest stayed on the dome-shaped web nearest the female and the loser stayed farthest from her or abandoned the web (Fig. 3b).
Males that established their webs closer to the female’s position had larger body mass than those located further above (paired t test: t = 3.759, df = 17, p < 0.01, Fig. 3). In the experiment in which two males were introduced, the probability of the larger male winning the dispute did not increase with increasing difference in mass between contestants (logistic regression: χ 2 = 0.001, df = 23, p = 0.97), but larger males won 72% of the contests (chi-squared test: χ 2 = 4.84, df = 1, p = 0.03).
In the test of effect of body size on fecundity, females with larger carapace area produced a greater number of eggs (linear regression: F 1,18 = 4.587, p = 0.046, R 2 = 0.45, Fig. 4), but the average diameter of eggs was similar (linear regression: F 1,18 = 0.289, p = 0.598). The carapace area was 6.45 ± 0.19 mm2, the total number of eggs was 67.15 ± 6.42, and the average egg diameter was 1.15 ± 0.02 mm2.
In the first trial of male preference, females with larger body mass formed new parings, whereas females with lower mass did not attract new males during the experiment (logistic regression: χ 2 = 9.691, df = 50, p < 0.002, Fig. 5). We did the analysis without the two outliers and found the same relationship (χ 2 = 8.059, df = 48, p = 0.004). The average mass of paired females (31.41 ± 2.14 mg) was 1.5 times higher than that of unpaired females (21.52 ± 1.83 mg). Furthermore, males with which they were paired had a similar body mass to the second males (paired t test: t = −0.043, df = 37, p = 0.966). The first males had an average body mass of 8.86 ± 0.36 mg, and for second males, it was 8.87 ± 0.38 mg. In the second trial, females naturally paired were larger than unpaired females (Mann-Whitney U test: U = 28, df = 35, p < 0.001). They had 1.4 times more body mass (36.85 ± 1.90 mg) than unpaired females (25.60 ± 1.25 mg). The magnitude of differences in female body mass was similar in both experiments.
Sex ratio and population density
In 2012, the OSR was unbiased during the breeding season, the female paring ratio increased 24.5 times from August to October and the total sex ratio also increased (3.4 times), but it was female-biased (Fig. 6). Population and female densities decreased gradually during the breeding season, whereas male density increased 2.1 times from August to October 2012 (Fig. 6). The OSR was also unbiased in October 2013 (OSR = 1.36, n = 99).
Discussion
Here, we studied M. porracea, a Neotropical orb-web spider that employs amphisexual parental care. In this system, males exhibit high reproductive investment by building their webs above those of females and taking care of offspring in the absence of mothers (Moura et al. 2017). Both behaviors are rarely found in spider species. In systems where males have a high investment in reproduction and parental care, they should be more selective than in systems without such investment (Kokko and Johnstone 2002; Parker 2006; Hoefler 2007; Edward and Chapman 2011). Therefore, we expected that M. porracea males will be choosy, preferring larger, more fecund females. The choosiness, however, may be constrained and dependent on their success in male-male competition. In intrasexual disputes, larger males often have advantages, leading to SAM (Masumoto 1999; Hoefler 2007; Bel-Venner et al. 2008). Therefore, we empirically evaluated the occurrence of assortative mating in M. porracea and its association with male mate choice. SAM occurred in the breeding season of the first year of our study (2012), but not in the second. This temporal variation in SAM were unexpected because our findings supported the male mate choice hypothesis: (1) larger males won most intrasexual disputes and preferred larger females, probably because (2) there was a positive correlation between female size and the number of eggs laid by them. We also found positive correlations between both measures that we selected to represent body size in males and females, suggesting that there is no reason to make a distinction between structural- and size-assortative mating, as previously suggested by Jiang et al. (2013).
In accordance to our results, most studies about SAM in spiders have detected advantages in intrasexual competition for large males, which are associated with a positive correlation between female size and its fecundity (Rubenstein 1987; Masumoto 1999; Hoefler 2007; Bel-Venner et al. 2008). In Z. x-notata, for example, large females were also more attractive to males (Bel-Venner et al. 2008), as in M. porracea. Thus, our findings provide support for the male mate choice hypothesis. There is evidence that male spiders may choose between females with different qualities (Hoefler 2007; Bel-Venner et al. 2008; Pruitt and Riechert 2009; Kralj-Fišer et al. 2013). However, the significance of male mate choice in sexual selection still is underestimated (see Barry and Kokko 2010). It is expected that higher investment by males in each mating and larger variation in female quality favor male mate choice. However, when female availability is low or the access to mates is sequential, and not simultaneous, males should not avoid mating opportunities with low-quality females (Edward and Chapman 2011), as observed by Barry and Kokko (2010) in the mantid Pseudomantis albofimbriata. In M. porracea, on the other hand, male investment in offspring care associated with variation in female fecundity according body size might favor male mate choice. Additionally, we found higher female density, lower female paring ratio and lower male density in the beginning of the breeding season suggesting that males had several opportunities to evaluate mates’ qualities (Fig. 6). Therefore, this scenario provides support for the hypothesis that male mate choice may drive SAM and the intensity of sexual selection on body size of M. porracea.
In addition, we detected SAM only in the 2012 breeding season. This temporal variation in occurrence of SAM between seasons must have occurred because of the period of the breeding season in which we quantified mate assortment, once we first estimated SAM in August 2012 and later in October 2013. Therefore, these differences between years may also reflect variation in population parameters affecting the strength of SAM throughout a season or between breeding seasons. Other animal species also exhibit variation in SAM, even with mate choice (Birkhead and Clarkson 1980; Miyashita 1994; Arnqvist et al. 1996; Bel-Venner et al. 2008; Mobley et al. 2014). This indicates that other processes associated with sexual selection, such as competition intensity for mating opportunities, may be involved in the establishment of SAM. The OSR, for example, could explain the probability of SAM occurrence (Arak 1983). In Z. x-notata, SAM occurred only in populations with male-biased OSR (Bel-Venner et al. 2008). The authors argued that males would be more selective when the costs of competition were higher and mating opportunities with high-quality females were scarce.
Larger increases in the bias of OSR, on the other hand, may weaken SAM. In monarch butterflies (Danaus plexippus, Nymphalidae), SAM was positive only early in the mating season (with slightly male-biased OSR), but it did not occur later in the mating season (with highly male-biased OSR) (Frey et al. 1998). However, general effects of OSR on SAM are still unclear, because OSR may not affect the probability SAM occurs in other species. For example, in the thrips Elaphrothrips tuberculatus (Hood, 1908) (Thysanoptera, Phlaeothripidae), SAM occurred only in 1 of 4 years of study, but sex ratio was slightly male-biased in all years (Crespi 1989b). Therefore, only OSR may not be a good measure to explain variation in SAM, relative to other measurements of male mating success (Klug et al. 2010, Moura and Peixoto 2013). In M. porracea, the OSR was non-biased and exhibited slight variations during the breeding season. Therefore, other sex ratios and population densities may provide better explanations for variations in SAM of M. porracea.
Female pairing ratio, total sex ratio, and male density increased during the reproductive season, while population and female density decreased (Fig. 6). In August 2012, males experienced a scenario with high availability of females (lower values of total sex ratio and higher values of female density) and weak competition intensity (lower values of female pairing ratio and male density). Because the availability of unpaired females was higher in this period, males would be more selective in choosing large potential mates because high-quality females were defensible and abundant. This situation and the advantage of large males during intrasexual contests for large females would strengthen SAM and is consistent with our findings of positive SAM in August 2012. In October 2012, however, males confronted a scenario with low availability of females (higher values of total sex ratio and lower values of female density) and strong competition intensity (higher values of female pairing ratio and male density). Therefore, males needed to move longer distances to find new females. Additionally, they might confront intense intrasexual disputes for mating opportunities and high spermatic competition. Thus, large females may become indefensible, and males would become less choosy and copulate with females irrespective of their size. SAM can be weakened or absent in these conditions, as we observed in breeding season 2013.
Most studies evaluated assortative mating at a single moment during the breeding season (e.g., Rubenstein 1987; Miyashita 1994; Masumoto 1999; Hoefler 2007; Bel-Venner et al. 2008; Kralj-Fišer et al. 2013). However, assortative mating could fluctuate in time during the breeding season (e.g., Adams et al. 1985; Arntzen 1999) and between seasons (e.g., Boag 1983; Dickerson et al. 2004), as we observed in M. porracea. These findings lead to an important question: is a single measure of SAM enough to estimate mate assortment in a population? Jiang et al. (2013) conducted a meta-analytic investigation of general patterns of assortative mating in animal populations using a single measure of assortative mating for each species. However, as we found in M. porracea, the strength of SAM may change during and/or between breeding seasons. Therefore, such approaches could over- or underestimate the strength of assortative mating.
M. porracea males exhibited an important role to the occurrence of SAM. However, this mating assortment may have between- and/or within-season variation. We provided evidences to support that this variation may depend on population parameters associated with the competition intensity for mating opportunities. Therefore, other species may also be susceptible to this variation. We highlighted that the mate choice hypothesis needs to include predictions involving competition intensity and mate availability and to consider its temporal variation for a more comprehensive understanding of SAM in animal populations.
References
Adams J, Greenwood P, Pollitt R, Yonow T (1985) Loading constraints and sexual size dimorphism in Asellus aquaticus. Behaviour 3(4):277–287
Anderson M (1994) Sexual selection. Princeton University Press, New Jersey
Arak A (1983) Male–male competition and male choice in anuran amphibians. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 181–210
Arnqvist G, Rowe L, Krupa JJ, Sih A (1996) Assortative mating by size: a meta-analysis of mating patterns in water striders. Evol Ecol 10:265–284
Arntzen JW (1999) Sexual selection and male mate choice in the common toad, Bufo bufo. Ethol Ecol Evol 11:407–414
Barry KL, Kokko H (2010) Male mate choice: why sequential choice can make its evolution difficult. Anim Behav 80:163–169
Bel-Venner MC, Dray S, Allainé D, Menu F, Venner S (2008) Unexpected male choosiness for mates in a spider. P Roy Soc Lond B Bio 275:77–82
Birkhead TR, Clarkson K (1980) Mate selection and precopulatory guarding in Gammarus pulex. Ethology 52:365–380
Boag PT (1983) The heritability of external morphology in Darwin’s ground finches (Geospiza) on Isla Daphne Major, Galapagos. Evolution 37:877–894
Bortolotti GR, González LM, Margalida A, Sánchez R, Oria J (2008) Positive assortative pairing by plumage colour in Spanish imperial eagles. Behav Process 78:100–107
Brown WD (1993) The cause of size-assortative mating in the leaf beetle Trirhabda canadensis (Coleoptera: Chrysomelidae). Behav Ecol Sociobiol 33:151–157
Burley N (1983) The meaning of assortative mating. Ethol Sociobiol 4:191–203
Cézilly F, Boy V, Tourenq CJ, Johnson AR (1997) Age-assortative pairing in the greater flamingo Phoenicopterus ruber roseus. Ibis 139:331–336
Christenson TE (1984) Alternative reproductive tactics in spiders. Am Zool 24:321–332
Clutton-Brock TH (2007) Sexual selection in males and females. Science 318:1882–1885
Crespi BJ (1989a) Causes of assortative mating in arthropods. Anim Behav 38:980–1000
Crespi BJ (1989b) Sexual selection and assortative mating in subdivided populations of the thrips Elaphrothrips tubercufatus (Insecta: Thysanoptera). Ethology 83:265–278
Dickerson BR, Willson MF, Bentzen P, Quinn TP (2004) Size-assortative mating in salmonids: negative evidence for pink salmon in natural conditions. Anim Behav 68(2):381–385
Dodson G, Marshall L (1984) Mating patterns in an ambush bug Phymata fasciata (Phymatidae). Amer Midl Nat 112:50–57
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Monographs in behavior and ecology. Princeton University Press, New Jersey
Edward DA, Chapman T (2011) The evolution and significance of male mate choice. Trends Ecol Evol 26:647–654
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Erlandsson J, Rolán-Alvarez E (1998) Sexual selection and assortative mating by size and their roles in the maintenance of a polymorphism in Swedish Littorina saxatilis populations. Hydrobiologia 378:59–69
Foellmer MW, Moya-Larano J (2007) Sexual size dimorphism in spiders: patterns and processes. In: Fairbairn DJ, Blanckenhorn WU, Szekely T (eds) Sex, size, and gender roles: evolutionary studies of sexual size dimorphism. Oxford University Press, New York, pp 71–81
Frey D, Leong KL, Peffer E, Smidt RK, Oberhauser K (1998) Mate pairing patterns of monarch butterflies (Danaus plexippus L.) at a California overwintering site. J Lep Soc 52:84–97
Gade MR, Hill M, Saporito RA (2016) Color assortative mating in a mainland population of the poison frog Oophaga pumilio. Ethology 122:1–8
Gutiérrez G, Lüddecke H (2002) Mating pattern and hatching success in a population of the Andean frog Hyla labialis. Amphibia-Reptilia 23:281–292
Harari AR, Handler AM, Landolt PJ (1999) Size assortative mating, male choice and female choice in the curculionid beetle Diaprepes abbreviatus. Anim Behav 58:1191–1200
Härdling R, Kokko H (2005) The evolution of prudent choice. Evol Ecol Res 7:697–715
Harris MR, Siefferman L (2014) Interspecific competition influences fitness benefits of assortative mating for territorial aggression in eastern bluebirds (Sialia sialis). PLoS One 9:e88668
Hoefler DC (2007) Male mate choice and size-assortative pairing in a jumping spider, Phidippus clarus. Anim Behav 73:943–954
Honěk A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66:483–492
Jiang Y, Bolnick DI, Kirkpatric M (2013) Assortative mating in animals. Am Nat 181:e125–e138
Johannesson K, Rolan-Alvarez E, Ekendahl A (1995) Incipient reproductive isolation between two sympatric morphs of the intertidal snail Littorina saxatilis. Evolution 49:1180–1190
Johnstone RA, Reynolds JD, Deutsch JC (1996) Mutual mate choice and sex differences in choosiness. Evolution 50:1382–1391
Jones AG, Moore GI, Kvarnemo C, Walker D, Avise JC (2003) Sympatric speciation as a consequence of male pregnancy in seahorses. Proc Natl Acad Sci U S A 100:6598–6603
Kirkpatrick M, Ravigné V (2002) Speciation by natural and sexual selection: models and experiments. Am Nat 159:S22–S35
Klug H, Heuschele J, Jennions MD, Kokko H (2010) The mismeasurement of sexual selection. J Evol Biol 33:447–462
Kokko H, Johnstone RA (2002) Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Philos Trans R Soc B 357:319–330
Kokko H, Rankin DJ (2006) Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philos Trans R Soc B 361:319–334
Kondrashov AS, Shpak M (1998) On the origin of species by means of assortative mating. P Roy Soc Lond B Bio 265:2273–2278
Kralj-Fišer S, Mostajo GAS, Preik O, Pekár S, Schneiderb JM (2013) Assortative mating by aggressiveness type in orb weaving spiders. Behav Ecol 24:824–831
Kvarnemo C, Simmons LW (1998) Male potential reproductive rate influences mate choice in a bushcricket. Anim Behav 55:1499–1506
Levi HW (1997) The American orb weavers of the genera Mecynogea, Manogea, Kapogea and Cyrtophora (Araneae: Araneidae). Bull Mus Comp Zool 155:215–255
Masumoto T (1999) Size assortative mating and reproductive success of the funnel-web spider, Agelena limbata (Araneae; Agelenidae). J Insect Behav 12:353–361
McLain DK (1982) Behavioral and morphological correlates of male dominance and courtship persistence in the blister beetle Epicauta pennsylvanica (Coleoptera: Meloidae). Am Midl Nat 107:396–403
McLain DK, Boromisa RD (1987a) Male choice, fighting ability, assortative mating and the intensity of sexual selection in the milkweed longhorn beetle, Tetraopes tetraophthalmus (Coleoptera, Cerambycidae). Behav Ecol Sociobiol 20:239–246
McLain DK, Boromisa RD (1987b) Stabilizing sexual selection and density-dependent correlates of copulatory success in the ambush bug, Phymata wolffii (Hemiptera: Reduviidae). Am Midl Nat 117:94–102
Michiels NK, Hohner A, Vorndran IC (2001) Precopulatory mate assessment in relation to body size in the earthworm Lumbricus terrestris: avoidance of dangerous liaisons? Behav Ecol 12:612–618
Miyashita T (1994) Size-related mating and mate guarding in the orb-web spider Nephila clavata (Araneae, Araneidae). J Insect Behav 7:289–296
Mobley KB, Abou Chakra M, Jones AG (2014) No evidence for size-assortative mating in the wild despite mutual mate choice in sex-role-reversed pipefishes. Ecol Evol 4:67–78
Moura RR, Peixoto PEC (2013) The effect of operational sex ratio on the opportunity for sexual selection: a meta-analysis. Anim Behav 86:675–683
Moura RR, Vasconcellos-Neto J, Gonzaga MO (2017) Extended male care in Manogea porracea (Araneae: Araneidae): the exceptional case of a spider with amphisexual care. Anim Behav 123:1–9
Ólafsdóttir GÁ, Ritchie MG, Snorrason SS (2006) Positive assortative mating between recently described sympatric morphs of Icelandic sticklebacks. Biol Lett 2:250–252
Parker GA (2006) Sexual conflict over mating and fertilization: an overview. Philos Trans R Soc B 361:235–259
Prenter J, MacNeil C, Elwood RW (2006) Sexual cannibalism and mate choice. Anim Behav 71:481–490
Pruitt JN, Riechert SE (2009) Male mating preference is associated with risk of pre-copulatory cannibalism in a socially polymorphic spider. Behav Ecol Sociobiol 63:1573–1580
Ridley M (1983) The explanation of organic diversity: the comparative method and adaptations for mating. Clarendon Press, Oxford
Ritchie MG (2007) Sexual selection and speciation. Annu Rev Ecol Evol Syst 38:79–102
Rittschof CC (2010) Male density affects large-male advantage in the golden silk spider, Nephila clavipes. Behav Ecol 21:979–985
Robertson JG (1990) Female choice increases fertilization success in the Australian frog, Uperoleia laevigata. Anim Behav 39:639–645
Rubenstein DI (1987) Alternative reproductive tactics in the spider Meta segmentata. Behav Ecol Sociobiol 20:229–237
Schulte KF, Uhl G, Schneider JM (2010) Mate choice in males with one-shot genitalia: limited importance of female fecundity. Anim Behav 80:699–706
Seehausen O, Van Alphen JJ, Witte F (1997) Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277:1808–1811
Taborsky B, Guyer L, Demus P (2014) ‘Prudent habitat choice’: a novel mechanism of size-assortative mating. J Evol Biol 27:1217–1228
Thomas F, Oget E, Gente P, Desmots D, Renaud F (1999) Assortative pairing with respect to parasite load in the beetle Timarcha maritima (Chrysomelidae). J Evol Biol 12:385–390
Venner S, Bernstein C, Dray S, Bel-Venner MC (2010) Make love not war: when should less competitive males choose low-quality but defendable females? Am Nat 175:650–661
Vieira MC, Peixoto PE (2013) Winners and losers: a meta-analysis of functional determinants of fighting ability in arthropod contests. Funct Ecol 27:305–313
Wong BBM, Jennions MD (2003) Costs influence male mate choice in a freshwater fish. Proc R Soc B 270:S36–S38
World Spider Catalog (2017). World Spider Catalog. Natural History Museum Bern, online at http://wsc.nmbe.ch, version 17.5, accessed on December 1, 2016.
Zimmer SM, Schneider JM, Herberstein ME (2014) Can males detect the strength of sperm competition and presence of genital plugs during mate choice? Behav Ecol 25:716–722
Acknowledgements
We thank Duratex S.A. for allowing our work in Fazenda Nova Monte Carmelo, Giancarlo Ângelo Ferreira, and Yuri Lima Vasconcelos Ferreira for providing transport to the study area in several occasions and Paulo Enrique C. Peixoto for suggestions on an initial draft of this manuscript. We also thank Sven Thatje, Simona Kralj-Fišer, and another anonymous reviewer for their contribution to improvement of the text. This project was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (research grant to R.R.M.), Instituto Nacional de Ciência e Tecnologia dos Hymenoptera Parasitoides da Região Sudeste (HYMPAR/Sudeste - CNPq/CAPES/Fapesp), Fundação de Amparo à Pesquisa de Minas Gerais (Proc. APQ-02104-14, CRA-30058/12, APQ-03202-13; APQ-02474-15), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (Proc. 306157/2014-4; 403733/2012-0; 445832/2014-2). Voucher specimens of Manogea porracea were deposited in the collection of Universidade Federal de Minas Gerais (curator A. J. Santos), Minas Gerais, Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Stano Pekar
Rights and permissions
About this article
Cite this article
Moura, R.R., Gonzaga, M.O. Temporal variation in size-assortative mating and male mate choice in a spider with amphisexual care. Sci Nat 104, 28 (2017). https://doi.org/10.1007/s00114-017-1448-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-017-1448-6