Abstract
The morphology of the arthropod Sanctacaris uncata, from the Middle Cambrian Burgess Shale of Canada, is reinterpreted based on a restudy of previously described material. Although originally considered a chelicerate-like arthropod, these affinities were dismissed based primarily on interpretations of the anterior appendages and hypotheses which considered the megacheirans (‘great-appendage’ arthropods) as putative ancestors of chelicerates. The similarities between megacheirans and chelicerates appear to be overstated however, and this study instead reaffirms the identity of putative chelicerate feature in S. uncata and similar arthropods such as Sidneyia and Emeraldella, both also from the Middle Cambrian Burgess Shale. Newly interpreted features, including the presence of pediform exites, multi-partite trunk exopods, and a trunk differentiated into an anterior limb-bearing area and a differentiated posterior limbless abdomen, were coded into an extensive phylogenetic data set of fossil and recent arthropods. In all analyses, Sanctacaris resolved as the basal-most member of total-group Euchelicerata (the least inclusive group including horseshoe crabs and arachnids but not pycnogonids), thus making it the oldest chelicerate in the fossil record. The vicissicaudates (including Sidneyia, Emeraldella, aglaspidids, and cheloniellids—all of which have previously been allied to chelicerates) resolved as sister-taxon to crown-group Chelicerata. This topology indicates that many purported chelicerate features, such as lamellar gills, and a differentiated posterior abdomen evolved sequentially in the chelicerate stem-lineage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With over 113,894 described species, the chelicerates (sea spiders, horseshoe crabs, and arachnids) represent one of the most species-rich clades on Earth today (Zhang 2011), second only to their primary prey, the hexapods (insects). Understanding the origin and early evolution of chelicerates has proven to be a difficult and contentious pursuit. Although molecular clock analyses indicate an early Cambrian origin for this clade (Lee et al. 2013; Rota-Stabelli et al. 2013), body fossils of crown-group euchelicerates are not recovered until the Early Ordovician (Van Roy et al. 2010), although unequivocal chelicerate trace fossils (Dunlop et al. 2004), and a putative larval pycnogonid are known from the late Cambrian (Waloszek and Dunlop 2002). A diversity of putative chelicerate ancestors have been identified from Cambrian Konservat-Lagerstätten. Older works tended to align chelicerates with artiopodans, a group of arthropods including trilobites and other trilobite-like arthropods, collectively known as the trilobitomorphs, and vicissicaudates, usually possessing a broad tergum, antennae, and bilobate exopods (Stein and Selden 2012). Within Artiopoda, chelicerates were typically allied to the vicissicaudates (Wills et al. 1995, 1998; Hou and Bergström 1997; Dunlop and Selden 1997; Bergström and Hou 2003; Edgecombe et al. 2011), a group consisting of cheloniellids, aglaspidids, and xenopods (Fig. 1(a,c,d,f,h); Ortega-Hernández et al. 2013); more recent studies have regarded megacheirans (‘short-great-appendage’ arthropods) as the putative sister-taxon of Chelicerata (Fig. 1(b,e,g)), based primarily on the morphology of their anterior-most appendage pair (Chen et al. 2004; Cotton and Braddy 2004; Scholtz and Edgecombe 2005; Haug et al. 2012).
Previous hypotheses regarding the relationships of fossil taxa, particularly artiopodans and megacheirans, to Chelicerata. Twenty common taxa were selected for ease of comparison. Megacheirans are represented by Jianfengia, Fortiforceps, Yohoia and Leanchoilia; trilobitomorph artiopodans by Retifacies, Kumaia, Cindarella, Naraoia and the trilobite Olenoides; and vicissicaudate artiopodans by Sidneyia, Emeraldella, Aglaspis and Cheloniellon. a Wills et al. (1995, fig. 1A; 1998, fig. 2.1). b Bousfield (1995, figs. 7, 10). Rather than proposing sister-taxon relationships, Bousfield (1995) postulated direct lineages. c Hou and Bergström (1997, figs. 87, 88). d Dunlop and Selden (1997, fig. 17.3). This analysis did not include megacheirans. e Chen et al. (2004, fig. 6B) and Haug et al. (2012, fig. 11). This analysis did not include artiopodans. f Bergström and Hou (2003, fig. 4). g Cotton and Braddy (2004, fig. 8). h Edgecombe et al. (2011, fig. 8)
Sanctacaris uncata from the middle Cambrian Burgess Shale was originally identified as a stem-chelicerate with putative chelicerate features including a cephalon bearing six appendage pairs, a cardiac lobe, and a similar pattern of tagmosis (Briggs and Collins 1988). As with other putative chelicerate allies, the affinities of Sanctacaris were rejected due to a lack of chelate frontal appendages (Bousfield 1995), the cephalic appendages were instead interpreted as a ‘limb-basket’ (Dewel and Dewel 1997) and this taxon resolved amongst a paraphyletic assemblage of megacheirans in a subsequent phylogenetic analysis (Budd 2002). A restudy of Sanctacaris was therefore undertaken. The significance of purported chelicerate features was explored, and new observations on Sanctacaris and other purported stem-group chelicerates were coded into an extensive phylogenetic analysis of panarthropods to ascertain their significance and explore the early evolution of Chelicerata.
Material and methods
Specimens and geological setting
All previously described material of S. uncata, reposited at the Royal Ontario Museum (ROM), Toronto, was examined. This material, which was originally described by Briggs and Collins (1988), is currently the only known material of S. uncata. These specimens were recovered from Unit 3 of the Collins Quarry exposure of the Kicking Horse Shale Member (Burgess Shale Formation) (Collins et al. 1983; Briggs and Collins 1988), situated on the western slope of Mount Stephen in Yoho National Park, British Columbia, Canada. Associated trilobites indicate this horizon belongs to the Polypleuraspis insignis Subzone of the Glossopleura Biozone (middle Cambrian, Series 3, Stage 5) (Fletcher and Collins 1998, 2003). This fauna is dominated by Alalcomenaeus, which represents 58 % of recovered specimens (Collins et al. 1983; Briggs and Collins 1999), and the bivalved arthropods Nereocaris briggsi (190 specimens) and Loricicaris spinocaudatus (28 specimens), which combined account for a further 8 % of specimens from this site (Legg and Caron 2014).
Specimens of the xenopods Emeraldella brocki (Bruton and Whittington 1983; Stein and Selden 2012) and Sidneyia inexpectans (Bruton 1981; Stein 2013), reposited at the ROM and the National Museum of Natural History (USNM), Smithsonian Institution, Washington D.C., were also examined for comparative purposes. Stratigraphical information regarding examined material is provided in Bruton and Whittington (1983) and Stein (2013).
Phylogenetic analysis
New observations made herein were coded into a modified version of a phylogenetic data set of panarthropods (Legg et al. 2013; Siveter et al. 2014). To the original data set of 314 taxa and 753 characters, 13 taxa were added, 14 characters were added and 16 were modified, either recoded or combined or divided into discrete characters, and three were removed completely resulting in a total data set of 327 taxa and 763 characters (see online supplementary material for details). Cladistic analysis was performed using TNT v. 1.1. (Goloboff et al. 2008b). All characters were treated as non-additive (unordered) and weighted using both equal and implied weighing with a variety of concavity constants (k = 2, 3, 10), a justification of which is given elsewhere (Goloboff et al. 2008a; Legg et al. 2013; Legg and Caron 2014). Most parsimonious trees (MPTs) were found using New Technology search options with 100 Random Addition Sequences using Ratchet (Nixon 1999), Sectorial Searches, Tree Drifting and Tree Fusing (Goloboff 1999).
Discussion
Morphological interpretation of S. uncata
This section is not intended as a complete redescription of S. uncata, but rather a discussion of key characteristics either in need of reinterpretation or deemed important for determining relationships of this taxon, particularly those pertaining to either artiopodan and/or chelicerate affinities.
The morphology of the anteriormost appendages has been a cynosure of arthropod systematics (Scholtz and Edgecombe 2005, 2006). The chelicerate affinities of Sanctacaris were rejected due to a lack of chelicerae (Bousfield 1995), a pivotal synapomorphy of Chelicerata (Lamsdell 2013). Based on comparisons with Offacolus kingi (Orr et al. 2000; Sutton et al. 2002), from the Silurian of Herefordshire, it was considered possible that the chelicerae may be present in Sanctacaris yet unobservable (Boxshall 2004). Still others have interpreted structures in the holotype (Figs. 2a,b and 3) originally identified as ‘antenna-like rami’ of the sixth cephalic appendage (Briggs and Collins 1988) as biramous deutocerebral antennae (Budd 2002). Frontal appendages, ‘great-appendages’, chelicerae, antennae or other uniramous anterior appendages could not be identified in any of the available material. Instead, the structures interpreted as part of a biramous antenna are herein interpreted as individual exites which belong to the posterior cephalic appendages (Figs. 2a,b and 3). Each ramus is distinguished from the other by a slight variation in relief with the posterior rami preserved above the anterior ones. This interpretation is further supported by their resemblance to exites preserved in other specimens (Fig. 2c). The distal tips of the posteriormost exites bear a cluster of setae (Figs. 2b and 3), reminiscent of those in the basal chelicerates Offacolus (Sutton et al. 2002) and Dibasterium durgae (Briggs et al. 2012).
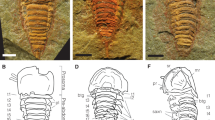
Sanctacaris uncata and Sidneyia inexpectans from the Burgess Shale: a, b Royal Ontario Museum (ROM) 43502b (holotype of Sanctacaris uncata), cephalic region. a Cephalic region, b enlargement of right cephalic appendages. c Cephalic appendages of ROM 43504b (S. uncata). d Cephalic appendages of ROM 920274a (Sidneyia inexpectans). Entire specimen figured in supplementary figure 1. e Posterior trunk, abdomen and telson of ROM 43506 (S. uncata). f Medial trunk region of ROM 43504b (S. uncata); arrows indicate bifurcation of putative axial lobes. g Medial trunk region showing proximal lamellal setae of ROM 43503 (S. uncata). h Distal exites of ROM 43504a (S. uncata). ab abdomen, ca II–VI cephalic appendages II–VI, ce compound eye, dex distal exite flap, en endopod, ex exite, ls lamellal setae, sb setal brush, st setae and tl telson
Camera lucida drawing of the appendages of the holotype of Sanctacaris uncata (ROM) 43502b. Accompanying photo in Fig. 1(b). a Camera lucida drawing. b Proposed homology of limb elements showing a gradual increase from five elements in the anterior appendages to seven in the posteriormost appendage. Endopods are in brown, basipodites in purple, exites in blue, and the compound eye in green. ce compound eye, cs cephalic shield, en clusters of endites, sb setal brush, and tp terminal endopod podomere
The putative limb-basket (sensu Bousfield 1995) has been interpreted as a pair of multi-ramous appendages, comparable to the short-great-appendages of megacheirans (Dewel and Dewel 1997; Budd 2002). An anterior clustering of the cephalic appendages has also been observed in other Palaeozoic arthropods, particularly the xenopods Sidneyia (Fig. 2d) and Emeraldella (Stein and Selden 2012). The reason for this is unknown but may be taphonomic, e.g. the appendages are pushed forward during burial, or due to moulting. Specimens preserved in this manner also tend not to have their antennae preserved (Fig. 2d), indicating that the lack of anterior appendages in Sanctacaris may not be a genuine absence. Each cephalic appendage of Sanctacaris possesses a distinct basipodite (Figs. 2a,b and 3) and is therefore not fused into a single multi-ramous appendage. Each appendage is associated with a pediform exite, but the nature of its attachment is unclear. The separation of these exites from the endopod in some specimens (Figs. 2a,b and 3) may indicate the exites were attached to the underside of the cephalon, as seen in Dibasterium (Briggs et al. 2012). The posterior cephalic endopod pairs of Sanctacaris resemble those of Sidneyia in possessing seven podomeres, the most distal of which bears an elongate endite on the medial-distal margins, and a subchelate terminal podomere (Fig. 2a,b). The anteriormost endopod of Sanctacaris possesses just five podomeres (Fig. 2a,b). A reduced anterior (first post-antenna/chelicera) endopod is also present in Sidneyia (Stein 2013), Emeraldella (Stein and Selden 2012) and chelicerates.
All appendages preserved in Sanctacaris are biramous; however, deutocerebral appendages in arthropods are universally uniramous (Boxshall 2013), thus indicating that either Sanctacaris has lost its anteriormost (deutocerebral) appendages through evolution or they were not preserved. Assuming, at least a single pair of unobserved anterior appendages then the cephalon of Sanctacaris bears six pairs of appendages, a characteristic feature of Euchelicerata (horseshoe crabs and arachnids) (Lamsdell 2013). Although Sanctacaris was originally described as possessing a cardiac lobe (Briggs and Collins 1988), another characteristic feature of Euchelicerata, this could not be identified in the available material. The cardiac lobe of unequivocal chelicerates is a sub-triangular bulge at the posterior of the cephalon. The equivalent area in Sanctacaris is sub-circular and much broader, more comparable to the interophthalmic area of extant horseshoe crabs, indicating the cephalon of Sanctacaris was also vaulted, but the eyes are located underneath the anterolateral margin of the cephalic shield.
The trunk of Sanctacaris consists of 11 segments, the posteriormost of which is limbless and has reduced lateral pleurae (Fig. 2e). An 11-segmented trunk is also present in aglaspidids (Hesselbo 1992) and the basal chelicerates Dibasterium (Briggs et al. 2012), Legrandella (Eldredge 1974) and possibly Weinbergina (Lamsdell 2013). Unlike Sanctacaris, however, the abdomens of these taxa all consist of three segments. The abdomens of cheloniellids and Emeraldella consist of a single segment (Stein and Selden 2012; Ortega-Hernández et al. 2013).
Although the axial region of the holotype of Sanctacaris demonstrates notable relief, it lacks distinct trilobation. Longitudinal ridges on the axial region may represent subaxial nodes, a feature common amongst basal chelicerates (Lamsdell 2013); however, in Sanctacaris, they are unevenly distributed and occasionally intersect transverse ridges. They could therefore be caused by post-mortem deformation rather than being a genuine morphological feature (Fig. 2f).
Trunk appendages occur on all but the posteriormost trunk segment. They are biramous, composed of a short endopod (the exact podomere count could not be determined) and an exite consisting of at least two parts, a proximal shaft bearing lamellar setae with accessory setules (Fig. 2g) and a distal lobe fringed with fine setae (Fig. 2h). The basipodite was not observed but is assumed to be present because the appendages are typically biramous and because basipodites are observed in similar appendages in the cephalon. A bi- or tripartite exite with a proximal shaft and distal lobe is characteristic of artiopods (Stein and Selden 2012; Stein 2013; Ortega-Hernández et al. 2013). A similar arrangement is also present in the opisthosomal appendages of extant horseshoe crabs, although the various elements have undergone fusion into a ridged operculum (Fig. 4). A subrhombic exite lobe, as seen in Sanctacaris (Fig. 2h), is also found in vicissicaudates, particularly Sidneyia (Stein 2013), Emeraldella (Stein and Selden 2012) and to a lesser extent Kwanyinaspis (Zhang and Shu 2005).
Putative homology of trunk exites in Cambrian arthropods and the book-gill operculae of extant chelicerates: a, b Second book-gill opercula of Limulus Polyphemus. a Museum für Naturkunde (ZMB) 32113; b interpretive sketch. c Interpretive sketch of a trunk appendage of Sidneyia inexpectans (redrawn from Stein (2013). Colours indicate putatively homologous elements: basipodite in purple, proximal exite lobe bearing lamellal setae in blue, distal exite lobe in green, telopodite (endopod) in orange, possible sternite in brown, and pre-basinal endite in red
New observations and interpretations made herein, particularly the identification of pediform exites tipped with a setal cluster and the presence of a short differentiated posterior tergite, are indicative of artiopodan affinities and seemingly support affinities with chelicerate arthropods (Briggs and Collins 1988). Furthermore, the high number of features shared with Sidneyia and Emeraldella, including previous observations which were confirmed herein, like the presence of a bipartite exopod shaft suggests a close relationship between these taxa, as recovered in a previous phylogenetic analysis (Legg et al. 2013).
Chelicerate-type features in other artiopodans
Given the number of similarities between Sanctacaris, a putative artiopodan, and chelicerates, we might expect other artiopodans to possess features indicative of chelicerate affinities. There is a wealth of literature documenting similarities between trilobites and trilobite-like taxa and chelicerates (Størmer 1944; Lauterbach 1980; Weygoldt 1986; Hou and Bergström 1997). Features previously deemed important include trilobation, genal spines, dorsal positioning of the lateral eyes and the presence of lamellar setae. The importance of these features has been dismissed by others however (Scholtz and Edgecombe 2005) as they are lacking in the majority of chelicerates, particularly pycnogonids and arachnids. The absence of these features from these taxa may actually be an apomorphic reversal, caused, in the case of the arachnids, by an adaptation to a terrestrial habitat, especially as many of these features are prevalent amongst basal members of Chelicerata (Lamsdell 2013).
As well as the aforementioned features, a number of other features are shared by artiopodans, particularly vicissicaudates, and chelicerates but are lacking in megacheirans and other putative stem-lineage euarthropods and stem-mandibulates. To the previous list we can add the presence of a marginal rim on the anterior of the cephalon, differentiation of the posteriormost somites into a short, moveable abdomen, tipped with an elongate, styliform, and potentially keeled telson, and the presence of multi-partite trunk exopods (discussed above).
Additionally, a prebasal endite has been identified in Sidneyia (Bruton 1981) and compared to the prebasal endites of xiphosurans and eurypterids (Boxshall 2004), although these structures were not considered homologous by others (Stein 2013) based on their assumed view of arthropod relationships. Given the structural similarities of the prebasal endites, which are all lacrimiform with a bulbous and spinose medial edge, and their common positioning, on the dorsolateral edge of a gnathobasic basis with an elongate dorsal flange, they are all coded as homologous in this study.
Segmental affinities of the anterior appendages of megacheirans
Recent works on the origins of chelicerates have been dominated by hypotheses regarding the chelate frontal appendages of megacheirans as homologous to the chelicerate chelicerae (Bousfield 1995; Chen et al. 2004; Cotton and Braddy 2004; Dunlop 2005; Scholtz and Edgecombe 2006; Haug et al. 2012; Tanaka et al. 2013), inferring that chelicerates could not have evolved from an antenna-bearing taxon such as the artiopodans. Similarities between short-great-appendages may be overstated however, and there are a number of lines of evidence which place reasonable doubt on their supposed homology with chelicerae.
Perhaps the most compelling line of evidence regarding the segmental affinities of the short-great-appendages has come from recent finds of fossilized neural tissues in the megacheiran Alalcomenaeus sp. (Tanaka et al. 2013). Based on alignment with Limulus and scorpions, the short-great-appendages were considered to innervate from the deutocerebral neuromere of the brain; however, the deutocerebral neuropils of Limulus are expressed anterior of the oesophageal foramen, whereas the neuropils corresponding to the short-great-appendages are located laterally of the oesophageal foramen, which may instead indicate a tritocerebral origin for these appendages. If this is the case, then deutocerebral neuropils are absent. A similar phenomenon occurs in other arthropods which have lost either their deuto- or tritocerebral appendages, such as hexapods (Strausfeld 2012). Pre-great-appendage antennae are present in other, presumably more primitive megacheirans (Legg et al. 2012, 2013; Legg 2013), such as Fortiforceps foliosa (figures 31C–D and 32A in Hou and Bergström 1997), and great-appendage-like appendages are also present in other antenna-bearing taxa including the bivalved arthropod Loricicaris spinocaudatus (figure 3C in Legg and Caron 2014), suggesting that more derived megacheirans, such as Alalcomenaeus, lost an antenna-bearing, presumably deutocerebral, somite.
Fuxianhuia bears two pairs of specialized cephalic appendages, deutocerebral antennae and tritocerebral ‘specialized post-antennal appendages’ (SPAs) (Ma et al. 2012; Yang et al. 2013). The SPAs of Fuxianhuia are geniculate like the short-great-appendages of megacheirans, possibly indicating they are homologous, although it should be noted that the SPAs of Fuxianhuia are not chelate. Geniculation was also used to unite megacheirans and chelicerates; however, many basal chelicerates, such as Dibsterium, Offacolus and possibly Weinbergina (Briggs et al. 2012; Lamsdell 2013), appear to lack this joint (figure 1C in Sutton et al. 2002 and figure 1C in Briggs et al. 2012). In fact, the anterior appendages of these taxa are elongated, composed of numerous podomeres, reminiscent of antennae. If these taxa indeed represent the most basal chelicerates, then it is reasonable to presume that the chelicerae actually originated from an antenniform appendage, akin to that of artiopodans. A similar hypothesis was also proposed by Sharma et al. (2013).
Character acquisition in the chelicerate stem-lineage
Phylogenetic analysis with equal character weighting resulted in 16 most parsimonious trees (MPTs) of 1924 steps (consistency index (CI) = 0.505; retention index (RI) = 0.871), and implied weighted analyses with a concavity constant of 2, 3 and 10 produced 28 MPTs of 179.96797 steps (CI = 0.499; RI = 0.867), 26 MPTs of 146.21588 steps (CI = 0.499; 0.867) and 17 MPTs of 66.65010 steps (CI = 0.503; RI = 0.869), respectively.
In all analyses, Sanctacaris resolved within crown-group Chelicerata (the least inclusive group including pycnogonids and euchelicerates) as the basal-most member of total-group Euchelicerata (Fig. 5). During analyses utilizing equal weighting and implied weighting with a concavity constant of 10, Sidneyia resolved as the sister-taxon of Sanctacaris. This relationship was supported by two synapomorphies: the presence of a paddle-shaped telson (ch. 198:1) and the differentiation of endopod endites into primary and secondary subsets (ch. 298:1). When analysed using implied weighting with a concavity constant of two or three Sidneyia instead resolved as a vicissicaudate, the entire group of which in turn is resolved as the sister-taxon of Chelicerata (Fig. 5). Both topologies indicate elongate, antenniform appendages were present in the chelicerate stem-lineage and were retained in selected crown-members such as Dibasterium and Offacolus. If this is the case, then we need to account for the position of Pycnogonida, whose position in the current analysis indicates a convergent origin of short, chelate appendages. The position of pycnogonids may, however, be a long-branch artefact caused by the extensive modification of the pycnogonid body plan.
The phylogenetic position of Sanctacaris uncata: (a) topology produced using implied character weighting with concavity constants of two and three and (b) topology produced using equal character weighting and implied character weighting with a concavity constant of ten. The megacheirans resolved as part of a paraphyletic assemblage outside of the arthropod crown-group. Trilobitomorpha includes trilobites and a diversity of trilobite-like, pygidium-bearing taxa including Cindarella (Petalopleura), Kumaia (Conciliterga) and Naraoia (Nektaspida). See Fig. 1 for alternative placements of these taxa
A sister-taxon relationship between chelicerates and vicissicaudates (regardless of the inclusion of Sidneyia) was supported by a number of synapomorphies mostly pertaining to tagmosis. In particular, this clade, termed Cheliceratomorpha (sensu Cotton and Braddy 2004) primitively possess a trunk composed of 12 segments (ch. 75:5), with a differentiated abdomen (ch. 93:1, 94:1), composed of three segments (ch. 95:2), although numerous variations on this body plan occur within derived members of this clade.
A bi- or tripartite exopod shaft (ch. 152:1) with lamellar setae restricted to the proximal shaft has previously been considered a diagnostic characteristic of artiopodans (Stein and Selden 2012); however, basal members including retifaciids, Kiisortoqia, Siriocaris, and Squamacula lack this feature. The retifaciids possess a flap-like exopod with extensive lamellar setae and appear to represent an intermediate stage between the simple flap-like exopod of non-artiopodans and the lamellate exopods of later artiopodans (Fig. 5). The fusion of the trunk limbs into operculae, as seen in the euchelicerate crown-group, may have been a crucial step in the diversification and terrestrialization of chelicerates as the operculae act as a protective cover for the respiratory lamellae, preventing them from desiccation during terrestrial excursions (Reisinger et al. 1991).
A single synapomorphy—the presence of an extensive cephalic doublure (ch. 24:1)—supports the monophyly of Artiopoda. This feature was excluded from prior diagnoses of this group (Stein and Selden 2012) but is retained in most members of this clade. This feature is lost within crown-group chelicerates, specifically within pycnogonids and arachnids. Other features previously used to link non-chelicerate artiopodans and chelicerates, such as short genal spines (ch. 38:1), an inflated posterior cephalon (ch. 31:1), a raised marginal cephalic rim (ch. 16:1) and a raised axial trunk region (ch. 71:1), show varying levels of homoplasy across tree topologies; however, these features tend to be found exclusively amongst artiopodans (including chelicerates) and may still serve as a good indicator of relationships between non-chelicerate artiopodans and chelicerates.
The topology obtained in this study indicates that a number of features prevalent amongst chelicerates appeared sequentially in the chelicerate stem-lineage (Fig. 5). Invoking a megacheiran ancestry for chelicerates involves an abrupt morphological change at the base of Chelicerata, whereby many of the features linking them to artiopodans are convergently acquired, thereby resulting in an unparsimonious view of their evolution.
References
Bergström J, Hou X-G (2003) Arthropod origins. Bull Geosci 78:323–334
Bousfield EL (1995) A contribution to the natural classification of Lower and Middle Cambrian arthropods: food-gathering and feeding mechanism. Amphipacifica 2:3–34
Boxshall GA (2004) The evolution of arthropod limbs. Biol Rev 79:253–300
Boxshall GA (2013) Arthropod limbs and their development. In: Minelli A, Boxshall G, Frusco G (eds) Arthropod biology and evolution. Molecules, development, morphology, Springer, pp. 241–267
Briggs DEG, Collins D (1988) A Middle Cambrian chelicerate from Mount Stephen, British Columbia. Palaeontology 31:779–798
Briggs DEG, Collins D (1999) The arthropod Alalcomenaeus cambricus Simonetta, from the Middle Cambrian Burgess Shale of British Columbia. Palaeontology 42:953–977
Briggs DEG, Siveter DJ, Siveter DJ, Sutton MD, Garwood RJ, Legg DA (2012) A Silurian horseshoe crab illuminates the evolution of chelicerate limbs. Proc Natl Acad Sci U S A 109:15702–15705
Bruton DL (1981) The arthropod Sidneyia inexpectans, Middle Cambrian, Burgess Shale, British Columbia. Phil Trans R Soc Lond B 295:619–653
Bruton DL, Whittington HB (1983) Emeraldella and Leanchoilia, two arthropods from the Burgess Shale, middle Cambrian, British Columbia. Phil Trans R Soc Lond B 300:553–582
Budd G (2002) A palaeontological solution to the arthropod head problem. Nature 417:271–275
Chen J-Y, Waloszek D, Maas A (2004) A new ‘great-appendage arthropod’ from the Lower Cambrian of China and homology of chelicerate chelicerae and raptorial antero-ventral appendages. Lethaia 37:3–20
Collins D, Briggs DEG, Conway MS (1983) New Burgess Shale fossil sites reveal Middle Cambrian faunal complex. Science 22:163–167
Cotton TJ, Braddy SJ (2004) The phylogeny of arachnomorph arthropods and the origin of Chelicerata. Trans R Soc Edinb Earth Sci 94:169–193
Dewel RA, Dewel WC (1997) The place of tardigrades in arthropod evolution. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman & Hall, London, pp 109–123
Dunlop JA (2005) New ideas about the euchelicerate stem-lineage. In: Deltshev C, Stoev P (eds) European arachnology 2005, pp. 9–23. Acta Zool Bulg Suppl 1
Dunlop JA, Selden PA (1997) The early history and phylogeny of the chelicerates. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman & Hall, London, pp 221–238
Dunlop JA, Anderson LI, Braddy SJ (2004) A redescription of Chasmataspis laurencii Caster & Brooks, 1956 (Chelicerata, Chasmataspidida) from the Middle Ordovician of Tennessee, USA, with remarks on chasmataspid phylogeny. Trans R Soc Edinb Earth Sci 94:207–225
Edgecombe GD, García-Bellido DC, Paterson JR (2011) A new leanchoiliid megacheiran arthropod from the lower Cambrian Emu Bay Shale, South Australia. Acta Palaeontol Pol 56:385–400
Eldredge N (1974) Revision of the Synziphosura (Chelicerata, Merostomata), with remarks on merostome phylogeny. Am Mus Novit 2543:1–41
Fletcher TP, Collins DH (1998) The Middle Cambrian Burgess Shale and its relationship to the Stephen formation in the Southern Canadian Rocky Mountains. Can J Earth Sci 17:400–418
Fletcher TP, Collins DH (2003) The Burgess Shale and associated Cambrian formations west of the Fossil Gully Fault Zone on Mount Stephen, British Columbia. Can J Earth Sci 40:1823–1838
Goloboff PA (1999) Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics 15:415–428
Goloboff PA, Carpenter JM, Salvador Arias J, Rafael Miranda Esquivel D (2008a) Weighting against homoplasy improves phylogenetic analysis of morphological data sets. Cladistics 24:758–773
Goloboff PA, Farris JS, Nixon KC (2008b) TNT, a free program for phylogenetic analysis. Cladistics 24:774–786
Haug JT, Waloszek D, Maas A, Liu Y, Haug C (2012) Functional morphology, ontogeny and evolution of mantis shrimp-like predators in the Cambrian. Palaeontology 55:369–399
Hesselbo SP (1992) Aglaspidida (Arthropoda) from the Upper Cambrian of Wisconsin. J Paleontol 66:885–923
Hou X-G, Bergström J (1997) Arthropods of the lower Cambrian Chengjiang fauna, southwest China. Fossils Strata 45:1–116
Lamsdell JC (2013) Revised systematics of the Palaeozoic ‘horseshoe crabs’ and the myth of monophyletic Xiphosura. Zool J Linn Soc 167:1–27
Lauterbach K-E (1980) Schlüsselereignisse in der Evolution des Grundplans der Arachnata (Arthropoda). Abh Verh Naturwiss Vereins Hamburg 26:293–320
Lee MSY, Soubrier J, Edgecombe GD (2013) Rates of phenotypic and genomic evolution during the Cambrian explosion. Curr Biol 23:1–7
Legg DA (2013) Multi-segmented arthropods from the middle Cambrian of British Columbia (Canada). J Paleontol 87:493–501
Legg DA, Caron J-B (2014) New Middle Cambrian bivalved arthropods from the Burgess Shale (British Columbia, Canada). Palaeontology 57:691–711
Legg DA, Sutton MD, Edgecombe GD, Caron J-B (2012) Cambrian bivalved arthropod reveals origin of arthrodization. Proc R Soc B 279:4699–4704
Legg DA, Sutton MD, Edgecombe GD (2013) Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nat Commun 4:2485
Ma X, Hou X-G, Edgecombe GD, Strausfeld NJ (2012) Complex brain and optic lobes in an early Cambrian arthropod. Nature 490:258–261
Nixon KC (1999) The parsimony ratchet, a new method for rapid parsimony analysis. Cladistics 15:407–414
Orr PJ, Siveter DJ, Briggs DEG, Siveter DJ, Sutton MD (2000) A new arthropod from the Silurian Konservat-Lagerstätte of Herefordshire, England. Proc R Soc Lond B 267:1497–1504
Ortega-Hernández J, Legg DA, Braddy SJ (2013) The phylogeny of aglaspidid arthropods and the internal relationships within Artiopoda. Cladistics 29:15–45
Reisinger PWM, Tutter I, Welsch U (1991) Fine structure of the gills of the horseshoe crabs Limulus Polyphemus and Tachypleus tridentatus and of the book lungs of the spider Eurypelma californicum. Zool Jahrb Abt Anat Ontog 121:331–357
Rota-Stabelli O, Daley AC, Pisani D (2013) Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr Biol 23:392–398
Scholtz G, Edgecombe GD (2005) Head, Hox and the phylogenetic position of trilobites. In: Koenemann S, Jenner R (eds) Crustacea and arthropod relationships. Taylor & Francis, Oxford, pp 139–165
Scholtz G, Edgecombe GD (2006) The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev Genes Evol 216:395–415
Sharma PP, Schwager EE, Giribet G, Jockusch EL, Extavour CG (2013) Distal-less and dashshund pattern both plesiomorphic and apomorphic structures in chelicerates: RNA interference in the harvestman Phalangium opilio (Opiliones). Evol Dev 15:228–242
Siveter DJ, Briggs DEG, Siveter DJ, Sutton MD, Legg DA, Joomun S (2014) A Silurian short-great-appendage arthropod. Proc R Soc Lond B 281 (in press)
Stein M (2013) Cephalic and appendage morphology of the Cambrian arthropod Sidneyia inexpectans. Zool Anz 253:164–178
Stein M, Selden PA (2012) A restudy of the Burgess Shale (Cambrian) arthropod Emeraldella brocki and reassessment of its affinities. J Syst Palaeontol 10:361–383
Størmer L (1944) On the relationships and phylogeny of fossil and recent Arachnomorpha. Skrift Norske Vidensk Acad I Oslo 5:1–158
Strausfeld NJ (2012) Arthropod brains: evolution, functional elegance, and structural significance. Harvard University Press, Cambridge, p 650
Sutton MD, Briggs DEG, Siveter DJ, Siveter DJ, Orr PJ (2002) The arthropod Offacolus kingi (Chelicerata) from the Silurian of Herefordshire, England: computer based morphological reconstructions and phylogenetic affinities. Proc R Soc Lond B 269:1195–1203
Tanaka G, Hou X-G, Ma X, Edgecombe GD, Strausfeld NJ (2013) Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 502:364–367
Van Roy P, Orr PJ, Botting JP, Muir LA, Vinther J, Lefebvre B, el Hariri K, Briggs DEG (2010) Ordovician faunas of Burgess Shale type. Nature 465:215–218
Waloszek D, Dunlop JA (2002) A larval sea spider (Arthropoda: Pycnogonida) from the Upper Cambrian ‘Orsten’ of Sweden, and the phylogenetic position of pycnogonids. Palaeontology 45:421–446
Weygoldt P (1986) Arthropod interrelationships—the phylogenetic-systematic approach. J Zool Syst Evol Res 24:19–35
Wills MA, Briggs DEG, Fortey RA, Wilkinson M (1995) The significance of fossils in understanding arthropod evolution. Verh Dtsch Zool Ges 88:203–215
Wills MA, Briggs DEG, Fortey RA, Wilkinson M, Sneath PHA (1998) An arthropod phylogeny based on fossil and recent taxa. In: Edgecombe GD (ed) Arthropod fossils and phylogeny. Columbia University Press, New York, pp 33–105
Yang J, Ortega-Hernández J, Butterfield NJ, Zhang X-G (2013) Specialized appendages in fuxianhuiids and the head organization of early arthropods. Nature 494:468–471
Zhang Z-Q (2011) Phylum Arthropoda von Siebold 1848. Zootaxa 3148:99–103
Zhang X-L, Shu D-G (2005) A new arthropod from the Chengjiang Lagerstätte, Early Cambrian, southern China. Alcheringa 29:185–194
Acknowledgments
Thanks go to J.-B. Caron and P. Fenton, both at the Royal Ontario Museum, Toronto (ROM), for access to material in their care. Thanks also to Nicola Woods (ROM) for providing additional photographs of Sanctacaris uncata, J. Lamsdell (Yale Peabody Museum) for providing additional photos of Sidneyia inexpectans, G. Edgecombe (Natural History Museum, London) and J. Dunlop (Museum für Naturkunde, Berlin) for the comments on an earlier version of this manuscript and X. Ma (NHM, London) and J. Ortega-Hernández for the critical discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 88 kb)
Supplementary figure 1
(DOCX 903 kb)
Rights and permissions
About this article
Cite this article
Legg, D.A. Sanctacaris uncata: the oldest chelicerate (Arthropoda). Naturwissenschaften 101, 1065–1073 (2014). https://doi.org/10.1007/s00114-014-1245-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-014-1245-4