Abstract
Sexual selection may operate on pre-copulatory, copulatory, and post-copulatory traits. An example of a copulatory target of sexual selection is the genitalic movements a male performs during copulation. These movements may function either to prevent sperm competition or to influence a female’s fertilization decision. Here we investigated how copulation duration, pedipalp movements, and abdominal movements that males of the pholcid spider Holocnemus pluchei produce during copulation influence sperm removal and/or patterns of successful sperm transfer. We compared mating events with virgin and mated females for differences in copulatory and post-copulatory behavior. We expected longer copulation duration, longer pedipalp movement duration, and more complex and frequent pedipalp and abdominal movements when males mated with mated females compared to virgin females. Except for abdominal movements, our results corroborated these predictions. Furthermore, when we investigated mating events with mated females, we observed sperm mass ejection from the female gonopore and physical removal of sperm by males’ procursi. Females with interrupted second mating events showed a significant reduction of stored sperm masses compared to females with completed mating events. We suggest that males use alternating pedipalp movements to remove most of the rival sperm stored by mated females prior to sperm transfer. Copulation duration and pedipalp movements can be further used to transfer sperm and/or as a form of genitalic copulatory courtship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several studies have shown that copulation duration in arthropods is longer than expected given that the basic function of copulation is the transfer of sperm from males to females (reviewed by Eberhard 2009). Thus, a long copulation suggests that functions other than sperm transfer may be operating. Long copulations are often adaptive because they improve a male’s fertilization success relative to rivals (reviewed by Simmons 2001). When females mate multiply, a fertilization pattern of “last-male precedence” may be accomplished by three processes: (1) an increase in ejaculate volume. Examples of this phenomenon are widespread (e.g., Pilastro et al. 2002; Wada et al. 2010; Kelly and Jennions 2011); (2) direct removal of previously stored rival sperm from the female’s sperm storage organs. Recent studies in varied taxa such as Cephalopoda (e.g., Naud et al. 2004; Wada et al. 2006, 2010) and Arthropoda (e.g., Waage 1979; Michiels 1989; Ono et al. 1989) have provided support for a diversity of sperm removal mechanisms; and (3) female stimulation that promotes ejection of rival sperm by the female (also known as sperm dumping). This last mechanism has been interpreted as a form of copulatory courtship (reviewed by Eberhard 1991, 2009, 2011). For example, it has been detected in some taxa that females bias fertilization toward males that execute specialized copulatory behavior (e.g., flies: Otronen and Siva-Jothy 1991; beetles: Edvardsson and Arnqvist 2000; spiders: Peretti et al. 2006). Genitalic copulatory courtship has also been documented in several taxa and often occurs during or following copulation (Otronen 1990; Eberhard 1994, 2001, 2009).
Spiders are useful models to uncover patterns of copulatory or post-copulatory sexual selection on traits like genitalic movements. The system has several highly tractable features: first, sperm are encapsulated (i.e., immobile) when transferred, and thus it is relatively easy to see which sex produces movements that allow sperm transport during their transfer (Eberhard 2004). Second, most copulatory movements are relatively stereotyped and usually occur on the female’s external surface, so it is possible to directly observe male genitalic movements. Such male movements include the rhythmic expansions of haematodochae, and twisting, vibrating, and repeated insertions and withdrawals of intromittent structures (Huber 1998). This allows relatively straightforward deductions of functional aspects of male genitalic structures (Eberhard 2004). Eberhard (2004) hypothesized that female genital morphology is a potent selective agent in the evolution of male copulatory strategies. Two types of female sperm storage organs are present in spiders (Coddington and Levi 1991). In non-haplogyne species, there may be multiple ducts for insemination and fertilization. In contrast, haplogyne species provide only a single duct for insemination and fertilization. Thus, sperm must enter and leave through the same duct (Foelix 1982). When females mate multiply, patterns of fertilization commonly follow last-male precedence in haplogyne species (Austad 1984). Haplogyne females store sperm in a single receptaculum to which the male genitalia have direct access. Such accessibility makes it possible for males to use their genitalia to remove sperm from previous males (physically or by other means, see Peretti and Eberhard 2010). In particular, haplogyne spiders are good candidates for research on cryptic choice because of their potential to eject sperm (Herbestein 2011).
Copulation in pholcids (i.e., cellar spiders) only takes place if the female adopts a horizontal position. The male then inserts both pedipalps into the center of the female gonopore and simultaneously moves both pedipalps horizontally (Schäfer and Uhl 2002). Copulation in these animals consists of a single long insertion of both male pedipalps at the same time. In functional terms, this insertion twists and squeezes the female (Huber and Eberhard 1997; Schäfer and Uhl 2002; Huber 2005). Given that these pedipalp movements are associated with fertilization success (Peretti et al. 2006), it has been suggested that pedipalps perform a copulatory courtship function (Huber and Eberhard 1997; Schäfer and Uhl 2002). In fact, sperm may leave the female gonopore during copulation (Uhl 1994; Huber and Eberhard 1997; Schäfer and Uhl 2002; Schäfer et al. 2008; Peretti and Eberhard 2010). However, whether the sperm movement is the result of active sperm removal by the male or is the result of sperm ejection by the female is a little explored issue (Huber 2005; Peretti and Eberhard 2010).
Female pholcid spiders lack a specific organ for storing sperm, so sperm is stored in regions of the external uterus (Huber 1995). In the pholcid Holocnemus pluchei, males continuously perform pedipalp movements and abdominal vibrations during copulation (Huber 1995). Females copulate with several males before laying eggs and there is last-male precedence (Kaster and Jakob 1997). Males carry out post-copulatory mate guarding to females by remaining close for ca. 14 h to prevent female remating. After 14 h, H. pluchei females are no longer receptive (Calbacho-Rosa et al. 2010).
Here we have investigated the nature and possible function of pedipalp movements and abdominal vibrations during copulation in the pholcid spider, H. pluchei. We tested the following hypotheses: (1) courtship duration, copulation duration, pedipalp movements, and abdominal vibrations will differ in mating events with virgin females versus mated females; (2) copulation duration is correlated with the frequency and duration of male pedipalp movements; and (3) different patterns of pedipalp movements performed during copulation have different functions that include sperm ejection, sperm deposition, and genitalic copulatory courtship. For hypothesis 1, we predicted that courtship and copulation duration will increase when mating with mated females. In other words, to increase fertilization success by outcompeting rival sperm, males should carry out processes that will take longer than if there was no rival sperm. For hypothesis 2, we expected more frequent and complex pedipalp and abdominal movements in mating events with mated females for a similar reason as put forward for hypothesis 1. For hypothesis 3, pedipalp function-specific movements will be expected depending on the functional context.
Methods
H. pluchei (Pholcidae) is a group-living spider that builds irregular webs containing one to 15 spiders of different age, size, and sex (Jakob 1991). Males commonly move between webs, and females often mate multiply (Jakob 1991). When multiply mated, last male sperm precedence is 74 % (Kaster and Jakob 1997; Calbacho-Rosa et al. 2010).
Collecting and rearing
We collected and used two sets of animals. The first set consisted of 60 females (collected in the penultimate instar to ensure status as virgins) and 75 adult males that were collected in the Ciudad Universitaria, Córdoba, Argentina (latitude: −31.40°, longitude: −64.18°, 384 m above sea level), between September 2008 and April 2010. This set was used for documenting male pedipalp movements and duration (i.e., the following three sections). We placed each individual in a plastic container (8 × 15 cm) containing paper as a surface for web building, with wet cotton ball as a water source, with a photoperiod of 12/12 h. Females were used after reaching sexual maturity. Males were used after at least 15 days in sexual isolation—this permitted sperm stores to be replenished in case any males had recently mated prior to capture. Juveniles and adults were fed on a weekly basis with Drosophila melanogaster adults and Tenebrio monitor larvae. The second set of animals consisted of 45 females (collected in the penultimate instar) and 60 adult males that were collected in the Ciudad Universitaria between September 2010 and March 2011. This set was used to determine the function of male pedipalp movement patterns by measuring sperm masses in females (i.e., “Results” section below). Animals were maintained as those of the first set.
Mating experiments
All interactions were recorded using a Nikon SMZ 1500 stereo microscope equipped with a Nikon Sight DS-FI1 digital camera. Careful positioning of the camera allowed close-up views of male pedipalp movements during copulation. Adult females were placed individually in boxes (8 × 12-cm height) 24 h before males to allow for web building. Each male was used only once. Events were transcribed from digital videos using J Watcher 0.9 (Blumstein et al. 2000).
Copulation duration and courtship according to female reproductive status
Two sets of females that differed in status were used: virgin individuals (n = 22; hereafter “virgin females”) and the same animals but after a single copulation (n = 22; hereafter “mated females”). Females are receptive for subsequent mating between 6 and 14 h after mating, therefore we initiated the “mated” trials after 6 h (Calbacho-Rosa et al. 2010). For each trial, we measured duration (in minutes) of copulation and courtship. We considered the initiation of courtship to be when individuals made first contact with their legs and the conclusion of courtship was recorded when copulation started. Females were replaced by new females if unresponsive for 20 min (despite male courtship). Unresponsive females were not used again. When mated females were unresponsive, data from when they were used as virgins were discarded. Therefore, we always used paired data from females: a first trial as a virgin and a second trial as a mated. After copulation, the mating male was removed. Each male was used only once (n = 44).
Pedipalp movement patterns, frequency, and duration according to female reproductive status
To answer whether there were differences across female reproductive status in male pedipalp movements during copulation, we used a subset of 15 females recorded in both virgin and mated trials. We recorded two patterns of pedipalp movements: alternating pedipalp movements and simultaneous pedipalp movements (for full description, see “Results” section). For alternating pedipalp movements, we recorded the frequency of occurrence, the absolute duration and relative duration (compared to the total duration of copulation). For simultaneous pedipalp movements, we measured the same variables. Frequency of occurrence corresponded to the number of pedipalp movements performed during mating, while absolute duration corresponded to the total time (in minutes) performing a movement. The relative duration corresponded to the percentage of the duration of copulation spent performing a movement. We also calculated frequency of occurrence and absolute duration of pedipalp movements in intervals of 3 min, to determine if the pedipalp movement patterns were constant or varied between mating events with females of different reproductive status (virgin vs. mated). Although this 3-min interval was chosen arbitrarily, it allowed us to detect whether simultaneous pedipalp movement patterns were constant or varying within the 50-min copulation duration range. A similar interval and approach have been used for other spiders (e.g., Schäfer and Uhl 2002). Finally, we estimated the frequency of male abdominal vibrations during trials with virgin and mated females.
Copulation duration and associated behaviors: a comparison between mating events with virgin females and mated females
We explored the effect of male behavior on copulation duration. For this we used the virgin vs. mated females setting previously described and explored within each female set (virgin and mated) how male pedipalp behavior predicts copulation duration (in minutes).
Testing a sperm removal function for male pedipalp movements
To test whether male pedipalp movements function to remove stored sperm, we used three groups of virgin females (n = 15 each). The first group was allowed to mate once (hereafter, the “one completed mating” group). The second group was allowed to mate twice (with a 6-h period in between each mating), but their second mating was interrupted when the male no longer produced a pattern of alternating pedipalp movements (hereafter, the “interrupted second mating” group). We interrupted mating events at this time, as preliminary observations suggested that at this stage of copulation males had not yet initiated sperm transfer (L. Calbacho-Rosa, personal observation). Thus, if pedipalp movements function to remove stored sperm, we ought to detect it at this stage. One way to find such effect is via measuring the stored sperm masses. Mating interruption was produced by gently shaking the container. A third group was allowed to complete two mating events also with a 6-h period in between each mating (Fig. 1) (hereafter, the “two completed mating events” group). After copulation, females were preserved in 80 % ethanol for subsequent estimation of relative sperm mass volume.
For quantification of relative sperm mass volume, females were dissected so that we could see the genital cavities covered with sperm masses. Sperm deposits were typically small round masses with similar height and mainly varied in diameter. We always confirmed that putative sperm masses contained sperm by observing each mass under light microscope (Nikon SMZ 1500 equipped with a Nikon Sight DS-FI1 digital camera). Masses could not be collected as they were too small and heavily attached to the genital cavity. Thus, our best logistic decision was to photograph the entire genital cavity. The photographer (I G-M) of each genital cavity was unaware of the female group to avoid bias. Then, boundaries of all masses for each female were measured using the software Image Tool® (UTHSCSA). To reduce the variance in sperm mass volume for all females, we assigned 100 % to those females that had their entire genital cavity covered by the sperm mass, which was then used as a reference data point for females that had less than 100 % of sperm (i.e., their genital cavity not entirely covered). This sperm value is regarded hereafter as “relative sperm mass volume.”
Statistical analyses
All analyses were carried out using generalized linear models (GLM) given that this procedure allows for response variables that have distributions other than a normal distribution, which was the case for some response variables in this study. Although response variables can be transformed to improve linearity and homogeneity of variance, this is not recommended. For the analysis of duration of copulation and courtship, we used two general linear models to see whether duration of copulation (model 1) and/or courtship (model 2), entered as response variables, differed when males mated with females of different status (virgin vs. mated) which was entered as a fixed factor. Female identity was entered as a random factor as each female was used twice. For the analysis of pedipalp movement patterns, we also used GLMs. In a first set of models, we investigated whether alternative pedipalp movements differed in frequency of occurrence (model 3), absolute duration (model 4), and relative duration (model 5) (these three terms entered as response variables, respectively) according to female status (virgin or mated; entered as factor). Female identity was entered as random factor. In a second set of models, we investigated whether simultaneous pedipalp movements also differed in the same three variables (thus, using models 1 to 3) with the same fixed (virgin or mated females) and random (female identity) factors. To assess the frequency of occurrence and absolute duration of pedipalp movements every 3 min with females of different reproductive status (virgin vs. mated), we examined boxplots (medians and quartiles) of each response variable across female reproductive status. For frequency of occurrence that males performed abdominal vibration according to female mating history, we also used a GLM again entering female identity as a random factor. To explore the relationship between male pedipalp behaviors and mating duration according to female mating history, we carried out GLMs. This was done to see whether frequency of occurrence (model 6) and absolute duration (model 7) for alternating pedipalp movements and frequency of occurrence (model 8) and absolute duration (model 9) for simultaneous pedipalp movements (these four as response variables) explained duration copulation (entered as covariable) according to female mating history (virgin and mated; entered as fixed factor). Female identity was entered as a random factor. Given that the interaction between terms “copulation duration” and “female mating history” was significant for models 6 to 9 (p < 0.0001), we proceeded with testing main effects for each of the four response variables separately. For testing for differences in relative sperm mass volume ejected according to female mating category, a GLM was also used. In this analysis, relative sperm mass volume values were entered as response variable and female mating category as factor. All GLMs used a logit link function to specify a binomial error structure. Post hoc comparisons were carried out using a Tukey test.
Results
Male copulation duration and courtship according to female reproductive status
Copulation duration was shorter with virgin females compared with mated females (F 1,42 = 18.66, p < 0.0001; Fig. 2). However, there was no difference in courtship duration across either type of female (F 1,42 = 0.128; p = 0.722; Fig. 2).
Pedipalp movement patterns, frequency, and duration according to female reproductive status
Two pedipalp movement patterns were observed. First, males sometimes moved a single pedipalp at a time in a non-rhythmic and alternating pattern (hereafter “alternating pedipalp movements”). This movement was carried out during the first 3 or 4 min in copulations with mated females and very sporadically in copulations with virgin females. During these movements, the male partially introduced the procursus of each pedipalp into the female’s gonopore. During these movements, the males did not insert either the dorsal or ventral apophyses genital bulbs into the gonopore (see related videos in Online Resources 1 and 2). In the second type of movement, males moved both pedipalps in a rhythmical and simultaneous manner (hereafter “simultaneous pedipalp movements”). In this movement, both pedipalps contracted and relaxed in a regular fashion (see Online Resource 3). This movement was commonly observed during copulations with virgin (n = 15) and mated females (n = 15). During this pattern of movement, the male introduced both the procursus and the two bulbal apophyses into the gonopore. The dorsal and ventral bulbal apophyses were inserted into the corresponding cavities of the external uterus.
Frequency of occurrence and absolute duration of simultaneous pedipalp movements were higher in mating events with mated females compared to mating events with virgin females (frequency of occurrence, F 1,26 = 15.136, p = 0.001, Fig. 3; absolute duration, F 1,26 = 16.704, p < 0.0001, Fig. 4). Nevertheless, no difference was found in the relative duration of simultaneous pedipalp movements in mating events with virgin females versus mated females (F 1,26 = 2.862, p = 0.104, Fig. 5). Frequency of occurrence (F 1,26 = 85.293, p < 0.0001, Fig. 3), absolute duration (F 1,26 = 25.071, p < 0.0001, Fig. 4) and relative duration (F 1,26 = 35.725, p < 0.0001, Fig. 5) of alternating pedipalp movements were greater in mating events with mated females than with virgin females. Simultaneous pedipalp movements varied between mating events. In mating events with virgin females, the frequency of occurrence was high during the first minutes, and decreased as the copulation continued (Fig. 6a). In contrast, in mating events with mated females, the frequency of occurrence of simultaneous pedipalp movements remained constant during copulation (Fig. 6a). A similar constant pattern was observed for the duration of simultaneous pedipalp movement in mating events with mated females (Fig. 6b).
a Frequency of occurrence of simultaneous pedipalp movements in copulations with virgin (n = 15) and mated females (n = 15), respectively in intervals of 3 min. b Duration of simultaneous pedipalp movements in copulations with virgin (n = 15) and mated females (n = 15), respectively in intervals of 3 min. Box plots indicate median values with quartiles
During the first minutes of copulation with mated females, a white irregular mass was seen coming out from the female genital opening (Fig. 7a). This mass was expelled some seconds after mating started and coincided with the phase of alternating pedipalp movements. As mentioned previously, the male exclusively performed this movement pattern on the area where the female stores the sperm from previous copulations, which is in the dorsal (i.e., internal) side of the epigyne, close to the genital opening (Fig. 7b). Some bits of sperm were attached to the male procursus (see related video in Online resources 1 and 2). Such mass never emerged during the phase of simultaneous pedipalp movements. When analyzed under a microscope, presence of encapsulated sperm was observed (Fig. 7c).
The frequency of occurrence of male abdominal vibration showed a tendency of being higher in copulations with mated females (29.285 ± 27.581) compared to virgin females (15.963 ± 7.065; F 1,26 = 3.29, p = 0.088).
Copulation duration and associated behaviors: a comparison between mating events with virgin females and mated females
Frequency (F 1,29 = 61.692, p < 0.0001) and duration (F 1,26 = 26.480, p < 0.0001) of alternative pedipalp movements and frequency (F 1,25 = 15.136, p = 0.001) and duration (F 2,26 = 12.459, p < 0.002) of simultaneous pedipalp movements significantly explained copulation duration according to female mating history. These results indicate that the frequency and duration of both pedipalp movements are positively associated with the duration of both copulations with virgin females and mated females.
Function of male pedipalp movement patterns
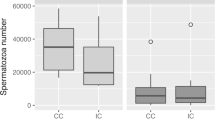
There were differences among female groups (one completed mating event vs. two completed mating events, vs. interrupted second mating events) in stored sperm mass volume (F 2,39 = 7.533, p = 0.002; Fig. 8): females of the “one completed mating event” group and those of the “two completed mating events” group did not differ (Tukey test, p > 0.05); however, both groups had considerably higher relative sperm mass volumes compared to the “interrupted second mating” group (Tukey tests for both comparisons, p < 0.05).
Discussion
In H. pluchei, we found that female reproductive status (virgin or mated) influenced male mating behavior in terms of the time spent in copulation and frequency and duration of pedipalp movement patterns. We also found that copulation duration is influenced by frequency and duration of pedipalp movement pattern (and, to a minor extent, abdominal vibration) and that different types of pedipalp movements are associated with different copulatory functions. These results are consistent with sexual selection theory: males which mate with mated females incur the increased risk of male–male competition (via sperm competition; Parker 1979) as opposed to mating with a virgin female. Thus, male mating behavior ought to differ if the copulating partner is a mated female. This pattern has wide support in the literature. Several examples can be found in the ability of males to control copulation duration according to female’s reproductive status in arthropods (e.g., Andrés and Cordero Rivera 2000; Marcotte et al. 2005; Solensky and Oberhauser 2009). In accordance with our prediction, males engaged in longer mating events and more complex pedipalp and abdominal movements when mating with mated females than with virgin females. Nevertheless, our results of copulation duration were inconsistent with prior results in other pholcids (e.g., copulation duration with virgin females was found to be longer in Pholcus phalangioides; Schäfer and Uhl 2002 and Physocyclus globosus; Huber and Eberhard 1997; Peretti et al. 2006; Peretti and Eberhard 2010). One explanation for the difference in results is that H. pluchei males could stimulate mated females using abdominal vibratory movements as a form of copulatory courtship. Such stimulation may induce the female to use the current male’s sperm for fertilization instead of previous mating males’ sperm. However, note that we detected a non-significant tendency of higher frequency of abdominal vibrations in mating events with mated females compared to mating events with virgin females. In P. globosus, males also perform abdominal vibrations during copulation, causing both the female and the web to shake (Peretti et al. 2006), which was also interpreted as a male stimulation to influence female fertilization decisions (Huber and Eberhard 1997). Contrary to our prediction, the duration of courtship in H. pluchei was not significantly affected by female mating history, a result that has also been found in P. phalangoides (Schäfer and Uhl 2002). However, it may not be duration of courtship per se but the nature of pedipalp movements what may affect fertilization. The frequency of occurrence and duration of simultaneous and alternating pedipalp movements were linked to the duration of copulations with both virgin and mated females. Our observations of pedipalp movement patterns in H. pluchei agree with those of Schäfer and Uhl (2002) in P. phalangioides, where the frequency of pedipalp movements in copulations with virgin and mated females is not constant. In P. globosus (Huber and Eberhard 1997), males also perform strong squeezing and twisting movements, which are more frequent at the onset and end of copulation.
Rhythmical pedipalps movements are common and highly diverse among Pholcids (reviewed in Huber and Eberhard 1997). Similar patterns of shifts in frequencies and durations of pedipalp insertions in copulations with virgin and mated females have been observed in many spider species (Elgar and Bathgate 1996; Huber and Eberhard 1997; Aisenberg and Costa 2005; Peretti et al. 2006; Schäfer et al. 2008; Aisenberg 2009). Although we found that frequency and duration also differed in H. pluchei according to female reproductive status, we also detected two pedipalp movement types: alternating pedipalp movements and simultaneous pedipalp movements. We interpret these movements as operating in different sperm competition-related functions. Alternating pedipalp movements—which are quickly alternated, almost superficial and disordered—coincide with sperm mass ejection from the female’s genital opening. We suggest that the function of this alternating movement is to remove previously stored, rival sperm. This hypothesis is based on: (a) the time of copulation in which sperm ejection occurred (at the beginning of the second copulation), (b) the specific region in which procursus of each pedipalps were inserted (the internal, dorsal, side of the epigyne that contains stored sperm), and (c) the fact that sperm were often attached to the male procursus (see videos as Online Resources 1 and 2). During alternating pedipalp movement, the two apophyses of the genital bulb remain completely outside the female. Therefore, it is not possible that the male transfers its own sperm during alternating pedipalp movements. Removal of rival sperm is a widespread trait and can be mechanically achieved using sexual (i.e., genitalic) as well as non-sexual morphological structures (reviewed by Leonard and Córdoba-Aguilar 2010).
Removal of sperm has been described for several arthropod species. For example, in odonates, a first stage takes place via removal of rival sperm, which is followed by sperm transfer (reviewed by Córdoba-Aguilar et al. 2003). In orthopterans, von Helversen and von Helversen (1991) also found that the sperm content of females whose second mating was interrupted was lower than that of females with one and two completed mating events. One alternative hypothesis to rival sperm removal in our study could be that pedipalp movements serve to stimulate the female’s sensory system so that she actively ejects her stored sperm. Related to this, in other spider species such as P. phalangioides (Uhl 1994; Schäfer and Uhl 2002; Schäfer et al. 2008), the ejection of sperm is also sometimes observed in virgin females. Interestingly, in P. globosus females eject sperm at the end of copulation (Huber and Eberhard 1997; Peretti and Eberhard 2010). We cannot discard a possible mechanism of cryptic female choice in our study subject, as has been hypothesized for other arthropods in cases where the female participates in sperm ejection (e.g., Eberhard 1994, 1996; Córdoba-Aguilar 1999; Snook and Hosken 2004; Burger 2007; Peretti and Eberhard 2010). In some species, sperm removal principally acts upon stored sperm from previous mating events (Otronen and Siva-Jothy 1991; Snook and Hosken 2004; Burger 2007), although in other species it belongs to the current mating partner (Eberhard 1996; Pizzari and Birkhead 2000; Rodríguez et al. 2004). Finally, one final possibility is that stored sperm is acted upon by both male-driven sperm removal processes and female-driven active ejection.
In pholcids, simultaneous pedipalp movements have been linked to a male stimulatory role (Huber and Eberhard 1997; Schäfer and Uhl 2002; Peretti and Eberhard 2010). During simultaneous pedipalp movements, pedipalp squeezing allows the procursus to enter the female gonopore. Since the procursus is not connected to the sperm duct, some have suggested that this pattern of movements could be a form of genital stimulation (Eberhard 1996; Peretti et al. 2006). In H. pluchei, simultaneous pedipalp movements involve strong squeezing and twisting movements. The procursi are then situated medially while the bulbal apophyses are inserted laterally into the external uterus. Huber (1995) also documented in this species that the dorsal and ventral bulbal apophyses are inserted into corresponding cavities, pressing the bulb at every turn against the female body and subsequently allows sperm to enter the gonopore. Thus, at least one function for simultaneous pedipalp movements is sperm transfer sensu stricto. However, although these movements have a higher frequency of occurrence and longer absolute duration in copulations with mated females compared with copulations with virgin females, the final sperm volume in twice-mated females is not significantly different from single-mated females. In addition, we can conclude that the second male transfers less sperm than the first male since we commonly observed that a part of the previous male’s sperm mass remained in the female even after the sperm removal phase ended. Possibly, the longer and more frequent simultaneous pedipalp movements carried out with females in their second mating events not only function to transfer more sperm but also as genitalic copulatory courtship. Genitalic copulatory courtship behavior is apparently quite common in spiders (Eberhard 1994, 2004; Huber 1998, 2005), but its effects have rarely been studied (Schäfer and Uhl 2002). The absolute number of pedipalp movements has been associated with paternity values in other pholcids: the higher the movement frequency, the higher the paternity (Huber and Eberhard 1997; Schäfer and Uhl 2002; Schäfer et al. 2008; Peretti et al. 2006; Peretti and Eberhard 2010).
In our study species, fertilization success depends to a large extent on the time males guard their mates (Calbacho-Rosa et al. 2010) so that there is a narrow window of opportunity for subsequent males to influence fertilization success. Based on the current results, we hypothesize that subsequent male success depends on the effectiveness of sperm removal and/or transfer. Thus, further sexual selection studies in H. pluchei could focus on elucidating the effect of pedipalp movements on fertilization success via sperm removal and/or transfer.
References
Aisenberg A (2009) Male performance and body size affect female re-mating occurrence in the orb-web spider Leucauge mariana (Araneae, Tetragnathidae). Ethology 115:1127–1136
Aisenberg A, Costa FG (2005) Females mated without sperm transfer maintain high sexual receptivity in the wolf spider Schizocosa malitiosa. Ethology 111:545–558
Andrés J, Cordero Rivera A (2000) Copulation duration and fertilization success in a damselfly: an example of cryptic female choice? Anim Behav 59:695–703
Austad SN (1984) Evolution of sperm priority patterns in spiders. In: Smith RL (ed) Sperm competition and the evolution of mating systems. Harvard University Press, Cambridge, pp 223–249
Blumstein D T, Evans C S, Daniel J C (2000) JWatcher. http://galliform.psy. mq.edu.au/jwatcher/. Accessed 20 Nov 2008
Burger M (2007) Sperm dumping in a haplogyne spider. J Zool (Lond) 273:74–81
Calbacho-Rosa L, Córdoba-Aguilar A, Peretti A (2010) Occurrence and duration of post-copulatory mate guarding in a spider with last sperm precedence. Behaviour 147:1267–1283
Coddington JA, Levi HW (1991) Systematics and evolution of spiders (Araneae). Annu Rev Ecol Syst 22:565–592
Córdoba-Aguilar A (1999) Male copulatory sensory stimulation induces female ejection of rival sperm in a damselfly. Proc R Soc Biol Sci Ser B 266:779–784
Córdoba-Aguilar A, Uhía E, Cordero Rivera A (2003) Sperm competition in Odonata (Insecta): the evolution of female sperm storage and rivals’ sperm displacement. J Zool (Lond) 261:381–398
Eberhard WG (1991) Copulatory courtship and cryptic female choice in insects. Biol Rev 66:1–31
Eberhard WG (1994) Evidence for widespread courtship during copulation in 131 species of insects and spiders, and implications for cryptic female choice. Evolution 48:711–733
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Eberhard WG (2001) The functional morphology of species-specific clasping structures on the front legs of male Archisepsis and Palaeosepsis flies (Diptera, Sepsidae). Zool J Linn Soc 133:335–368
Eberhard WG (2004) Why study spider sex: special traits of spiders facilitate studies of sperm competition and cryptic female choice. J Arachnol 32:545–556
Eberhard WG (2009) Postcopulatory sexual selection: Darwin’s omission and its consequences. Proc Natl Acad Sci USA 106:10025–10032
Eberhard WG (2011) Experiments with genitalia: a commentary. Trends Ecol Evol 26:17–21
Edvardsson M, Arnqvist G (2000) Copulatory courtship and cryptic female choice in red flour beetles Tribolium castaneum. Proc R Soc Biol Sci Ser B267:559–563
Elgar MA, Bathgate R (1996) Female receptivity and male mate-guarding in the jewel spider Gasteracantha minax Thorell (Araneidae). J Insect Behav 9:729–738
Foelix RF (1982) The biology of spiders. Harvard University Press, Cambridge
Herbestein ME (2011) Spider behaviour: flexibility and versatility. Cambridge University Press, Cambridge
Huber BA (1995) Copulatory mechanism in Holocnemus pluchei and Pholcus opilionoides, with notes on male cheliceral apophyses and stridulatory organs in Pholcidae (Araneae). Acta Zool (Stockh) 76:291–300
Huber BA (1998) Genital mechanics in some neotropical pholcid spiders (Araneae: Pholcidae) with implications for systematics. J Zool (Lond) 244:587–599
Huber BA (2005) Sexual selection research on spiders: progress and biases. Biol Rev 80:363–385
Huber BA, Eberhard WG (1997) Courtship, copulation, and genital mechanics in Physocyclus globosus (Araneae, Pholcidae). Can J Zool 74:905–918
Jakob EM (1991) Costs and benefits of group living for pholcid spiderlings: losing food, saving silk. Anim Behav 41:711–722
Kaster JL, Jakob EM (1997) Last-male sperm priority in a haplogyne spider (Araneae: Pholcidae): correlations between female morphology and patterns of sperm usage. Ann Entomol Soc Am 90:254–259
Kelly CD, Jennions MD (2011) Sexual selection and sperm quantity: meta-analyses of strategic ejaculation. Biol Rev 86:863–884
Leonard J, Córdoba-Aguilar A (eds) (2010) The evolution of primary characters in animals. Oxford University Press, Oxford
Marcotte M, Delisle J, McNeil JN (2005) Impact of male mating history on the temporal sperm dynamics of Choristoneura rosaceana and C. fumiferana females. J Insect Physiol 51:537–544
Michiels NK (1989) Morphology of male and female genitalia in Sympetrum danae (Sulzer), with special reference to the mechanism of sperm removal during copulation (Anisoptera: Libellulidae). Odonatologica 18:21–31
Naud MJ, Hanlon RT, Hall KC, Shaw PW, Havenhand JN (2004) Behavioural and genetic assessment of reproductive success in a spawning aggregation of the Australian giant cuttlefish, Sepia apama. Anim Behav 67:1043–1050
Ono T, Siva-Jothy MT, Kato A (1989) Removal and subsequent ingestion of rivals’ semen during copulation in a tree cricket. Physiol Entomol 14:195–202
Otronen M (1990) Mating behavior and sperm competition in the fly, Dryomyza anilis. Behav Ecol Sociobiol 26:349–356
Otronen M, Siva-Jothy MT (1991) The effect of postcopulatory male-behavior on ejaculate distribution within the female sperm storage organs of the fly, Dryomyza anilis (Diptera, Dryomyzidae). Behav Ecol Sociobiol 29:33–37
Parker GA (1979) Sexual selection and sexual conflict. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition in insects. Academic Press, New York, pp 123–166
Peretti AV, Eberhard WG (2010) Cryptic female choice via sperm dumping favours male copulatory courtship in a spider. J Evol Biol 23:271–281
Peretti AV, Eberhard W, Briceño D (2006) Copulatory dialogue: female spiders sing during copulation to influence male genitalic movements. Anim Behav 72:413–421
Pilastro A, Scaggiante M, Rasotto MB (2002) Individual adjustment of sperm expenditure accords with sperm competition theory. Proc Natl Acad Sci U S A 99:9913–9915
Pizzari T, Birkhead T (2000) Female feral fowl eject sperm of subdominant males. Nature 405:787–789
Rodríguez V, Windsor D, Eberhard WG (2004) Tortoise beetle genitalia and demonstration of a sexually selected advantage for flagellum length in Chelymorpha alternans (Chrysomelidae, Cassidini, Stolaini). In: Jolivet P, Santiago-Blay JA, Schmitt M (eds) New developments in the biology of Chrysomelidae. Academic Publishing, The Hague, SPB, pp 739–748
Schäfer MA, Uhl G (2002) Determinants of paternity success in the Pholcus phalangioides (Pholcidae, Araneae): the role of male and female mating behaviour. Behav Ecol Sociobiol 51:368–377
Schäfer MA, Misof B, Uhl G (2008) Effects of body size of both sexes and female mating history on male behaviour and paternity success in a spider. Anim Behav 76:75–86
Simmons LW (2001) Sperm competition and its evolutionary consequences in the insects. Princeton University Press, Princeton, New Jersey
Snook RR, Hosken DJ (2004) Sperm death and dumping in Drosophila. Nature 428:939–941
Solensky MJ, Oberhauser KS (2009) Male monarch butterflies, Danaus plexippus, adjust ejaculates in response to intensity of sperm competition. Anim Behav 77:465–472
Uhl G (1994) Genital morphology and sperm storage in Pholcus phalangioides (Fuesslin, 1775) (Pholcidae: Araneae). Acta Zool (Stockh) 75:1–12
von Helversen D, von Helversen O (1991) Pre-mating sperm removal in the bush cricket Metaplastes Ramme 1931 (Orthoptera, Tettigonoidea, Phaneropteridae). Behav Ecol Sociobiol 28:391–396
Waage JK (1979) Dual function of the damselfly penis: sperm removal and sperm transfer. Science 203:916–918
Wada T, Takegaki T, Mori T, Natsukari Y (2006) Reproductive behavior of the Japanese spineless cuttlefish Sepiella japonica. Venus 65:221–228
Wada T, Takegaki T, Mori T, Natsukari Y (2010) Sperm removal, ejaculation and their behavioral interaction in male cuttlefish in response to female mating history. Anim Behav 79:613–619
Acknowledgments
We thank Margarita Chiaraviglio and Cristina Sciocia for suggestions on the manuscript. The authors also acknowledge D. Vrech and P. Olivero for help during collecting and rearing. Financial support was provided by the Consejo Nacional de Investigaciones Científicas y Técnicas, FONCYT, and Secretaria de ciencia y técnica–Universidad Nacional de Córdoba of Argentina and Agencia Nacional de Investigación, España. Four reviewers and Chris Anderson provided highly useful comments to previous versions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by: Sven Thatje
Electronic supplementary material
Below is the link to the electronic supplementary material.
Male carrying out alternating pedipalp movements, overview (MPG 3214 kb)
Male carrying out alternating pedipalp movements, detailed view (MPG 1720 kb)
Male carrying out simultaneous pedipalp movements (MPG 4272 kb)
Rights and permissions
About this article
Cite this article
Calbacho-Rosa, L., Galicia-Mendoza, I., Dutto, M.S. et al. Copulatory behavior in a pholcid spider: males use specialized genitalic movements for sperm removal and copulatory courtship. Naturwissenschaften 100, 407–416 (2013). https://doi.org/10.1007/s00114-013-1038-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-013-1038-1