Abstract
The distribution of parasites is often characterised by substantial aggregation with a small proportion of hosts harbouring the majority of parasites. This pattern can be generated by abiotic and biotic factors that affect hosts and determine host exposure and susceptibility to parasites. Climate factors can change a host’s investment in life-history traits (e.g. growth, reproduction) generating temporal patterns of parasite aggregation. Similarly, host age may affect such investment. Furthermore, sex-biased parasitism is common among vertebrates and has been linked to sexual dimorphism in morphology, behaviour and physiology. Studies exploring sex-biased parasitism have been almost exclusively conducted on polygynous species where dimorphic traits are often correlated. We investigated the effects of season and life-history traits on tick loads of the monogamous eastern rock sengi (Elephantulus myurus). We found larger tick burdens during the non-breeding season possibly as a result of energetic constraints and/or climate effects on the tick. Reproductive investment resulted in increased larval abundance for females but not males and may be linked to sex-specific life-history strategies. The costs of reproduction could also explain the observed age effect with yearling individuals harbouring lower larval burdens than adults. Although adult males had the greatest larval tick loads, host sex appears to play a minor role in generating the observed parasite heterogeneities. Our study suggests that reproductive investment plays a major role for parasite patterns in the study species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The distribution of parasites across hosts is often characterised by heterogeneities with a small number of hosts harbouring the majority of parasites (Wilson et al. 2001; Poulin 2007). Such heterogeneities are caused by differences between individual hosts in exposure and susceptibility to parasites. Variance in exposure and susceptibility can be linked to abiotic as well as biotic factors acting on parasites and their hosts. Climate variables such as temperature and rainfall may affect the survival of parasites and thus, determine host exposure (Randolph 2004). Energy and nutrient availability varies seasonally and influences the resources accessible to hosts for parasite defence (Nelson et al. 2002; Altizer et al. 2006; Martin et al. 2008). As a result, hosts may suffer more from disease and parasites during winter when a period of reduced availability of resources coincides with increased thermoregulatory demands (Nelson 2004). Alternatively, seasonal variation in abiotic factors (e.g. temperature) may result in greater parasite burdens during warmer months (e.g. Randolph et al. 2002). Seasonal patterns in parasite burdens can furthermore be caused when the proportion of susceptible individuals in the host population varies throughout the year. This may be the case during reproduction as it is one of the most energetically costly life-history traits and, accordingly, immune responses are often compromised during reproduction. This may also be attributed to increases in immunosuppressive hormones (i.e. testosterone). Indeed, elevated parasite loads in reproductively active animals have been reported for a number of mammal species (e.g. Christe et al. 2000; Mougeot et al. 2004; Pelletier et al. 2005; Lourenco and Palmeirin 2008). The recruitment of naive hosts (i.e. those that have not developed immunity yet) during the breeding season may further contribute to the establishment of seasonal patterns of parasitism and can also introduce age-dependent heterogeneities (Cattadori et al. 2005; Cornell et al. 2008). However, parasite loads can also be larger in older hosts when parasites accumulate over time or when survival is reduced in younger individuals with high parasite loads compared to older individuals with similar burdens (Soliman et al. 2001; Hawlena et al. 2006).
The degree of parasitism is often affected by host sex and male-biased parasite loads are common in vertebrates (Moore and Wilson 2002; Klein 2004; Morand et al. 2004; Krasnov et al. 2005). The causes for such sex biases in parasite burdens have been linked to morphological, behavioural as well as physiological mechanisms. If host size is equated with patch size for a parasite, then larger hosts may be able to sustain larger numbers of parasites. Consequently, males will harbour greater parasite numbers in species with a male-biased sexual size dimorphism (Moore and Wilson 2002; Krasnov et al. 2005; Harrison et al. 2010). Behavioural differences, such as sex-specific ranging patterns, have also been linked to sex biases in parasite burdens (e.g. Morand et al. 2004; Krasnov et al. 2005; Scantlebury et al. 2010). Alternatively, it has been suggested that the immunosuppressive properties of testosterone may be responsible for male-biased parasite loads (Folstad and Karter 1992; Zuk and McKean 1996; Hughes and Randolph 2001; Klein 2004).
In species with polygynous mating systems, it is often difficult to distinguish between the alternative hypotheses explaining sex biases in parasite burdens as sex differences in body mass, ranging patterns and androgen levels tend to be highly correlated (e.g. Olsson et al. 2000; Mougeot et al. 2005). However, the majority of studies on sex-biased parasitism in mammals have been conducted on species with polygynous mating systems, some of which exhibit no sexual dimorphism (e.g. Morand et al. 2004; Krasnov et al. 2005; Harrison et al. 2010; Scantlebury et al. 2010). In comparison, the only study assessing parasite loads in a monogamous mammal, characterised by a lack of dimorphism with respect to body mass and home range, found female bias in parasitism with the authors suggesting a link to greater reproductive effort in females (Porteus and Pankhurst 1998).
Sengis (or elephant shrews) belong to the order Macroscelidea within the superorder Afrotheria, an ancient monophyletic group that comprises elephants, hyraxes, tenrecs, golden moles, sirenians and aardvarks (Skinner and Chimimba 2005). This order is endemic to Africa and encompasses 17 species belonging to four genera all of which are believed to be monogamous (Rathbun 1979). Indeed, field evidence suggests large home range overlap between pairs and little overlap among neighbours as well as a lack of sexual dimorphism for a number of sengi species (e.g. Rathbun 1979; FitzGibbon 1997; Ribble and Perrin 2005; Rathbun and Rathbun 2006; Schubert et al. 2009a). The monogamous mating system of sengis is thought to be the result of male mate guarding, and although attempts by males to include unmated females in their home range have been reported repeatedly, they were only of short duration (Rathbun 1979; Fitzgibbon 1997; Ribble and Perrin 2005; Rathbun and Rathbun 2006; Schubert et al. 2009a). Sengis are known to carry a large number of ectoparasites, particularly ticks (e.g. Fourie et al. 1995; 2005). However, until now reports on sengi parasites have been largely descriptive and have not considered the effects of life-history traits on parasite loads. We investigated the role of reproductive effort on tick burdens using data collected from a population of eastern rock sengis (Elephantulus myurus) collected monthly over 1 year. We hypothesized that if (a) reproductive effort is the main factor affecting tick loads, tick burdens should vary seasonally (breeding vs. non-breeding period). Similarly, (b) adults should carry greater tick burdens than yearling animals as the latter have a limited investment in reproduction. In addition, (c) sex biases in tick burdens were predicted if susceptibility and/or exposure to ticks is linked to sex-specific traits (i.e. behaviour, physiology) while this was not expected to be the case if patch size (i.e. body size) was the key factor limiting tick burdens. Since reproductive investment may differ between the sexes, we furthermore evaluated the effect of reproductive status (i.e. pregnancy) and investment in reproductive tissues (i.e. testes size) on tick burdens of females and males, respectively.
Materials and methods
Sengis were collected at Goro Game Reserve in the Limpopo Province, South Africa (22°58′S, 29°25′E) under permit number CPM-333-00002 from the Department of Environmental Affairs, Limpopo Province. Animals were trapped with Sherman live traps baited with a mixture of oats, peanut butter and sardines, and set overnight around rocky outcrops. Sengis were caught on a monthly basis from September 2007 to August 2008 as part of a study of their reproductive biology that aimed to collect ten individuals (five of each sex) per month (Medger 2010). The mass of animals was recorded to the nearest 0.1 g on the day of capture using a digital balance (Scout Pro SPU123; Ohaus Corporation, USA). Animals were housed in standard rodent cages with wood shavings as bedding and maintained on a diet of Pronutro (high protein cereal; Pioneer Foods Ltd, South Africa), canned dog food (Promeal Ltd, South Africa), meal worms as well as grated apples and carrots with water freely available in the field and during transport to the laboratory. All animals were searched for ticks no later than 3 days after arrival in the laboratory, and ticks were removed using tweezers and stored in 70% ethanol until identification. Ticks were identified, counted and their developmental stage recorded as larvae, nymph or adult under a stereo-microscope. In an unrelated study of wild-caught sengis kept in the laboratory with their natural tick burdens, significant numbers of Rhipicephalus near warburtoni did not drop off their hosts until a minimum of 7 days had elapsed. Furthermore, animals sampled immediately after capture in the field exhibited similar tick abundances to those reported in the current study (H. Lutermann, unpublished data). Hence, we are confident that the tick burdens obtained with the methods described correspond to those in the field. Animals were euthanised and dissected as part of another study on their reproductive biology that also recorded the presence of embryos for females and testes mass (to the nearest 0.0001 g) for males (Medger 2010). Animals were classified in six relative age classes based on the degree of maxillary molar tooth wear and eruption (Medger 2010). Based on their annual distribution, animals of age classes one to four were likely be in their first year and thus regarded as yearlings, while animals of the remaining two age classes were considered adults. All procedures were approved by the animal ethics committee of the University of Pretoria (EC028-07).
Statistical analyses
Firstly, we evaluated the degree of aggregation of both tick larvae and nymphs among their hosts by calculating the moment k using the maximum likelihood approach implemented in the program Quantitative Parasitology 3.0 (Rózsa et al. 2000). In addition, we assessed variation in larval and nymphal tick abundances by employing generalized linear models (GLZs) with a negative binomial distribution and a log-link function with season (breeding, non-breeding), host sex and age class (i.e. yearling, adult) as independent variables. Sample size limitations precluded a meaningful analysis considering monthly data. We used an information-theoretic approach that allows testing a suit of hypotheses simultaneously and calculated the Akaike values (AIC) for all possible models (Burnham and Anderson 2002). Models were ranked based on their AICs from lowest to highest to evaluate their fit (ΔAIC) and AICs were corrected for small sample size (ΔAICC, Burnham and Anderson 2002). Following Symonds and Moussalli’s (2011) recommendations, we furthermore calculated the Akaike weight (ω i ) that allows evaluating the probability that a given model has the best fit. In addition, we calculated the evidence ratio (ER) as a measure of how much more likely one model is compared to another one (Symonds and Moussalli 2011). Based on the presence of pregnant and lactating females in the study population, we defined the period from August to March as breeding season and the remainder of the year (April to July) as non-breeding season. All two- and three-way interactions were included in the model. Initially, body mass was included in the models. However, since this covariate received little support, we only report models excluding body mass.
Males and females frequently employ sex-specific life-history strategies to maximise their fitness, and the physiological costs of pregnancy and lactation are generally assumed to be substantially greater than the costs of sperm production (Rolff 2002). To evaluate possible differential effects of sex-specific investment strategies, we repeated the above analysis separately for the two sexes. For males, we added testes mass (corrected for body mass) as covariate in the GLZ. For females, we included reproductive status (i.e. pregnant/non-pregnant) as variable. As, by definition, pregnant females only occurred during the breeding season, this analysis was restricted to this period. All statistical analyses were carried out in R 2.12.2 (www.r-project.org). Results are presented as means and confidence interval (CI).
Results
A total of 113 elephant shrews (58 females, 55 males) were assessed for their tick burdens. The total number of ticks collected was 22,334 with larvae (20,664) by far exceeding the number of nymphs (1,670). With the exception of one specimen (Haemaphysalis elliptica), all ticks collected belonged to one species, which we choose to call Rhipicephalus sp. near warburtoni. This was based on the examination of adult ticks, moulted from engorged nymphs collected from the sengis as well as preliminary phylogenetic analyses. It is a three-host tick and while the immature stages prefer eastern rock sengis, all stages of development are likely to be found on scrub hares (Lepus saxatilis). To date, R. sp. near warburtoni has only been collected in the Limpopo Province of South Africa. The immature stages of this tick were present on sengis in all months with an overall prevalence of 100% and 98.2% for larvae and nymphs, respectively. Peak abundances were observed during July for both larvae (339.1, CI 200.38–477.82) and nymphs (28.8, CI 20.34–37.26). The lowest burdens for larvae were recorded in February (23.6, CI 11.76–35.44) and in October for nymphs (2.5, CI 1.3–3.7). On average, animals harboured 187.3 (CI 154.36–220.29) larvae and 14.8 (CI 12.74–17.01) nymphs. The distribution of both larvae and nymphs was significantly aggregated (larvae—k = 0.926, χ 2 = 15.45, df = 19, p < 0.05; nymphs—k = 1.521, χ 2 = 23.10, df = 14, p < 0.05).
Factors affecting tick abundance
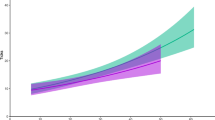
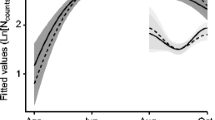
The two best models explaining variation in the abundance of tick larvae contained all main effects as well as the interactions between season, age and sex; together they had a cumulative ω i of 52% (Table 1). The full model had the best fit; however, its AIC differed little from the next best model and it was only 1.26 times more likely than the second best model (Table 1). In comparison, the third best model was already 2.7 times less likely. As Fig. 1 suggests, the larval burdens varied with season (Fig. 1) and were three times as high during the non-breeding (n = 40, 299.7, CI 247.08–359.57) compared to the breeding season (n = 73, 95.1, CI 90.50–157.04). It did not differ between the sexes (females—166.03, CI 126.14–205.93, males—209.78, CI 156.00–263.56) but was greater for adults compared to yearlings (yearling—n = 42, 148.88, CI 98.24–199.52, adults—210.07, CI 166.96–263.18). The cumulative ω i was 78.99% and 72.06% for the interaction between season and age, and sex and age, respectively. Compared to 43.99% for the interaction between season and sex, this suggests that the effect of the former two made a greater contribution to generating the observed variance. Figure 2a suggests greater larval burdens for adults compared to yearling animals during the breeding but not the non-breeding season. Furthermore, Fig. 2b indicates that adult males harboured a substantially higher abundance of larvae than yearling males, while a similar difference was not apparent among females.
For the abundance of nymphs, the best model contained only season as variable and had a 44% probability of being the best model (Table 2). At the same time, it was 2.57 times more likely than the second best model and 6.72 times more likely than the third best model (Table 2), suggesting that other variables contributed little to the observed variation. Indeed, nymphal burdens were almost twice as high during the non-breeding (n = 40, 20.3, CI 17.17–23.61) compared to the breeding season (n = 73, 11.4, CI 9.27–14.43). For all two-way interaction terms, the cumulative probability lay below 0.12% and that for the three-way interaction term was below 0.01.
Effects of reproductive effort
During the breeding season, the model including only the reproductive status of a female had a 61% probability of being the best model (Table 3). Pregnant females had greater larval abundances (n = 16, 160.44, CI 49.94–337.36) than non-pregnant females (n = 22, 72.44, CI 41.83–103.05). The best model was furthermore 2.64 times more likely than the model containing age and reproductive status (Table 3). For males, the best models did not contain testes mass and it had a 58% probability of being the best model (Table 4). However, it was only 1.48 times more likely than the full model. At the same time, it was 26.15 times more likely the best model than the third model containing only the main effects season and age (AICC = 684.13, ω i = 0.02), suggesting that the interaction between season and age has a strong effect on the larval tick burdens among males.
Discussion
In accordance with previous studies (Fourie et al. 1992, 2005), sengis in the current study carried large numbers of tick larvae and nymphs. Ticks were present during all months and despite large variances in the abundance of immature ticks, clear seasonal patterns were apparent for both immature stages. Tick burdens were significantly greater during the non-breeding season contradicting our hypothesis that increases in reproductive effort result in decreased parasite defence. However, it has repeatedly been suggested that the seasonal cessation of breeding activity is linked to the limited availability of nutrients and energy during cold periods, and this may also affect immune function (Nelson et al. 2002; Martin et al. 2008). Indeed, the non-breeding period in our study species coincides with the coldest months of the year (April to July). This, in conjunction with the observation that torpor bouts, an energy-saving strategy, occur more frequently during this period in E. myurus (Mzilikazi and Lovegrove 2004), could be an indication of energetic constraints that may also lower resistance to tick infestation. Climate factors such as temperature and rainfall are known to affect tick abundances (Randolph 2004). Such abiotic constraints on the ticks may also explain why we did not find support for the hypothesis that greater tick burdens may be associated with reproductive effort per se. Questing ticks suffer from water loss that can be markedly increased when experiencing high temperatures and low humidity (Needham and Teel 1991; Benoit and Denlinger 2010). As the breeding season of the study species coincides with high temperatures and rainfall, this could account for the observed patterns (Lutermann et al. 2012). The overlap in breeding season and climatic conditions also makes it difficult to distinguish between factors acting directly on the ticks and the effect of host reproductive effort.
Although tick burdens were not elevated during the breeding season, our sex-specific analyses suggest that, at least for females, the reproductive investment associated with pregnancy results in significantly increased larval abundances. This finding is consistent with studies from other mammal species, reporting that females harbour larger ectoparasite loads during pregnancy due to a compromised immune function (Christe et al. 2000; Lourenco and Palmeirin 2008). On the other hand, this could also be linked to increases in foraging activity to meet the greater energetic demands of pregnancy and lactation but results in higher parasite exposure. That a relationship between reproductive effort and parasite loads was not apparent in males is consistent with the notion that sperm production is comparatively ‘cheap’ and thus, investment in reproductive tissue should result in less immune-compromising effects for males. Across both sexes, the effects of reproductive effort may have contributed to the observed age bias in larval abundances and in accordance with our hypothesis yearling individuals sustained lower tick burdens during the breeding season. Similar effects of reproductive investment have been well documented in other species (Christe et al. 2000; Mougeot et al. 2004; Pelletier et al. 2005; Lourenco and Palmeirin 2008) and are likely to explain our finding.
We can largely exclude body mass as a contributing factor to the observed tick burdens, suggesting that any sex bias in tick burdens should be related on physiological or behavioural traits. Adult males appeared to carry the greatest larval abundances. This bias is particularly marked in comparison to yearling males, suggesting that it may be related to reproductive activity. Although E. myurus is socially monogamous, male home ranges are consistently larger and more variable than those of females as a result of male forays into home ranges of temporarily unmated females (Ribble and Perrin 2005). Such differences in ranging behaviour have frequently been suggested to increase the exposure to parasites (e.g. Morand et al. 2004; Krasnov et al. 2005; Boyer et al. 2010; Scantlebury et al. 2010). In addition, males may experience physiological stress during the breeding season as a result of mate guarding that is associated with significant short-term body mass loss as reported for other sengi species (Schubert et al. 2009b). Both of the above strategies are likely to play a greater role for adult males, and this may explain the observed age effect.
In conclusion, the abundance of tick larvae and nymphs were markedly increased during the non-breeding season. This could be a result of changes in energy and nutrient availability to the host and, consequently, a compromised immune function. However, it may also be caused by more favourable climate conditions for larval ticks during this period. Larval tick loads were greater for adult compared to yearling individuals, possibly as a result of reproductive effort. This was directly related to the reproductive effort of maintaining a pregnancy for females, while a similar relationship with investment in reproductive tissue could not be found for males. Reproduction-related behavioural traits may have a greater contribution in causing these patterns in males. Host sex appeared to play only a minor role in generating heterogeneities of larval burdens as would be expected in a monogamous species.
References
Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P (2006) Seasonality and the dynamics of infectious diseases. Ecol Lett 9:467. doi:10.1111/j.1461-0248.(2005)00879.x
Benoit JB, Denlinger DL (2010) Meeting the challenges of on-host and off-host water balance in blood-feeding arthropods. J Insect Physiol 56:1366–1376. doi:10.1016/j.jinsphys.2010.02.014
Boyer N, Réale D, Marmet J, Pisanu B, Chapuis J-L (2010) Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus. J Anim Ecol 79:538–547. doi:10.1111/j.1365-2656.2010.01659.x
Burnham KP, Anderson DR (2002) Model selection and multimodel inference, 2nd edn. Springer, New York
Cattadori IM, Boag B, Bjornstad ON, Cornell SJ, Hudson PJ (2005) Peak shift and epidemiology in a seasonal host–nematode system. Proc R Soc B 272:1163–1169. doi:10.1098/rspb.2004.3050
Christe P, Arlettaz R, Vogel P (2000) Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis). Ecol Lett 3:207–212
Cornell SJ, Bjornstad ON, Cattadori IM, Boag B, Hudson PJ (2008) Seasonality, cohort-dependence and the development of immunity in a natural host–nematode system. Proc R Soc B 275:511–518. doi:10.1098/rspb.2007.1415
Fitzgibbon CD (1997) The adaptive significance of monogamy in the golden-rumped elephant-shrew. J Zool 242:167–177
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
Fourie LJ, Horak IG, van den Heever JJ (1992) The relative importance of rock elephant shrews Elephantulus myurus and Namaqua rock mice Aethomys namaquensis for economically important ticks. S Afr J Zool 27:108–114
Fourie LJ, Du Toit JS, Kok DJ, Horak IG (1995) Arthropod parasites of elephant-shrews, with particular reference to ticks. Mammal Rev 25:31–37
Fourie LJ, Du Toit JS, Kok DJ, Horak IG (2005) Elephant shrews as hosts of immature ixodid ticks. Onderstepoort J Vet Res 72:293–301
Harrison A, Scantlebury M, Montgomery WI (2010) Body mass and sex-biased parasitism in wood mice Apodemus sylvaticus. Oikos 199:1099–1104. doi:10.1111/j.1600-0706.2009.18072.x
Hawlena H, Abramsky Z, Krasnov BR (2006) Ectoparasites and age-dependent survival in a desert rodent. Oecologia 148:30–39. doi:10.1007/s00442-005-0345-4
Hughes VL, Randolph SE (2001) Testosterone depresses innate and acquired resistance to ticks in natural rodent hosts: a force for aggregated distributions of parasites. J Parasitol 87:49–54
Klein SL (2004) Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol 26:247–264
Krasnov BR, Morand S, Hawlena H, Khokhlova IS, Shenbrot GI (2005) Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia 146:219–217. doi:10.1007/s00442-005-0189-y
Lourenco SI, Palmeirin JM (2008) Which factors regulate the reproduction of ectoparasites of temperate-zone cave-dwelling bats? Parasitol Res 104:127–134. doi:10.1007/s00436-008-1170-6
Lutermann H, Medger K, Horak IG (2012) Abiotic and biotic determinants of tick burdens in the eastern rock sengi (Elephantulus myurus). Med Vet Entomol. doi:10.1111/j.1365-2915.2011.01002.x
Martin LB, Weil ZM, Nelson RJ (2008) Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil Trans R Soc Lond B 363:329–339. doi:10.1098/rstb.2007.2142
Medger K (2010). The reproductive biology of two small southern African mammals, the spiny mouse, Acomys spinosissimus (Rodentia: Muridae) and the Eastern rock elephant-shrew, Elephantulus myurus (Macroscelidea: Macroscelididae). PhD thesis. University of Pretoria
Moore SL, Wilson K (2002) Parasites as a viability cost of sexual selection in natural populations of mammals. Science 297:2015–2018
Morand S, Goüy de Belloco J, Stanko M, Miklisovà D (2004) Is sex-biased ectoparasitism related to sexual size dimorphism in small mammals of Central Europe? Parasitology 129:505–510. doi:10.1017/S0031182004005840
Mougeot F, Irvine JR, Seivwright L, Redpath SM, Piertney SB (2004) Testosterone, immunocompetence, and honest sexual signaling in male red grouse. Behav Ecol 15:930–937. doi:10.1093/beheco/arh087
Mougeot F, Redpath SM, Piertney SB (2005) Elevated spring testosterone increases parasite intensity in male red grouse. Behav Ecol 17:117–125. doi:10.1093/beheco/arj005
Mzilikazi N, Lovegrove BG (2004) Daily torpor in free-ranging rock elephant shrews, Elephantulus myurus: a year-long study. Physiol Biochem Zool 77:285–296
Needham GR, Teel PD (1991) Off-host physiological ecology of ixodid ticks. Ann Rev Entomol 36:659–681
Nelson RJ (2004) Seasonal immune function and sickness responses. Trends Immunol 25:187–192
Nelson RJ, Demas GE, Klein SL, Kriegsfeld LJ (2002) Seasonal patterns of stress, immune function, and disease. Cambridge University Press, Cambridge
Olsson M, Wapstra E, Madsen T, Silverin B (2000) Testosterone ticks and travels: a test of the immunocompetence-handicap hypothesis in free-ranging male sand lizards. Proc R Soc B 267:239–2343. doi:10.1098/rspb.2000.1289
Pelletier F, Page KA, Ostiguy T, Festa-Bianchet M (2005) Fecal counts of lungworm larvae and reproductive effort in bighorn sheep, Ovis canadensis. Oikos 110:473–480
Porteus IS, Pankhurst SJ (1998) Social structure of the mara (Dolichotis patagonium) as a determinant of gastro-intestinal parasitism. Parasitology 116:269–275
Poulin R (2007) Evolutionary ecology of parasites. Princeton University Press, Princeton
Randolph SE (2004) Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 129:S37–S65. doi:10.1017/S0031182004004925
Randolph SE, Green RM, Hoodless AN, Peacey MF (2002) An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int J Parasitol 32:979–989
Rathbun GB (1979) The social structure and ecology of elephant shrews. Adv Ethol 20:1–79
Rathbun GB, Rathbun CD (2006) Social structure of the bushveld sengi (Elephantulus intufi) in Namibia and the evolution of monogamy in the Macroscelidea. J Zool 269:391–399. doi:10.1111/j.1469-7998.2006.00087.x
Ribble DO, Perrin MR (2005) Social organization of the Eastern elephant shrew (Elephantulus myurus): the evidence for mate guarding. Belg J Zool 135:167–173
Rolff J (2002) Bateman’s principle and immunity. Proc R Soc Lond B 269:867–872. doi:10.1098/rspb.2002.1959
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86:228–232
Scantlebury M, Maher McWilliams M, Marks NJ, Dick JTA, Edgar H, Lutermann H (2010) Effects of life history traits on parasitism in Grey squirrels (Sciurus carolinensis). J Zool 282:246–255. doi:10.1111/j.1469-7998.2010.00734.x
Schubert M, Pillay N, Ribble DO, Schradin C (2009a) The round-eared sengi and the evolution of social monogamy: factors that constrain males to live with a single female. Ethology 115:972–985. doi:10.1111/j.1439-0310.2009.01684.x
Schubert M, Schradin C, Rödel HG, Pillay N, Ribble DO (2009b) Male mate guarding in a socially monogamous mammal, the round-eared sengi: on costs and trade-offs. Behav Ecol Sociobiol 64:257–264. doi:10.1007/s00265-009-0842-2
Skinner JD, Chimimba CT (2005) The mammals of the southern African subregion, 3rd edn. Cambridge University Press, Cambridge
Soliman S, Marzouk AS, Main AJ, Montasser AA (2001) Effect of sex, size, and age of commensal rat hosts on the infestation parameters of their ectoparasites in a rural area of Egypt. J Parasitol 87:1308–1316
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel interference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Wilson K, Bjornstad ON, Dobson AP, Merler S, Poglaen G, Randolph SE, Read AF, Skorping A (2001) Heterogeneities in macroparasite infections: patterns and processes. In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP (eds) The ecology of wildlife diseases. Oxford University Press, Oxford, pp 6–44
Zuk M, McKean KA (1996) Sex differences in parasite infections: patterns and processes. Int J Parasitol 26:1009–1024
Acknowledgements
We thank the management, particularly D. Dewsnap, and staff of the Goro Game Reserve for permission to collect animals in the reserve and their continuous support. A. Harwood, A. Köhler, W. Matten, K. Ntuli, A. Prins, L. Riato and M. Sebonego are thanked for help during the field work. The manuscript was greatly improved by the comments of three anonymous reviewers. KM acknowledges a doctoral grant from the National Research Foundation (NRF), HL a Research Fellowship from the University of Pretoria and IGH funding from the University of Pretoria and the NRF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Lutermann, H., Medger, K. & Horak, I.G. Effects of life-history traits on parasitism in a monogamous mammal, the eastern rock sengi (Elephantulus myurus). Naturwissenschaften 99, 103–110 (2012). https://doi.org/10.1007/s00114-011-0874-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-011-0874-0