Abstract
A postanal tail is a major synapomorphy of the phylum Chordata, which is composed of three subphyla: Vertebrata, Cephalochordata, and Tunicata (Urochordata). Among tunicates, appendicularians are the only group that retains the tail in the adult, and the adult tail functions in locomotion and feeding in combination with a cellulose-based house structure. Given the phylogenetic position of tunicates, the appendicularian adult tail may possess ancestral features of the chordate tail. We assess the ultrastructural development of the tail epidermis of the appendicularian Oikopleura dioica. The epidermis of the larval tail is enclosed by the larval envelope, which is a thin sheet similar to the outer tunic layer of ascidian larvae. The epidermis of the adult tail seems to bear no tunic-like cellulosic integuments, and the tail fin is a simple folding of the epidermis. Every epidermal cell, except for the triangular cells at the edge of the tail fin, has a conspicuous matrix layer of fibrous content in the apical cytoplasm without enclosing membranes. The epidermis of the larval tail does not have a fibrous matrix layer, suggesting the production of the layer during larval development and metamorphosis. Zonulae adhaerentes firmly bind the epidermal cells of the adult tail to one another, and the dense microfilaments lining the cell borders constitute a mechanical support for the cell membranes. The intracellular matrix, cell junctions, and cytoskeletons probably make the tail epidermis a tough, flexible shell supporting the active beating of the oikopleuran adult tail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The subphylum Tunicata (Urochordata) is one of the three subphyla of the phylum Chordata, and recent molecular phylogenies indicate that the Tunicata is the closest sister group to ours (i.e., subphylum Vertebrata) among the extant taxa (Delsuc et al. 2006; Putnam et al. 2008). All tunicates produce a cellulosic extracellular matrix outside the epidermis; this is one of the most remarkable synapomorphies of this taxon because cellulose synthesis has never been found in any other metazoans to date. The ability to make cellulose arose once in the tunicate ancestor via horizontal gene transfer from a bacterial lineage (Matthysse et al. 2004; Nakashima et al. 2004; Sagane et al. 2010). Ascidians and thaliaceans (salps, doliolids, and pyrosomes) have a gelatinous, leathery, or cartilaginous matrix called the tunic that covers their epidermis. Cellulosic components in the tunic have been well documented in ascidians (e.g., Van Daele et al. 1992; Helbert et al. 1998; Nakashima et al. 2008), and the presence of cellulose was also demonstrated in the tunic of salps, doliolids, and pyrosomes (Belton et al. 1989; Okamoto et al. 1996; Hirose et al. 1999). Recent genomic surveys of ascidians uncovered the presence of a cellulose synthase (CesA) gene that is similar in structure to bacterial CesA genes rather than those of higher plants (Matthysse et al. 2004; Nakashima et al. 2004).
The class Appendicularia (Larvacea) is one of the three classes of Tunicata, together with ascidians and thaliaceans. Although no appendicularians have a tunic in the adult stage, specialized epidermal cells called oikoplastic cells in the anterior trunk secrete a “house,” an extracellular apparatus that concentrates and filters food particles (see Flood and Deibel 1998). The appendicularian house and the larval envelope were shown to contain cellulose by electron diffraction analysis and Fourier transform infrared spectroscopic microscopy (Kimura et al. 2001; Nakashima et al. 2011). Recently, two CesA genes were found in the appendicularian Oikopleura dioica Fol 1872 (Sagane et al. 2010; Nakashima et al. 2011); one of the two genes is involved in making the larval envelope and the other in making the house of adults. Also, recent studies reported that a hexagonal epidermis covering the posterior trunk and tail, as well as the house and larval envelope, shows an immunohistochemical signal for the carbohydrate-binding module (CBM) family 3, with a strong affinity for cellulose and chitin (Goldstein et al. 1993; Nakashima et al. 2011). Notably, the tail and posterior trunk epidermis have a CBM-positive component, although this area has neither tunic nor house-secreting cells. This fibrous matrix is distributed in the epidermal cells as a scalelike structure, indicating that the epidermal cells in the appendicularian tail have some unique structures among tunicates. Bone and Mackie (1975) also described a “fibrous or cuticular layer” in the epidermal cells of O. dioica and Oikopleura labradoriensis Lohmann 1892, and the authors mentioned the possibility that this layer in the epidermis is made up of tunic fibrils (i.e., cellulose) based on the fibrous fine structure. The same structures were described as “web of fibrous material in the apical cytoplasm” (Burighel et al. 1989) and “apical mesh-like sheet” (Stach 2007) in the tail epidermal cells of O. dioica.
From the viewpoint of chordate phylogeny, the tail has been a highly debated organ in tunicates (e.g., Garstang 1928; Nishino and Satoh 2001) ever since the discovery of the notochord in ascidians (Kowalevsky 1866). A postanal tail is a major synapomorphy of the phylum Chordata, and thus it may be useful to survey the similarity and diversity of tail morphology among tunicate taxa to better understand their phylogenetic relationships. Appendicularians are the only tunicate group that uses a tail throughout life; the adult tail is an active motor organ for swimming, feeding, and expanding the house rudiment (see Bone and Mackie 1975; Flood and Deibel 1998). The adult appendicularian tail differs considerably from those of ascidian larvae in the presence of a cellular fin and the absence of a tunic. Whereas fine structures of oikopleuran tail epidermis have been examined in both adults and larvae (Bone and Mackie 1975; Bone 1985; Burighel et al. 1989; Stach 2007), the intracellular matrix in the apical cytoplasm has not been described as the scalelike structure. In the present study, we examined the epidermal morphology of the tail in the larvae and adults of O. dioica focusing on the cytodifferentiation of epidermal cells and the details of the intracellular matrix, which is a potentially cytoskeletal structure supporting the tail epidermis. Cell junctions of the tail epidermis are also more complex in the adult than those in the larva. It is possible that the adult tail of the ancestral chordate is retained in appendicularians and it was lost in the other tunicate taxa through the diversification of tunicates. In this case, the traits identified in this study may represent a terminal differentiation of epidermal cells in the tailed bodies of Tunicata that is hidden in other tunicates whose tailed body plans are eliminated through metamorphosis.
Materials and methods
Animals
O. dioica was collected near the Seto Marine Biological Laboratory, Tanoura port (Kii Peninsula, Wakayama, Japan), Sakoshi port, or Aioi Bay (both in Hyogo, Japan) and cultured in 5- or 10-L beakers with constant stirring at 18°C as described previously (Fujii et al. 2008; Nishida 2008). Some hatching larvae (stage 1, Fenaux 1998) and adults (days 3–5 at 20°C) were transferred to plastic dishes and rinsed with artificial seawater (Rohtomarine; Rei-Sea, Tokyo, Japan) before fixation.
Light and fluorescence microscopy
Living adults were anesthetized using 0.01% (weight/volume) tricaine (MS-222, A5040; Sigma, St. Louis, MO, USA) and examined under a dissection microscope (SZX16; Olympus, Tokyo, Japan and CoolSNAP digital photo system; Nikon, Tokyo, Japan). For a whole-mount photograph of the hatching larva, animals were fixed with 4% paraformaldehyde in 0.5 M NaCl and 0.1 M MOPS (pH 7.5) for 1 day at 4°C. After rinses with phosphate-buffered saline (PBS) containing 0.1% Tween 20, the specimens were photographed via Nomarski differential interference contrast (DIC) view (Olympus BX61 and DP72 digital camera). Several images were captured for each specimen with slight differences in the focal planes, and the multiple images were combined to increase the depth of field using the post-processing image software Helicon Focus Pro 4.2.7 (Helicon Soft, Ltd., Kharkov, Ukraine).
The microfilaments in the epidermal cells were visualized using BODIPY FL phallacidin (B607; Invitrogen, Carlsbad, CA, USA). At days 3–5, adults were relaxed on ice and then fixed using the same fixative as above at 4°C for 2 days. After several washes with PBS and two washes with 0.1 M Tris buffer (pH 8.0), the specimens were soaked in BODIPY FL phallacidin (1 U/200 µL of the Tris buffer) for 1 h at room temperature (∼20°C). After washes with PBS, the tail epidermis was observed under a fluorescence light microscope (the system mentioned above) through an objective (×20, na = 0.70) and “WIB” filter cube (Olympus).
Electron microscopy
The hatching larvae and adults were fixed with 2.5% glutaraldehyde–0.45 M sucrose–0.1 M cacodylate buffer (pH 7.5) at 4°C for 2 h. The specimens were briefly rinsed with the same buffer, postfixed with 1% osmium tetroxide–0.1 M cacodylate buffer (pH 7.5) at 4°C for 1.5 h, then dehydrated through an ethanol series.
For transmission electron microscopy (TEM), the specimens were embedded in an epoxy resin. Sections were stained with uranyl acetate and lead citrate and examined under a transmission electron microscope (JEM-1011; JEOL, Tokyo, Japan) at 80 kV.
For scanning electron microscopy (SEM), some adult specimens were dehydrated through an ethanol series, immersed in t-butanol and freeze-dried, sputter coated with gold–palladium, and examined under a scanning electron microscope (JSM-6060LV; JEOL) at 15 kV. Some other adults were embedded in styrene resin and sectioned until the desired structures were exposed. Then the resin was removed by soaking the specimens in acetone (1 h each through three changes). After a brief rinse with ethanol, the specimens were processed for SEM observation as described above.
Results
Larvae
Newly hatched larvae of O. dioica were about 250 μm long (100 μm trunk and 150 μm tail), and each larva was enveloped in a thin, acellular membrane referred to as a larval envelope in this report (Fig. 1a). While the larval envelope tightly wraps most parts of the tail, it forms a caudal appendage at the distal end of the tail. In the cross sections of the tail near the caudal appendage, the envelope formed a finlet (Fig. 1b, c). The larval envelope is an electron-dense layer about 5 nm thick, with fine projections (∼50 nm in height) on its surface. The finlet is a simple folding of the envelope, without supporting structures in the lumen. In cross section, the finlet surface has fine projections, as found on the larval envelope wrapping the tail body (Fig. 1c).
Newly hatched larvae of Oikopleura dioica (a DIC, b–g TEM). a Whole-mount larva. The larval envelope entirely covers the larva (white arrows) and forms a caudal appendage at the distal end of the tail (white arrowhead). Scale bar 100 μm. b Cross section of the tail near the caudal appendage. The larval envelope forms the finlets. Scale bar 2 μm. c Cross section of the finlet. The epidermal cell has a cellular process at the base of the finlet (double arrows). Scale bar 0.5 μm. Fine projections occur on the surface of the envelope (inset: scale bar 0.1 μm). d Semi-sagittal section of the tail. The tail consists of epidermal cell (ec), immature muscle mesoderm (me), and immature notochord (nc), and they all contain vesicular bodies with tiny vesicles (ve). Scale bar 2 μm. e Enlargement of the ectoderm. A tight junction (tj) exists between the ectodermal cells. Scale bar 0.5 μm. f, g Secretion of the contents of the vesicular bodies into the space between the ectoderm and the larval envelope. Cellular projections (double arrows) often contain small vesicles. Scale bars 0.5 μm. Arrows, finlets; arrowheads, larval envelope
At this stage, the tail had a three-layered structure: immature notochord cells surrounded by immature muscle cells, with the epidermis entirely covering them (Fig. 1d). Cytodifferentiation was not prominent in these tissues, whereas tight junctions were found among the epidermal cells (Fig. 1e, f). Round or elliptical vesicular bodies are often found not only in the epidermis but also in immature notochord and muscle cells (Fig. 1d, e). The vesicular body is about 1 μm in diameter and contains many tiny vesicles of 50 nm diameter or less. The contents of some of the vesicular bodies appear to be released from the epidermal cells into the space between the larval envelope and the epidermis (Fig. 1f, g). The epidermal cells also have cellular projections that contain small vesicles (Fig. 1c, g).
Adults
At the end of the larval stage, the animal sheds its larval envelope and the anterior part of the trunk starts to secrete the house. Other than the house produced by the trunk epidermal cells, the adult Oikopleura appears to have no integumentary matrices overlying its body (Fig. 2a). While the appendicularian tail rotates 90° during development, the dorsal side of the tail is defined by the presence of the neural tube in this report. In cross sections of the tail of mature adults, the notochord is laterally sandwiched by muscle cells of about 12 μm thickness, and the simple squamous epidermis entirely covers them (Figs. 2b, c and 3a). The fin is a simple folding of the epidermis. While an epidermal cell is usually about 0.2 μm thick, it is thicker at the base of the fin (asterisks in Fig. 2d, e). On the muscle cells facing the notochord and sinus, filamentous extracellular matrices cover the cell surface (Fig. 2e), while a gap is often found between the notochord and muscle cells (asterisk in Fig. 2f). In contrast, the muscle cells are tightly attached to the epidermis through the basal lamina (Fig. 2f). In the muscle cells, the muscle fibers occupy half of the cytoplasm on the notochord/sinus side, and wedge-shaped mitochondria are distributed side by side in the middle portion of the cells, while the remaining part of the cytoplasm (i.e., epidermal side) is filled with ribosomes (Fig. 2f).
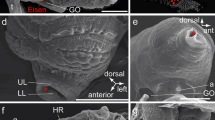
Adult of O. dioica. a Whole-mount adult (dorsal view). The tail elongates to the left. Scale bar 0.5 mm. b, c Cross section of the tail (b histological section stained with toluidine blue; c SEM). Epidermis of the tail forms the fin. Scale bars 20 μm. d, e Cross section of the fin base (d TEM, e SEM). Thin epidermis (facing arrowheads) is overlaid on the muscle cells (mu) and thickens at the fin base (asterisk). Scale bars 5 μm. f Cross section of the epidermis (ep)–muscle (mu) of the tail body (TEM). A gap exists between muscle and notochord cells (asterisk). Scale bar 0.5 μm. ep epidermis, mt mitochondrion, nc notochord, ne nerve cord, si sinus
Epidermal cells in the tail of adult O. dioica (a, b SEM, c–f TEM, g epifluorescence). a Squamous epidermal cells have a polygonal shape. Scale bar 10 μm. b Enlargement of the border between the epidermal cells. Some protrusions are seen on the cell surface (arrows). Scale bar 1 μm. c Cross section of an epidermal cell showing the fibrous matrix in the apical cytoplasm (facing arrowheads) and mitochondrion (mt). Scale bar 0.2 μm. d–f Narrow tight junction (white arrowhead), zonulae adhaerentes (black arrows), and possible gap junctions (white arrows) between the epidermal cells covering the muscle cells (d) and those of the fin (e, f). Facing arrowheads indicate the intracellular matrix layer. Scale bars 0.1 μm. g The polygonal cell borders are stained with BODIPY FL-conjugated phallacidin in the tail fin (fluorescence micrograph). Several bundles of microfilaments can be seen in the epidermal cells. Scale bar 50 μm. ba basal lamina, mt mitochondrion, mu muscle cell
Each epidermal cell is a polygonal plate and the tail is covered with these plates without any chinks (Fig. 3a). The border between the epidermal cells forms a ridge, and small protrusions occur on the flat surface of the epidermis (Fig. 3b). The epidermal cells always have a layer of fibrous matrix in the apical cytoplasm (arrowheads in Fig. 3c) and often contain mitochondria. The cell membrane on the basal side is flat and adheres to the muscle cells through a thin basal lamina. Some fuzzy materials appear on the apical cell surface. The epidermal cells composing the fin have the same morphology as those covering the muscle band; the fibrous matrix is always found in the apical cytoplasm. The fin epidermal cells also contain mitochondria, and the cell membrane of the basal side is lined with basal lamina. The lumen of the fin is loosely filled with materials similar to those of the basal lamina. While the ridge between the epidermal cells is not prominent in sections, zonulae adhaerentes are found in the lateral membranes (black arrows in Fig. 3d–f). The zonulae adhaerentes are always located at the apical part of the cells where the intracellular matrix layer is, suggesting an interconnection between them. A narrow tight junction can be detected as a narrowing of the gap between the lateral membranes (white arrowhead in Fig. 3e), but it is often obscure in oblique sections. In some sections, the two lateral membranes are closely apposed, probably forming gap junctions (white arrows in Fig. 3e, f). Phallacidin staining visualized the polygonal shape of epidermal cells in the fin (Fig. 3g), indicating that the lateral cell membranes are lined with microfilaments that are probably associated with the zonulae adhaerentes. Aside from the lining of the cell border, several bundles of microfilaments exist in the epidermal cells (Fig. 3g) that are aligned approximately perpendicular to the long axis of the tail. The bundles are various in length and rarely penetrate the entire cell diameter from one side to the other.
In cross sections at the edge of the fin, a triangular epidermal cell links the left and right epidermal sheets (Fig. 4a). This edge cell does not have an intracellular matrix, whereas the adjacent epidermal cells do (Fig. 4b). The apical cell surface is covered with fuzzy material. Tight junctions occur between the edge cell and adjacent epidermal cells (arrowhead in Fig. 4b), but zonulae adhaerentes are not observed (arrowhead in Fig. 4b). Beneath the edge cell is a minute cytoplasmic profile of only 0.1 μm or less in diameter, which is probably a nerve fiber running beneath the edge cells rostrocaudally (arrow in Fig. 4b). The base and lateral border of the edge cells were heavily stained with phallacidin, indicating the dense distribution of microfilaments there (Fig. 4c).
Edge of the fin in the tail of O. dioica (TEM). a Triangular cells linking left and right epidermal sheets. Arrowheads indicate the junction with adjacent epidermal cells. Scale bar 0.5 μm. b Enlargement of the junction between the edge cell and adjacent cells. The intracellular matrix layer is found in the adjacent epidermal cells (facing arrowheads) but not in the edge cell. The arrow indicates the minute cytoplasmic profile beneath the edge cell. Scale bar 0.2 μm. c Microfilaments in the fin stained with phallacidin conjugated with BODIPY FL (fluorescence micrograph). The borders of the edge cells are heavily stained. Scale bar 50 μm. lu lumen of the fin
Discussion
The apical intracellular matrix is a scalelike covering of the epidermis
Alkaline treatment of the adult Oikopleura resulted in a thin sheet consisting of hexagonal scalelike structures (Nakashima et al. 2011). The shape of these scalelike plates corresponds to the polygonal shape of the epidermal cells (Fig. 3a), while the tail epidermis has no extracellular matrix (Fig. 3c–f). Accordingly, the scalelike structures were identified as the intracellular fibrous matrix of the epidermis. These scalelike plates consist of meshwork embedded in amorphous matrix, and this meshwork has an affinity to the CBM family 3 even after acetic/nitric acid hydrolysis treatment following Updegraff (1969, see Nakashima et al. 2011). Therefore, the intracellular matrix is believed to consist of some component(s) resistant to alkaline treatment and acid hydrolysis for cellulose purification. However, this is not a strict demonstration of the presence of cellulose, as CBM family 3 has affinity not only to cellulose but also to some other polysaccharides, such as chitin (see Goldstein et al. 1993), and some non-cellulosic components sometimes remain after the acetic/nitric acid hydrolysis. Most proteins should be excluded by this treatment, but we would not deny the presence of proteinaceous components if they are tightly combined or masked by other robust molecules, such as cellulose or chitin. However, neither electron diffraction nor Fourier transform infrared spectroscopic microscopy could demonstrate the presence of cellulose due to technical reasons, and at the very least, both of the two CesA genes so far identified in O. dioica were not expressed in the tail epidermis (Sagane et al. 2010; Nakashima et al. 2011).
Therefore, we are not able to identify the CBM-positive component in the scalelike plates, although we know that it is stable through the treatments for cellulose purification and is CBM positive. Even if it is cellulose, chitin, or another polysaccharide, intracellular accumulation of such a fibrous matrix, without surrounding endomembranes, is a remarkable feature of the Oikopleura epidermis. The chemical composition of the fibrous matrix in the tail epidermis should be investigated to consider the potential diversification of cellulosic integuments, which comprises a major synapomorphy for tunicates. Recent development of genomic study in O. dioica will potentially provide the molecular basis to determine this unique feature (see Denoeud et al. 2010).
A tough shell composed of a scalelike intracellular matrix and intercellular junctions
In the adult tail, the simple squamous epidermis entirely covers the tail and forms the fin as well. The epidermal cells are thin, polygonal plates, except for the cells at the fin base, and they are not overlain by extracellular matrix. In these cells, the apical cell membrane is always lined with the thick, fibrous matrix layer, or the scalelike plate, which may have cytoskeletal functions. Moreover, several bundles of microfilaments are oriented perpendicular to the long axis of the tail. These bundles probably strengthen the epidermal cells together with the scalelike matrix.
Burighel et al. (1989) reported that the tail epidermal cells of O. dioica are joined by narrow tight junctions under which lie zonulae adhaerentes and gap junctions. The presence of gap junctions was also indicated by Bone and Mackie (1975) and Bone (1985) who demonstrated that the epidermal cells of Oikopleura propagate electrical impulses. The combination, arrangement, and fine structures of these cell junctions of our present observation agree well with the preceding study. Phallacidin staining showed that the lateral cell membrane is lined with bundles of microfilaments that could combine with the zonulae adhaerentes. We also found that the scalelike apical matrix has a close association with the zonulae adhaerentes.
This animal lacks a tunic covering the whole body, so that this intracellular matrix layer and the tight reciprocal attachment of epidermal cells should make the tail epidermis a tough sheet, like a scale armor. The muscle cells in the adult tail are firmly attached to the epidermis via the basal lamina, but only loosely to the notochord. The epidermis is thus subjected to repetitive muscle contractions. To support the active movement of the tail, the epidermis should be a tough sheet. This sheet may provide mechanical support against shear stress on the surface of epidermis, which should not be ignored for a swimmer in an aquatic milieu with a low Reynolds number. At the same time, the epidermal sheet should be flexible enough to allow the undulating tail beat. This may be the reason why the bundles of microfilaments in the epidermal cells are oriented perpendicular to the long axis of the tail. These oriented cytoskeletons can provide mechanical support for the cellular fin, like fish fin rays, and also keep flexibility for the dynamic changes in cell length along the axis. The triangular edge cells of the fin are in turn subjected to reciprocal slippage of the left and right epidermal sheets during the tail beats, and consistent with this finding, they lack the intracellular matrix layer but develop a different sort of junction having a heavier microfilament lining at the interface with the epidermal sheet. In the present study, the extracellular matrices lining the fin epidermis appeared to fill the lumen loosely in TEM observations, but they have well-organized arrangement in SEM observation by Flood (1975).
Development of the epidermal shell
Newly hatched larvae of O. dioica are entirely covered with a thin, electron-dense membrane, i.e., larval envelope. Fenaux (1998) described the envelope as a “thin acellular membrane” and suggested that it is comparable to the tunic of ascidian larvae. Stach (2007) also supposed that this structure is the cuticle of the tunic of Oikopleura larvae, and he suggested the homology between this extracellular covering and the tunic of ascidian larvae. These interpretations would be strongly supported by the presence of cellulose in the caudal appendage, which is a posterior extension of the larval envelope (Nakashima et al. 2011). In ascidians, tadpole larvae always have two layers in the tunic, an outer and an inner tunic layer (see Burighel and Cloney 1997). The outer layer is a thin tunic layer entirely covering the larva and has no cellular component, whereas the inner layer is thicker and denser than the outer layer and contains various tunic cells in its matrix. Since the ascidian larva sloughs off the outer tunic layer during metamorphosis, the larval envelope of Oikopleura would correspond to the outer tunic layer of ascidians rather than the inner one. In cross sections of the larval envelope including the finlet, one can see fine projections of about 50 nm high on the surface (Fig. 1c). Moreover, the tunic of some ascidians and salps has minute protrusions 100 nm high or lower on the cuticular surface (Hirose et al. 1997, 1999), although the functions of these protrusions are unknown.
In the larva, the cells constituting the tail epidermis appear to remain undifferentiated, except for the presence of tight junctions between the epidermal cells. Vesicular bodies are often found in the tail cells, and those in epidermal cells sometimes release their vesicular contents into the space between the larval envelope and the epidermis, as described by Stach (2007). The functions of the released vesicles are still unknown, but they may be involved in the formation, growth, or shedding of the larval envelope, although the vesicular bodies are also found in the immature notochord and muscle cells. These vesicles may be involved in the formation of intercellular space (central lumen) during notochord development (see Nishino et al. 2001) and also the cavity of the tail sinus. In any case, vesicular bodies are not present in the tail of adult specimens. The tail epidermis of the newly hatched larva has neither an intracellular matrix layer nor zonulae adhaerentes, which are found in the adult tail epidermis, suggesting that the formation of these subcellular structures occurs in the period of larval development and metamorphosis. The tight junctions between the larval tail epidermis (Fig. 1e, f) are longer and more prominent than the very narrow junctions in the adult tail epidermis (Fig. 3e, see also Burighel et al. 1989; Stach 2007). This may indicate that the tight junctions in the larval tail function as both permeability barriers and cell adhesion, and the latter function is entrusted to zonulae adhaerentes in the adult tail. Interestingly, oikoplast cells in the adult trunk are laterally joined by wide tight junctions (Burighel et al. 1989).
Phylogenetic perspective
In the tailed bodies of tunicates, the tail epidermis varies considerably in morphology. In ascidian larvae, the tail epidermis is entirely covered with a tunic that forms the fins, and the epidermal cells have tight junctions but not zonulae adhaerentes (reviewed by Burighel and Cloney 1997). These character states are similar to those of the tail epidermis in an appendicularian larva. In contrast, the epidermis of the tail in the appendicularian adult forms the fins, lacks an extracellular covering, and has an intracellular matrix and well-developed zonulae adhaerentes. While most tunicates eliminate the tail through metamorphosis, appendicularians retain the tail throughout life as a motor organ for free swimming, pumping water containing food particles through the house, and for expanding the house rudiment (see Bone and Mackie 1975). Whereas the characteristics of the larval epidermis are comparable between appendicularians and ascidians, the adult epidermis of the appendicularian exhibits highly specialized features, including scalelike intracellular plates and elaboration of the zonulae adhaerentes. Some phylogenetic studies have proposed that appendicularians are basal to other metamorphic tunicates (Holland et al. 1988; Holland and Holland 1989; Wada 1998; Nishino and Satoh 2001; Delsuc et al. 2006). Under this phylogenetic hypothesis, the inner tunic layer of the ascidian larva and the tunic of adult ascidians and thaliaceans are regarded to be apomorphies, while the features of the appendicularian adult tail demonstrated in the present study represent a terminal differentiation of the tail epidermis of tunicates that is lost in ascidians and thaliaceans. Development of a full set of junctional complexes in appendicularians from an immature state with a simple tight junction may support this line of discussion. In contrast, the basal positioning of appendicularians has recently been questioned by several investigations (Zeng and Swalla 2005; Stach 2007; Stach et al. 2008). Stach et al. (2008) suggested that the occurrence of tail rotation during Oikopleura development offers evidence to place the appendicularians as a sister group to aplousobranchian ascidians.
Determining plesiomorphy in this context would be valuable for resolving this issue, i.e., to compare the epidermis in cephalochordates and/or primitive vertebrates (e.g., cyclostomes) with the tail epidermis of appendicularians. In both cephalochordates and cyclostomes, the epidermis is lined with a thick, collagenous lamella that serves as the mechanical support for the epidermis, while secretory mucous vesicles occupy the apical cytoplasm of epidermal cells (e.g., Downing and Novales 1971; Ruppert 1997). This may indicate that the presence of a matrix layer in the apical cytoplasm is a unique feature of appendicularians among chordates. In contrast, the several types of cell junctions would be a common feature for chordates. Various cellular junctions are actually found in the epidermis of cephalochordates and cyclostomes, e.g., the epidermis of cephalochordates has zonulae adhaerentes and septate junctions although it lacks tight junctions and gap junctions (Lane et al. 1987; Ruppert 1997). One must also observe the tail epidermis in other appendicularian species to determine the generality of the intracellular matrix and cellular junctions in Appendicularia and to identify the chemical content of the intracellular scalelike matrix. Such comparative studies of epidermal structures will uncover useful findings to clarify the history of diversification of appendicularians and other tunicate taxa.
References
Belton PS, Tanner SF, Cartier N, Chanzy H (1989) High-resolution solid-state 13C nuclear magnetic resonance spectroscopy of tunicin, an animal cellulose. Macromolecules 22:1615–1617
Bone Q (1985) Epithelial action potentials in Oikopleura (Tunicata: Larvacea). J Comp Physiol A 156:117–123
Bone Q, Mackie GO (1975) Skin impulses and locomotion in Oikopleura (Tunicata: Larvacea). Biol Bull 149:267–286
Burighel P, Cloney R (1997) Urochordata: Ascidiacea. In: Harrison F, Ruppert E (eds) Hemichordata, Chaetognatha, and the invertebrate chordates. Microscopic anatomy of invertebrates, vol. 15. Wiley-Liss, New York, pp 221–347
Burighel P, Lane NJ, Martinucci GB, Dallai R (1989) Junctional diversity in two regions of the epidermis of Oikopleura dioica (Tunicata, Larvacea). Cell Tissue Res 257:529–535
Delsuc F, Brinkmann H, Chourrout D, Philippe H (2006) Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 493:965–968
Denoeud F, Henriet S, Mungpakdee S et al (2010) Plasticity of animal genome architecture unmasked by rapid evolution of a pelagic tunicate. Science 330:1381–1385
Downing SW, Novales RR (1971) The fine structure of the lamprey epidermis. I. Introduction and mucous cells. J Ultrastruct Res 35:282–294
Fenaux R (1998) Life history of the Appendicularia. In: Bone Q (ed) The biology of pelagic tunicates. Oxford University Press, Oxford, pp 151–159
Flood PR (1975) Scanning electron microscope observations on the muscle innervation of Oikopleura dioica Fol (Appendicularia, Tunicata) with notes on the arrangement of connective tissue fibres. Cell Tissue Res 164:357–369
Flood PR, Deibel D (1998) The appendicularian house. In: Bone Q (ed) The biology of pelagic tunicates. Oxford University Press, Oxford, pp 105–124
Fujii S, Nishio T, Nishida H (2008) Cleavage pattern, gastrulation, and neurulation in the appendicularian, Oikopleura dioica. Dev Genes Evol 218:69–79
Garstang W (1928) The morphology of the Tunicata and its bearing on the phylogeny of the Chordata. Q J Microsc Sci 72:51–187
Goldstein MA, Takagi M, Hashida S, Shoseyov O, Doi RH, Segel IH (1993) Characterization of the cellulose-binding domain of the Clostridium cellulovorans cellulose-binding protein A. J Bacteriol 175:5762–5768
Helbert W, Nishiyama Y, Okano T, Sugiyama J (1998) Molecular imaging of Halocynthia papillosa cellulose. J Struct Biol 124:42–50
Hirose E, Lambert G, Kusakabe T, Nishikawa T (1997) Tunic cuticular protrusions in ascidians (Chordata, Tunicata): a perspective of their character-state distribution. Zool Sci 14:683–689
Hirose E, Kimura S, Itoh T, Nishikawa J (1999) Tunic morphology and cellulosic components of pyrosomas, doliolids, and salps (Thaliacea, Urochordata). Biol Bull 196:113–120
Holland ND, Holland LZ (1989) Fine structural study of the cortical reaction and formation of the egg coats in a lancelet, Branchiostoma floridae. Biol Bull 176:111–122
Holland LZ, Gorsky G, Fenaux R (1988) Fertilization in Oikopleura dioica (Tunicata, Appendicularia): acrosome reaction, cortical reaction, and sperm-egg fusion. Zoomorphology 108:229–243
Kimura S, Ohshima C, Hirose E, Nishikawa J, Itoh T (2001) Cellulose in the house of the appendicularian Oikopleura rufescens. Protoplasma 216:71–74
Kowalevsky A (1866) Entwicklungsgeschichte der einfachen Ascidien. Mem Acad Impériale Sci St-Petersbourg 10:1–19
Lane NJ, Dallai R, Martinucci GB, Burighel P (1987) Cell junctions in amphioxus (Cephalochordata): a thin section and freeze-fracture study. Tissue Cell 19:399–411
Matthysse A, Deschet K, Williams M, Marry M, White A, Smith W (2004) A functional cellulose synthase from ascidian epidermis. Proc Natl Acad Sci U S A 101:986–991
Nakashima K, Yamada L, Satou Y, Azuma J, Satoh N (2004) The evolutionary origin of animal cellulose synthase. Dev Genes Evol 214:81–88
Nakashima K, Sugiyama J, Satoh N (2008) A spectroscopic assessment of cellulose and the molecular mechanisms of cellulose biosynthesis in the ascidian Ciona intestinalis. Mar Genom 1:9–14
Nakashima K, Nishino A, Horikawa Y, Hirose E, Sugiyama J, Satoh N (2011) The crystalline phase of cellulose changes under developmental control in a marine chordate. Cell Moll Life Sci 68:1623–1631
Nishida H (2008) Development of the appendicularian Oikopleura dioica: culture, genome, and cell lineages. Dev Growth Differ 50:S239–S256
Nishino A, Satoh N (2001) The simple tail of chordates: phylogenetic significance of appendicularians. Genesis 29:36–45
Nishino A, Satou Y, Morisawa M, Satoh N (2001) Brachyury (T) gene expression and notochord development in Oikopleura longicauda (Appendicularia, Urochordata). Dev Genes Evol 211:219–231
Okamoto T, Sugiyama J, Itoh T (1996) Structural diversity of Ascidian crystalline celluloses. Wood Res 83:27–29
Putnam NH, Butts T, Ferrier DEK et al (2008) The amphioxus genome and the evolution of the chordate karyotype. Nature 453:1064–1072
Ruppert EE (1997) Cephalochordata (Acrania). In: Harrison F, Ruppert E (eds) Hemichordata, Chaetognatha, and the invertebrate chordates. Microscopic anatomy of invertebrates, vol. 15. Wiley-Liss, New York, pp 349–504
Sagane Y, Zech K, Bouquet JM, Schmid M, Bal U, Thompson EM (2010) Functional specialization of cellulose synthase genes of prokaryotic origin in chordate larvaceans. Development 137:1483–1492
Stach T (2007) Ontogeny of the appendicularian Oikopleura dioica (Tunicata, Chordata) reveals characters similar to ascidian larvae with sessile adults. Zoomorphology 126:203–214
Stach T, Winter J, Bouquet JM, Chourrout D, Schnabel R (2008) Embryology of a planktonic tunicate reveals traces of sessility. Proc Natl Acad Sci U S A 105:7229–7234
Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32:420–424
Van Daele Y, Revol JF, Gaill F, Goffinet G (1992) Characterization and supramolecular architecture of the cellulose-protein fibrils in the tunic of the sea peach (Halocynthia papillosa, Ascidiacea, Urochordata). Biol Cell 76:87–96
Wada H (1998) Evolutionary history of free-swimming and sessile lifestyles in urochordates as deduced from 18S rDNA molecular phylogeny. Mol Biol Evol 15:1189–1194
Zeng L, Swalla BJ (2005) Molecular phylogeny of the protochordates: chordate evolution. Can J Zool 83:24–33
Acknowledgment
We are indebted to Dr. Hiroki Nishida (Osaka University) and Dr. Nori Satoh (Okinawa Institute of Science and Technology Promotion Corporation) for providing facilities and encouragements. We would like to express our sincere thanks to the reviewers for their detailed, kind, and constructive comments. This study is partially supported by grants-in-aid for young scientists (B) to KN (10422924) and AN (20770046) from MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Nakashima, K., Nishino, A. & Hirose, E. Forming a tough shell via an intracellular matrix and cellular junctions in the tail epidermis of Oikopleura dioica (Chordata: Tunicata: Appendicularia). Naturwissenschaften 98, 661–669 (2011). https://doi.org/10.1007/s00114-011-0815-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-011-0815-y